Identification of dihydropyridines that reduce cellular tau levels†
Christopher G.
Evans
a,
Umesh K.
Jinwal
b,
Leah N.
Makley
a,
Chad A.
Dickey
b and
Jason E.
Gestwicki
*a
aDepartment of Pathology and the Life Sciences Institute, University of Michigan, 210 Washtenaw Ave., Ann Arbor, MI 48109, USA. E-mail: gestwick@umich.edu; Tel: +1 734-615-9537
bDepartment of Molecular Medicine, University of South Florida, 4001 E. Fletcher Ave., MDC 36, Tampa, FL 33613, USA. E-mail: cdickey@health.usf.edu; Tel: +1 813-396-0639
First published on 16th November 2010
Abstract
A series of dihydropyridines were identified that have an effect on the accumulation of tau, an important target in Alzheimer's disease. The dihydropyridine collection was expanded using the Hantzsch multicomponent reaction to develop preliminary structure–activity relationships.
Tau is a microtubule-binding protein that accumulates in a number of neurodegenerative disorders, including frontotemporal dementia and Alzheimer's disease (AD).1 These diseases are collectively termed tauopathies and they are characterized by aggregation of hyperphosphorylated tau. The presence of abnormal tau correlates with neuron loss and memory deficits.2 Therefore, selectively reducing its levels might be an effective strategy.
Efforts towards that goal have largely focused on either inhibitors of tau aggregation,3 inhibitors of its phosphorylation4 or compounds that stimulate its degradation.5 Each of these strategies is potentially promising, but many of the compounds identified to date have relatively modest activity. For example, methylene blue (MB), which both inhibits tau aggregation3 and stimulates its degradation through heat shock protein 70 (Hsp70),5 has an EC50 value of approximately 10 μM. Other promising compounds, such as the Hsp90 inhibitors 17-AAG and EC1012, reduce tau levels but they also produce a robust stress response, which is expected to diminish their long-term efficacy.5,6 Thus, new compounds that counteract tau accumulation are still of interest.
While conducting cell-based screens for small molecules that impact tau levels, we identified the 1,4-dihydropyridine 4a (data not shown). Based on this finding, we sought to synthesize a focused collection to facilitate characterization of structure–activity relationships (SAR). Accordingly, we were attracted to the Hantzsch multicomponent reaction because of its high atom economy and suitability for combinatorial synthesis. This reaction produces the dihydropyridine core scaffold from an aldehyde, amine, and two 1,3-dicarbonyls in a single step (Scheme 1). Also, it has good functional group tolerance and there are known stereoselective routes.7
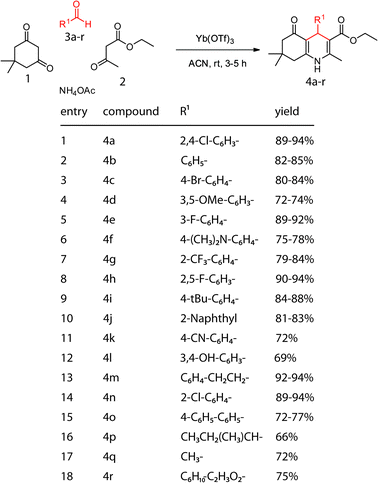 | ||
| Scheme 1 Variation of the aldehyde in the Hantzsch reaction to expand the diversity of the dihydropyridine collection. | ||
To generate a dihydropyridine collection, we first explored a series of aldehydes that were functionalized with ether bulky aromatics or smaller, alkyl groups (Scheme 1). To maintain the general structure of the initial compound, dimedone 1 (1.5 equiv.), ethylacetoacetate 2 (1 equiv.), and Yb(OTf)3 (10 mol%) were mixed in acetonitrile. After stirring for 10 minutes, the aldehyde (1.0 equiv.) and ammonium acetate (1.0 equiv.) were added. The reactions then proceeded for 3–5 hours, after which they were poured into saturated NaCl, washed with ethylacetate and the products re-crystallized from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 water
3 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ethanol. Using this approach, compounds 4a–r were obtained in moderate to good yields (69–94%).
ethanol. Using this approach, compounds 4a–r were obtained in moderate to good yields (69–94%).
To expand the diversity in this collection, we took advantage of published methods8 to exchange the ester for a thioester on compounds 4a and 4b. Briefly, these examples were refluxed in toluene with 2.2 equivalents of Lawesson's reagent for 1 h. The resulting products, 5a and 5b, were filtered through Celite and purified as above in good yield (Scheme 2).
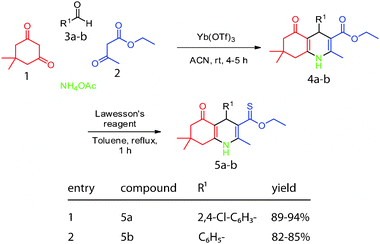 | ||
| Scheme 2 Introduction of a thioester into the dihydropyridines. | ||
To test whether modifications to the heterocyclic amine could be tolerated, we combined dimedone with aryl or alkyl amines in acetonitrile to form the enamine.9 After 30 minutes, ethylacetoacetate (1.0 equiv.), 2,4-dicholoro benzaldehyde 3a (1.0 equiv.) and 10% Yb(OTf)3 were added and the reaction was allowed to proceed for an additional 4–5 hours. This procedure generated compounds 7a–d with yields ranging from 71–82% (Scheme 3).
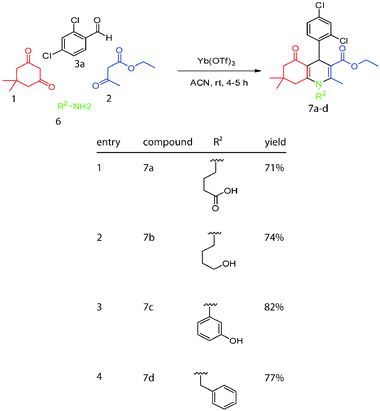 | ||
| Scheme 3 Substitutions of the amine in the dihydropyridine. | ||
To further diversify the scaffold, we next varied the identity of the 1,3-dicarbonyls (8 and 9; Scheme 4). Specifically, we used indanedione and 2,4-pentanedione in place of dimedone to produce derivatives 10a and 10b in good yields. On the other side of the molecule, we substituted either methylacetoacetate or benzylacetoacetate for ethylacetoacetate to produce 10c and 10d in 82 and 85% yield, respectively.
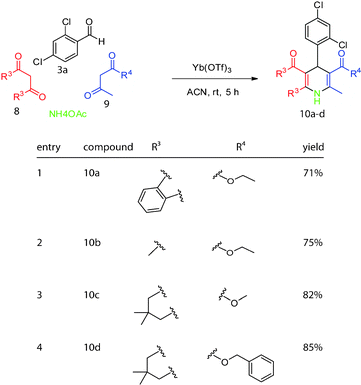 | ||
| Scheme 4 Substitutions of 1,3-dicarbonyls to add diversity. | ||
Finally, to fully exploit the strengths of the Hantzsch reaction we varied multiple components simultaneously. Using the reaction conditions and starting materials employed earlier, we made derivatives 11a–11k (Scheme 5). These compounds include examples, such as 11b and 11c, which contain β-ketoamides. Together, these efforts produced a library of 39 functionalized dihydropyridines. At this stage, no attempt was made to separate the enantiomers.
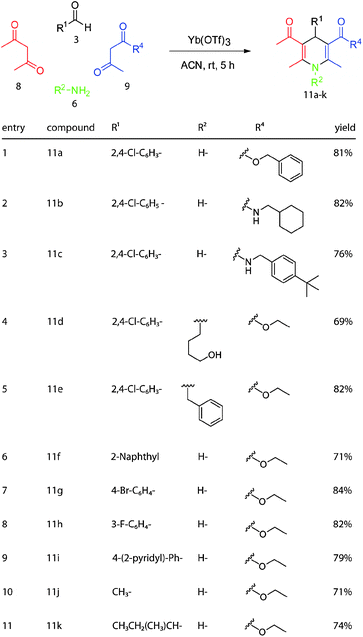 | ||
| Scheme 5 Multiple components of the Hantzsch reaction were simultaneously exchanged to create dihydropyridines with increased diversity. | ||
With this collection in hand, we treated cultured IMR32 neuroblastoma cells for 24 h with 100 μM compound and measured endogenous tau levels by Western blot. Some of the compounds, such as 11b–f, were found to be toxic under these conditions and excluded from further analysis. We then compared the remaining examples to the benchmark compounds MB and 17-AAG, which reduced tau levels by approximately 50 to 70% (Fig. 1).5,6 Based on those values, we imposed an arbitrary threshold of 25% to focus on the most active compounds in the dihydropyridine collection. This analysis focused attention on compounds 4p, 11a and 11g, which reduced tau levels by at least 25%. Interestingly, we also identified examples, such as 4a–b, 4d, 10b–c, 11j and 11k, which increased tau levels by at least 25%. Previous efforts have also noted compounds that increase tau levels and both types of molecules have been useful probes of tau biology.5a–b
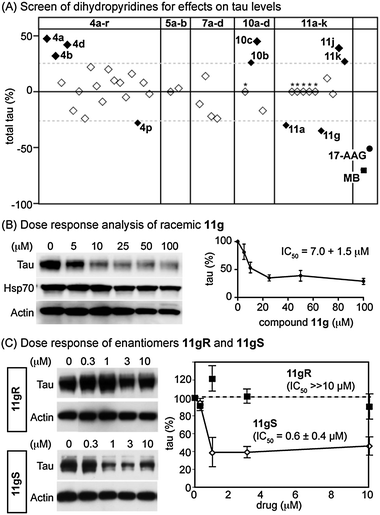 | ||
| Fig. 1 Screening results for the dihydropyridines. (A) IMR32 cells were treated for 24 h with 100 μM compound, followed by Western blots for total tau. Quantification of these blots is shown versus a vehicle control (1% DMSO). Also shown are two positive controls, methylene blue (MB) and 17-AAG. Arbitrary activity cut-offs are shown at ±25% (dotted line). Inactive compounds are shown as open symbols. Active compounds are shown in solid symbols and are labeled. *Toxic compounds. (B) Dose response analysis of 11g. (C) Testing of the enantiomers of 11g. The S enantiomer (open symbols) had activity while the R enantiomer (closed symbols) was inactive. Results are the average of triplicates and the error bars are SEM. | ||
An analysis of these results suggested some preliminary SAR. Specifically, large substitutions on the aldehyde such as naphthyl (4j) or p-diphenyl (4o) were not tolerated. Likewise, conversion of the ester to a thioester in 5a and 5b reduced activity, as did any modification of the heterocyclic amine (compounds 7a–d). Modest substitutions of an ethyl to methyl group in the diketone (e.g. from 4a to 10c) had marginal effects but larger groups, such as the benzyl ester in 10d, abolished activity. Interestingly, minimally functionalized benzyls in the aldehyde position, such as 4a, 4b and 4d, produced the most potent stimulators of tau accumulation, while smaller alkyl groups, such as in 4p, tended to produce compounds that reduced tau levels. This finding suggests a way of converting a compound from one that causes tau accumulation to one that leads to reductions. However, the impact of the aldehyde position also seemed to be influenced by the identity of the 1,3-diketone. For example, if dimedone was used, compounds 4a and 4b promoted tau accumulation, but replacing it for a methyl diketone, as in 11a and 11g, produced relatively strong inhibitors. Together, these findings reveal patterns of substitution that promote either tau degradation or accumulation.
Following this initial screening, we selected compound 11g for in-depth studies. In dose dependence experiments, we found that this compound reduced tau levels by ∼70% with an IC50 of 7.0 ± 1.5 μM (Fig. 1B), a potency comparable to some of the best, known anti-tau compounds.5 To test whether 11g activates a cellular stress response, we examined the levels of stress-inducible Hsp70 by Western blot and found that its levels were not significantly increased, suggesting that 11g does not act through this pathway (Fig. 1B). Next, we generated the two enantiomers of 11g (11gS and 11gR), using an organocatalytic procedure.7a In the IMR32 cells, only 11gS had activity, with an IC50 value of ∼ 600 nM (Fig. 1C). The cellular target of these dihydropyridines is currently unclear, but the relatively potent activity of 11gS suggests that further studies are warranted.
C.G.E. was supported by the NIH (GM008353). We also acknowledge support from the Abe and Irene Pollin CBD Fund, the Alzheimer's Association (IRG-09-130689) and NIH (NS059690 and AG031291).
Notes and references
- (a) T. L. Spires-Jones, W. H. Stoothoff, A. de Calignon, P. B. Jones and B. T. Hyman, Trends Neurosci., 2009, 32, 150 CrossRef CAS; (b) S. Jeganathan, M. von Bergen, E. M. Mandelkow and E. Mandelkow, Biochemistry, 2008, 47, 10526 CrossRef CAS.
- H. Braak and E. Braak, Acta Neuropathol., 1991, 82, 239 CrossRef CAS.
- (a) S. Taniguchi, N. Suzuki, M. Masuda, S. Hisanaga, T. Iwatsubo, M. Goedert and M. Hasegawa, J. Biol. Chem., 2005, 280, 7614 CAS; (b) C. M. Wischik, P. C. Edwards, R. Y. Lai, M. Roth and C. R. Harrington, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 11213 CrossRef CAS.
- M. P. Mazanetz and P. M. Fischer, Nat. Rev. Drug Discovery, 2007, 6, 464 CrossRef CAS.
- (a) J. Koren, 3rd, U. K. Jinwal, D. C. Lee, J. R. Jones, C. L. Shults, A. G. Johnson, L. J. Anderson and C. A. Dickey, J. Cell. Mol. Med., 2009, 13, 619 CrossRef; (b) U. K. Jinwal, Y. Miyata, J. Koren, 3rd, J. R. Jones, J. H. Trotter, L. Chang, J. O'Leary, D. Morgan, D. C. Lee, C. L. Shults, A. Rousaki, E. J. Weeber, E. R. Zuiderweg, J. E. Gestwicki and C. A. Dickey, J. Neurosci., 2009, 29, 12079 CrossRef CAS; (c) S. Oddo, A. Caccamo, B. Tseng, D. Cheng, V. Vasilevko, D. H. Cribbs and F. M. LaFerla, J. Neurosci., 2008, 28, 12163 CrossRef CAS.
- C. A. Dickey et al., J. Clin. Invest., 2007, 117, 648 CrossRef.
- (a) C. G. Evans and J. E. Gestwicki, Org. Lett., 2009, 11, 2957 CrossRef CAS; (b) J. C. Legeay, J. Y. Goujon, J. J. Vanden Eynde, L. Toupet and J. P. Bazureau, J. Comb. Chem., 2006, 8, 829 CrossRef CAS; (c) A. M. Maurya and R. A. Kumar, Tetrahedron, 2007, 63, 1946 CrossRef CAS.
- B. A. Vigante, Y. Y. Ozols, B. S. Chekavichus and G. Y. Dubur, Khim. Geterotsikl. Soedin., 1988, 9, 1232.
- (a) O. I. el-Sabbagh and H. M. Rady, Eur. J. Med. Chem., 2009, 44, 3680 CrossRef CAS; (b) S. J. Tu, Y. Zhang, R. H. Jia, B. Jiang, Y. J. Zahang and S. J. Ji, Tetrahedron Lett., 2006, 47, 6521 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization of compounds. See DOI: 10.1039/c0cc02253e |
| This journal is © The Royal Society of Chemistry 2011 |
