Catalysts by the meter: rapid screening approach of N-heterocyclic carbene ligand based catalysts†‡
Carolin
Lang
,
Ute
Gärtner
and
Oliver
Trapp
*
Ruprecht-Karls-Universität Heidelberg, Organisch-Chemisches Institut, Im Neuenheimer Feld 270, 69120 Heidelberg, Germany. E-mail: trapp@oci.uni-heidelberg.de; Fax: +49 (0) 6221 544904; Tel: +49 (0) 6221 548470
First published on 8th September 2010
Abstract
Here, we demonstrate a versatile screening platform for NHC ligand based catalysts by coating fused-silica micro capillaries with a bonded 1,3-bismesityl-2-imidazolidinylidene ligand. Such micro capillaries can be efficiently converted into (pre)-catalysts from various organometallic precursors by solid-phase chemistry techniques and can be quantitatively screened using on-column reaction chromatography.
N-Heterocyclic carbenes (NHCs) are highly versatile ligands1–3 and are of major focus in the development of highly active catalysts4–7 in a broad range of reactions with superior properties in regard to stability and use in various reaction media and conditions.8,9 NHCs have been immobilized10 on a variety of soluble and insoluble polymers and inorganic materials, i.e. on silica.11–13 Advantages of immobilization14,15 are the recyclability of catalysts, easy removal from reaction mixtures, and minimisation of leaching of expensive catalysts, which is of great importance for the practicability of catalysts in industrial applications.
Here, we present a novel strategy utilizing the permanent immobilisation of NHC pre-ligands on the inner surface of fused-silica micro capillaries (id 250 μm) with a defined film thickness (between 50 to 1000 nm) of the polymeric matrix.
Such NHC pre-ligand modified fused-silica capillaries can be converted to NHC ligands and with suitable organometallic precursors to pre-catalysts utilizing all advantages of solid-phase organic chemistry, i.e. high reagent concentrations, simple removal of non-reacted reagents, and analytical techniques at the same time (cf.Fig. 1).
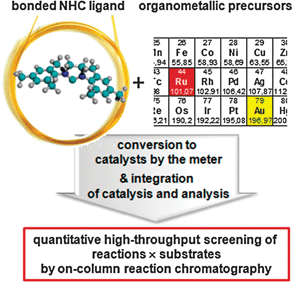 | ||
| Fig. 1 Strategy utilizing immobilised NHC ligands in fused-silica micro capillaries to create NHC ligand based catalysts (here Ru and Au catalysts) for high-throughput investigations by on-column reaction chromatography. | ||
Reactions can be directly monitored by implementing such capillaries in chromatographic systems. Furthermore, the integration of catalysis and analysis by on-column chromatography allows rapid and precise high-throughput measurements of kinetic and thermodynamic data, which recently was made possible by the derivation of mathematical functions to access kinetic parameters from such experiments.16 These data are important to tune reaction conditions and to understand reactions on a molecular level.17–21 On the other hand this approach can be also switched to a preparative micro reactor mode22–25 by continuous feeding of reagents into the catalytically active capillary.17,26
To immobilise the prototype of NHC ligands, a 1,3-bismesityl-2-imidazolidinylidene ligand, we considered a similar strategy used by Blechert et al.10 to modify the backbone of the 2-imidazolidinylidene moiety via an ether function, which allows us to introduce terminal alkene chains, which can react under benign conditions in a hydrosilylation with hydridopolysiloxanes. Polysiloxanes are ideal polymeric supports, because solvent properties can be easily tuned, they are inert, are commonly used as stationary phases in gas chromatography, and can show positive effects in catalysis.27
All three investigated pathways are starting with 1,3-bismesityl-2-imidazolidinylidene, which is converted into the allylether in 91% yield, but can be also converted into ligand precursors with various spacer lengths. A total spacer length of about 5 to 10 Å is ideal, because intramolecular interactions of the ligand with the polymer backbone caused by gauche conformations28 of the linker are reduced and there is still sufficient flexibility of the ligand.29 Similar to the strategy developed by Blechert et al.10 in pathway A (cf.Scheme 1) 1-(allyloxy)-N,N′-dimesityl-2,3-diamino-propane 2 was cyclised with triethyl orthoformate to give the imidazolium salt 3 in 96% yield, which was consecutively immobilized to 4 by hydrosilylation using the Karstedt catalyst under ultrasonication. Pt is removed by treatment with activated charcoal. In the next step the carbene is formed by treatment with potassium tert-butoxide followed by a reaction with Grubbs 1st generation catalyst to form the bonded Grubbs 2nd generation30catalyst 5. However, we found that the polymer supported catalyst 5 prepared by pathway A was very sensitive to decomposition which could be explained by the combination of free base and remaining Si–H groups in the polysiloxane, which are necessary for a permanent bonding of the polymer to glass surfaces via condensation with surface silanol-groups. Therefore the strategy was changed to a base free route in pathways B and C (cf.Scheme 1). In pathway B the diamine 2 is first immobilized viahydrosilylation to hydridomethyldimethyl-polysiloxane to form polymer 6, which was monitored by 1H-NMR spectroscopy (cf. Fig. S6 in the ESI‡). The bonded diamine 6 is then cyclised with pentafluorobenzaldehyde to the immobilised 2-(pentafluorophenyl)imidazolidine 7.31,32 The advantage is that the bonded carbene can be thermally generated, which can be immediately converted to form the bonded Grubbs 2nd generation catalyst 5 by ligand exchange against the tricyclohexylphosphine ligand. The formation of the bonded Grubbs 2nd generation catalyst was monitored by 31P-NMR spectroscopy (cf. Fig. S7 in the ESI‡). In contrast to the polymeric Grubbs 2nd generation catalyst 5 obtained via pathway A this material is stable, which can be attributed to the absence of base. Coating of the inner surface of fused silica capillaries with the catalytically active polymer 5 turned out to be highly challenging, because of the large active glass surface (high surface to volume ratio) and the immobilization of the polymer to the surface which affords higher temperatures. Therefore the optimized reaction conditions were transferred at a very early stage to the fused-silica capillary as solid-phase reaction vessel in pathway C (cf.Scheme 1). Polymeric diamine 6 was coated onto the inner surface of a fused-silica capillary (id 250 μm) with a film thickness of 250 nm. The polymer film was then immobilized onto the glass surface by heating the capillary to 180 °C (40 °C for 5 min, then heating up to 180 °C for 10 min at a heating rate of 0.5 K min−1) under slow nitrogen flow. This polymer is highly reactive and is permanently bonded to the glass surface via Si–O–Si bonds. The permanently bonded polymeric diamine 8 is then converted with pentafluorobenzaldehyde to the permanently bonded polymeric 2-(pentafluorophenyl)imidazolidine 9. Surface modified capillaries 9 are characterised by excellent long term stability and easy activation to the carbene, to form together with organometallic precursors, NHC ligand based catalysts by the meter, which is successfully demonstrated in the next steps. Heating modified capillaries 9 in a water bath to 60 °C results in the formation of the immobilized free carbene which reacts with Grubbs 1st generation catalyst by ligand exchange to give permanently bonded polymeric Grubbs 2nd generation catalyst capillary 10. Here, we utilize several advantages, which include solid-phase chemistry at high reagent concentrations, excess of reagents, easy removal of reagents by flushing the capillary with solvents, and requiring only short capillary pieces to synthesize the desired catalytically active column under defined and reproducible conditions. Furthermore, capillaries compatible to chromatographic separation columns offer the possibility of in situ reaction monitoring by installing capillary column 9 between a pre-separation column for thermal equilibration, followed by a separation column connected to EI GC/MS. This approach has also the advantage that the system can be simply checked against catalytic activity of the sample injector and separation columns by performing measurements with and without the catalytically active capillary. For the here studied reactions and reaction conditions no catalytic activity of the injector or separation capillary was observed. Heating the capillary from 40 to 80 °C with a heating rate of 4 K min−1 results in the observation of a broad peak (wh ≈ 2 min) with a characteristic EI MS fragmentation pattern (m/z = 168 (100%) and 99 (50%)) for pentafluorobenzene. Permanently bonded polymeric Grubbs 2nd generation catalyst capillaries 10 show some remarkable properties in comparison to Grubbs 2nd generation catalyst dissolved in poly(dimethylsiloxane) and coated onto the inner surface of fused-silica capillaries.17 These capillaries are moisture and air insensitive, can be installed several times over weeks without any loss of activity or leaching (detected by MS), and are stable even at elevated temperatures of 105 °C for several hours.
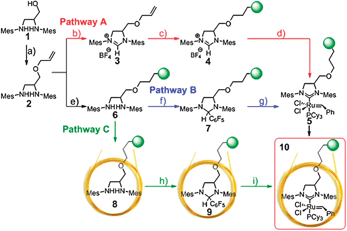 | ||
Scheme 1 (a) 3-Bromo-prop-1-ene, NaH, THF, 24 h, 64 °C. (b) NH4BF4, HC(OEt)3. (c) Hydridomethyldimethylpolysiloxane, Karstedt catalyst, THF, ultrasonication for 3 h. (d) KOtBu, THF, rt, 1 h, (Cy3P)2Cl2Ru(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHPh), toluene, 80 °C, 4 h. (e) Hydridomethyldimethylpolysiloxane, Karstedt catalyst, THF ultrasonication for 3 h. (f) Pentafluorobenzaldehyde, AcOH, 24 h. (g) (Cy3P)2RuCl2CHPh, toluene, 80 °C, 4 h. (h) Pentafluorobenzaldehyde, AcOH, n-pentane, 60 °C. (i) (Cy3P)2RuCl2CHPh, n-pentane, 60 °C. CHPh), toluene, 80 °C, 4 h. (e) Hydridomethyldimethylpolysiloxane, Karstedt catalyst, THF ultrasonication for 3 h. (f) Pentafluorobenzaldehyde, AcOH, 24 h. (g) (Cy3P)2RuCl2CHPh, toluene, 80 °C, 4 h. (h) Pentafluorobenzaldehyde, AcOH, n-pentane, 60 °C. (i) (Cy3P)2RuCl2CHPh, n-pentane, 60 °C. | ||
Injection of N,N-diallyltrifluoroacetamide at 50 °C and 80 kPa leads to the formation of N-trifluoroacetamide-3-pyrroline in 61.0% yield, considering a response factor of 1.66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the signal intensities of the reactant to product (cf.Fig. 2a).33 The contact time Δt of the reactant on the catalytically active column is only 1.75 s, which corresponds to a reaction rate constant 0.54 s−1 and an activation barrier ΔG#(323.15 K) of 81 kJ mol−1. These experiments elucidate the high activity of the permanently bonded polymeric Grubbs 2nd generation catalyst capillary 10.
1 in the signal intensities of the reactant to product (cf.Fig. 2a).33 The contact time Δt of the reactant on the catalytically active column is only 1.75 s, which corresponds to a reaction rate constant 0.54 s−1 and an activation barrier ΔG#(323.15 K) of 81 kJ mol−1. These experiments elucidate the high activity of the permanently bonded polymeric Grubbs 2nd generation catalyst capillary 10.
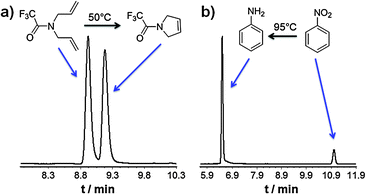 | ||
| Fig. 2 On-column reaction gas chromatographic experiments using (a) a 10 cm permanently bonded polymeric Grubbs 2nd generation catalyst capillary 10 to achieve on-column ring closing metathesis of N,N-diallyltrifluoroacetamide to N-trifluoroacetamide-3-pyrroline at 50 °C, and (b) a 10 cm permanently bonded polymeric 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene gold(I) chloride to hydrogenate nitrobenzene to aniline at 95 °C. | ||
To demonstrate the versatility of the ‘catalyst by the meter’ approach, we converted modified capillaries 9 into permanently bonded polymeric 1,3-bis(2,4,6-trimethyl-phenyl)imidazol-2-ylidene gold(I) chloride complex 10 by treatment with the Au(I) complex AuCl·Me2S.34,35
Over the last decade spectacular achievements using gold complexes and nanoparticles in homogeneous and heterogeneous catalysis, respectively, have been reported.36–41
An only 10 cm long capillary coated with polymeric 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene gold(I) chloride 11 (Scheme 2) was then used as hydrogenation catalyst to prove loading with Au. It is assumed that the Au(I) complex decomposes and agglomerates to nanoparticular Au under hydrogen atmosphere.39,41 The on-column reaction gas chromatographic experiment shows the conversion of nitrobenzene to aniline in 86.2% yield (cf.Fig. 2b). The contact time Δt of the reactant on the catalytically active column is 2.5 s, which corresponds to a reaction rate constant 0.79 s−1 and an activation barrier ΔG#(368.15 K) of 91.5 kJ mol−1.
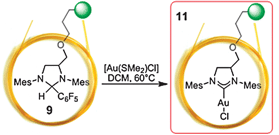 | ||
| Scheme 2 Preparation of permanently bonded polymeric 1,3-bis(2,4,6-trimethylphenyl)imidazol-2-ylidene gold(I) chloride 11 by the ‘catalyst by the meter’ approach. | ||
In conclusion, we have demonstrated a novel strategy to investigate catalytic reactions by bonding ligands to polysiloxanes and permanently coating such polymeric ligand systems onto the inner surface of fused-silica capillaries. Such capillaries are easily converted to catalytically active separation columns using solid phase chemistry strategies. This has been successfully demonstrated by tethering 2-(pentafluorophenyl)imidazolidine to the capillary surface and generating free carbene ligands on demand. Using various organometallic precursors allows preparing catalysts by the meter which can be quantitatively investigated by on-column reaction chromatography to obtain kinetic data in high-throughput fashion with high precision and to elucidate reaction mechanisms.
Generous financial support by the Deutsche Forschungsgemeinschaft (DFG) for an Emmy Noether grant (TR 542/3) and the SFB 623 ‘Molecular Catalysts: Structure and Functional Design’ is gratefully acknowledged.
Notes and references
- S. Diez-Gonzalez, N. Marion and S. P. Nolan, Chem. Rev., 2009, 109, 3612–3676 CrossRef CAS.
- O. Schuster, L. Yang, H. G. Raubenheimer and M. Albrecht, Chem. Rev., 2009, 109, 3445–3478 CrossRef CAS.
- C. Samojiowicz, M. Bieniek and K. Grela, Chem. Rev., 2009, 109, 3708–3742 CrossRef CAS.
- R. H. Grubbs, Handbook of Metathesis, Wiley VCH, Weinheim, 2003 Search PubMed.
- T. M. Trnka and R. H. Grubbs, Acc. Chem. Res., 2001, 34, 18–29 CrossRef CAS.
- T. N. Tekavec and J. Louie, Top. Organomet. Chem., 2007, 21, 159–192 CAS.
- R. H. Grubbs, Angew. Chem., Int. Ed., 2006, 45, 3760–3765 CrossRef CAS.
- J. A. Love, M. S. Sanford, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 10103–10109 CrossRef CAS.
- H. Clavier and S. P. Nolan, Chem.–Eur. J., 2007, 13, 8029–8036 CrossRef.
- S. C. Schürer, S. Gessler, N. Buschmann and S. Blechert, Angew. Chem., Int. Ed., 2000, 39, 3898–3901 CrossRef CAS.
- W. J. Sommer and M. Weck, Coord. Chem. Rev., 2007, 251, 860–873 CrossRef CAS.
- S. T. Nguyen, R. H. Grubbs and J. W. Ziller, J. Am. Chem. Soc., 1993, 115, 9858 CrossRef CAS.
- M. Mayr, M. R. Buchmeiser and K. Wurst, Adv. Synth. Catal., 2002, 344, 712–719 CrossRef CAS.
- C. Coperet and J.-M. Basset, Adv. Synth. Catal., 2007, 349, 78–92 CrossRef CAS.
- M. R. Buchmeiser, Chem. Rev., 2008, 108, 303–321.
- O. Trapp, Anal. Chem., 2006, 78, 189–198 CrossRef CAS.
- O. Trapp, S. K. Weber, S. Bauch and W. Hofstadt, Angew. Chem., Int. Ed., 2007, 46, 7307–7310 CrossRef CAS.
- O. Trapp, S. K. Weber, S. Bauch, T. Bäcker, W. Hofstadt and B. Spliethoff, Chem.–Eur. J., 2008, 14, 4657–4666 CrossRef CAS.
- O. Trapp, Chem. Today, 2008, 26, 26–28 Search PubMed.
- O. Trapp, J. Chromatogr., A, 2008, 1184, 160–190 CrossRef CAS.
- O. Trapp, Electrophoresis, 2010, 31, 786–813 CrossRef CAS.
- G. Jas and A. Kirschning, Chem.–Eur. J., 2003, 9, 5708–5723 CrossRef CAS.
- O. Flögel, J. D. C. Codee, D. Seebach and P. H. Seeberger, Angew. Chem., 2006, 118, 7157–7160 CrossRef.
- H. R. Sahoo, J. G. Kralj and K. F. Jensen, Angew. Chem., Int. Ed., 2007, 46, 5704–5708 CrossRef.
- F. E. Valera, M. Quaranta, A. Moran, J. Blacker, A. Armstrong, J. T. Cabral and D. G. Blackmond, Angew. Chem., Int. Ed., 2010, 49, 2478–2485 CrossRef CAS.
- N. Wang, T. Matsumoto, M. Ueno, H. Miyamura and S. Kobayashi, Angew. Chem., Int. Ed., 2009, 48, 4744–4746 CrossRef CAS.
- M. S. Decluea and J. S. Siegel, Org. Biomol. Chem., 2004, 2, 2287–2298 RSC.
- M. Raiza, J. Wegmann, S. Bachmann and K. Albert, Angew. Chem., Int. Ed., 2000, 39, 3486–3489 CrossRef.
- H. Cousin, O. Trapp, V. Peulon-Agasse, X. Pannecoucke, L. Banspach, G. Trapp, Z. Jiang, J. C. Combret and V. Schurig, Eur. J. Org. Chem., 2003, 3273–3287 CrossRef CAS.
- J. A. Love, M. S. Sanford, M. W. Day and R. H. Grubbs, J. Am. Chem. Soc., 2003, 125, 10103–10109 CrossRef CAS.
- G. W. Nyce, S. Csihony, R. M. Waymouth and J. L. Hedrick, Chem.–Eur. J., 2004, 10, 4073–4079 CrossRef CAS.
- A. P. Blum, T. Ritter and R. H. Grubbs, Organometallics, 2007, 26, 2122–2124 CrossRef CAS.
- O. Trapp, S. Bremer and S. K. Weber, Anal. Bioanal. Chem., 2009, 395, 1673–1679 CrossRef CAS.
- P. D. Fremont, N. M. Scott, E. D. Stevens and S. P. Nolan, Organometallics, 2005, 24, 2411–2418 CrossRef CAS.
- Y. Horino, T. Yamamoto, K. Ueda, S. Kuroda and F. D. Toste, J. Am. Chem. Soc., 2009, 131, 2809–2811 CrossRef CAS.
- A. Hoffmann-Röder and N. Krause, Org. Biomol. Chem., 2005, 3, 387–391 RSC.
- W.-C. Li, M. Comotti and F. Schüth, J. Catal., 2006, 237, 190–196 CrossRef CAS.
- A. S. K. Hashmi, Nature, 2007, 449, 292–293 CrossRef CAS.
- A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180–3211 CrossRef.
- N. Wang, T. Matsumoto, M. Ueno, H. Miyamura and S. Kobayashi, Angew. Chem., Int. Ed., 2009, 48, 4744–4746 CrossRef CAS.
- A. Corma, C. Gonzalez-Arellano, M. Iglesias and F. Sanchez, Appl. Catal., A, 2009, 356, 99–102 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details and further characterisation data. See DOI: 10.1039/c0cc02306j |
| This journal is © The Royal Society of Chemistry 2011 |
