Tools for metal ion sorting: in vitro evidence for partitioning of zinc and cadmium in C. elegans metallothionein isoforms†‡
Oksana I.
Leszczyszyn
a,
Sukaina
Zeitoun-Ghandour
b,
Stephen R.
Stürzenbaum
b and
Claudia A.
Blindauer
*a
aDepartment of Chemistry, University of Warwick, Coventry, UK. E-mail: c.blindauer@warwick.ac.uk; Tel: +44 (0)2476528264
bDivision of Pharmaceutical Science, School of Biomedical and Health Sciences, King's College London, UK.
First published on 28th September 2010
Abstract
In vitro evidence for the isoform-specific partitioning of cadmium and zinc ions between the two C. elegans metallothioneins is presented. This observation is discussed in terms of isoform-specific affinities towards zinc and cadmium and the implications of our study on the in vivo roles of C. elegans metallothioneins.
Ensuring the adequate uptake of essential metal ions whilst preventing the accumulation of toxic metal ions is a problem faced by all organisms, above all those in direct contact with the lithosphere. This dichotomy is particularly challenging for Zn2+ and Cd2+, which share similar coordination chemistries. Many studies have capitalised upon this similarity to characterise zinc binding sites in proteins;1 however, other examples demonstrated that Cd2+ substitution at some Zn2+ binding sites is not isomorphous, causing structural change and misfolding2 and/or loss of activity.3 Either way, the ability to displace Zn2+ at crucial sites endows Cd2+ with toxicity, leading to disruption of signalling pathways,4 of DNA repair5 and of gene regulation.6 Consequently, mechanisms for metal ion homeostasis that differentiate between essential Zn2+ and toxic Cd2+ are imperative for survival—yet our understanding of how this discrimination is achieved is limited.
Metallothioneins (MTs) are key players in essential metal homeostasis and detoxification, largely due to their favourable metal binding properties and expression profiles that are regulated in a temporal and spatial fashion.7 The genomes of many organisms contain multiple MT isoforms; for example, the human genome encodes a cluster of 14 MT genes8 and Drosophila has four MT genes.9 The purpose of multiple isoforms is still unclear, but metal specificity has emerged as one possibility.10 The nematode Caenorhabditis elegans (C. elegans) provides an excellent system to study isoform-specificity of MT genes, as its fully sequenced genome contains only two isoforms, CeMT-1 and CeMT-2 (mtl-1 and mtl-2).11 Both genes are induced in the gut by exposure to elevated levels of Cd2+, and in addition, CeMT-1 is constitutively expressed in three cells of the lower pharyngeal bulb.11,12 A recent study suggested that CeMT-1 and CeMT-2 prevent in vivo accumulation of Zn2+ and Cd2+ ions.13 Deletion of the CeMT-1 gene massively increased Zn accumulation, whilst deletion of CeMT-2 had a more pronounced effect on Cd accumulation. This in vivo selectivity, together with in vivo speciation data, provided evidence that different metabolic pathways exist for these closely similar ions, and that the two MTs play a role in their differentiation. Significantly, the metal specificity observed at the organismal level was also reflected at protein level; specifically in the relative affinities of the isoforms towards Zn2+ and Cd2+.13 Moreover, model calculations based on thermodynamic data suggested that although both C. elegans MTs bound Cd2+ more strongly than Zn2+, Cd2+ would preferentially bind to CeMT-2 over CeMT-1. Thus, the simultaneous presence of both MT isoforms in cytosols may facilitate discrimination between Zn2+ and Cd2+.
In order to test this hypothesis, we have employed electrospray ionisation mass spectrometry (ESI-MS) to directly observe Zn2+ and Cd2+ binding preferences in a system where both isoforms and both metal ions are present. ESI-MS has previously been used to study competitive binding of Zn2+ or Cd2+ to mouse MT-2 and MT-3 isoforms,14 but the direct competition between Zn2+ and Cd2+ for two different MTs has not been reported.
To establish that both proteins can be observed and quantified in a mixture, an aliquot of a solution containing equimolar amounts (with respect to binding site concentration) of Zn-loaded CeMT-1 and CeMT-2 isoforms were mixed and directly infused into an ESI mass spectrometer (Fig. 1).
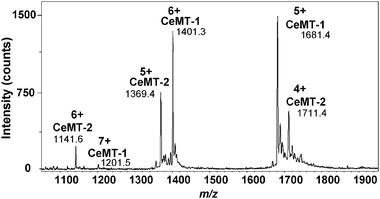 | ||
| Fig. 1 Charge state envelopes of a mixture of zinc-loaded C. elegans MT isoforms. The Zn binding site concentrations are equivalent (25 μM CeMT-1 and 29 μM CeMT-2 in 10 mM ammonium acetate, pH 7.4, 10% methanol). Deconvolution yields Zn7–CeMT-1 (75 aa; observed neutral mass: 8402.4 ± 0.9 Da; theoretical neutral mass: 8402.6 Da) and Zn6–CeMT-2 (65 aa; observed neutral mass: 6842.4 ± 1.0 Da; theoretical neutral mass: 6843.1 Da) as major species, with both proteins missing their initiation methionine. | ||
The discrete charge state distributions allow definitive identification of the individual MT isoforms; with +7, +6, +5 charge state signals observed for Zn7–CeMT-1 and +6, +5, +4 for Zn6–CeMT-2. In general, the signals corresponding to CeMT-1 metallospecies had a higher intensity than those of CeMT-2, despite the latter being more concentrated. Evidently, the ionisation efficiencies of the two proteins are somewhat different and therefore, our subsequent experimental design avoids comparing absolute intensities of the two isoforms.
Further aliquots of the isoform mixture were incubated with increasing amounts of Cd2+ and, after removal of excess metal ions, were analysed by ESI-MS (Fig. S1, ESI‡). The +5 charge state of CeMT-1 and +4 charge state of CeMT-2 were selected for data evaluation. For both isoforms, signals of all possible mixed Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn stoichiometries were observed during the titration (Fig. 2 and Tables S1 and S2, ESI‡).
Zn stoichiometries were observed during the titration (Fig. 2 and Tables S1 and S2, ESI‡).
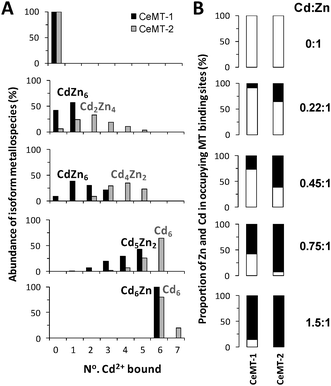 | ||
| Fig. 2 Distribution of Zn2+ and Cd2+ ions in C. elegans MT isoforms. (A) Profile of metallospecies observed for CeMT-1 (black) and CeMT-2 (grey) at each titration point, plotted against the number of bound Cd2+. The abundance of each metallospecies was calculated from MS intensities, expressed as a percentage of the corresponding total isoform signal intensity. (B) Proportion of Zn2+ (white) and Cd2+ (black) occupying total binding sites in the respective MT isoform at each titration point, derived from percentages shown in (A) (see ESI‡ for details). | ||
As more Cd2+ becomes available to the isoforms, a successive exchange of Zn2+ for Cd2+ at MT binding sites occurs (Fig. 2A). Crucially, the levels of metal exchange by CeMT-1 and CeMT-2 were different, with higher Cd2+ contents observed for CeMT-2. For example, Cd4Zn2 was the most prominent CeMT-2 metallospecies compared with CdZn6 for CeMT-1 at the 0.45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Cd
1 Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn titration point. Furthermore, when the distribution of metal ions over all MT binding sites was considered, an isoform-specific partitioning of Cd2+ and Zn2+ ions was observed (Fig. 2B). Clearly, at each titration point more Cd2+ ions were bound to CeMT-2 than to CeMT-1.
Zn titration point. Furthermore, when the distribution of metal ions over all MT binding sites was considered, an isoform-specific partitioning of Cd2+ and Zn2+ ions was observed (Fig. 2B). Clearly, at each titration point more Cd2+ ions were bound to CeMT-2 than to CeMT-1.
In the presence of excess Cd2+, CeMT-2 was fully Cd-loaded (Cd6); however, CeMT-1 retained the Cd6Zn metallospecies. Even when pure CeMT-1 was incubated with excess Cd2+, the same species dominated (Fig. 3A). Indeed, in our previous study, holo-cadmium species were produced by stripping of Zn2+ and reconstitution of the apo-protein.13 Furthermore, the “in vivo” and in vitro formation of the Cd6Zn metallospecies has also been reported by Bofill et al.,15 and was also observed in our laboratory, when CeMT-1 was isolated from Cd-supplemented cultures (Fig. 3B).
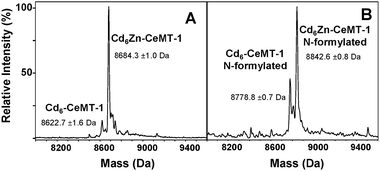 | ||
| Fig. 3 Deconvoluted ESI mass spectra of Cd6Zn–CeMT-1 produced by (A) incubation of Zn-loaded protein with a 10-fold excess of CdCl2 (with respect to protein concentration) and (B) over-expression in E. coli growing in Luria–Bertani medium supplemented with CdCl2. No Cd7 species are observed in either spectrum. | ||
These observations indicate that the formation of this species is independent of expression system or purification methods, occurs both in vivo and in vitro when both Zn2+ and an excess of Cd2+ are present, and is an intrinsic property of the CeMT-1 isoform. In principle, the reluctance to exchange metal ions into a particular site may have kinetic or thermodynamic reasons. For example, in the cyanobacterial MT SmtA, one site is kinetically inert towards metal exchange.16 For CeMT-1, the “in vivo” formation of the Cd6Zn metallo species from the unfolded apo-protein argues against a kinetic basis for metal preferences and thus favours thermodynamic origins. Control experiments (Fig. S2, ESI‡) have confirmed that Cd6Zn is a thermodynamic product.
Complete displacement of Zn2+ by Cd2+ at all-thiolate binding sites, such as those found in MTs, is consistent with the principle that thiolate ligands inherently possess a greater affinity for soft Cd2+ ions over borderline Zn2+.17 The average stability constants for Cd2+ and Zn2+ binding in both CeMT-1 and CeMT-2 reflect this trend (Table 1)13 leading to preferential binding of Cd2+ in both isoforms.
However, the average log KCd values for CeMT-1 and CeMT-2 differ by almost two orders of magnitude from each other, whilst the two log KZn are identical (Table 1). Ultimately, differences between CeMT-1 and CeMT-2 are encoded in their primary sequences. Over the first 56 residues, the two proteins share 60% identity and the positions of 18 cysteines are conserved (Fig. S3, ESI‡). In addition to this cysteine-rich common core, the C-terminal extension present in CeMT-1 contains four extra ligands, which are likely to be involved in the coordination of the seventh metal ion. Studies involving truncated sequences suggest two domains with [M3Cys9] clusters for CeMT-2, and also two domains for CeMT-1 but with four metals in the C-terminal domain. The contribution of three histidines to metal coordination in CeMT-1 was substantiated by chemical modification.15 To gain insight into the origins of (i) the reluctance of CeMT-1 to bind a seventh equivalent of Cd2+ in the presence of Zn2+ and (ii) the remarkable difference in log KCd values between Cd7–CeMT-1 and Cd6–CeMT-2, the in vitro affinities towards Zn2+ and Cd2+ of the Cd6Zn–CeMT-1 species were determined (Table 1; see also ESI‡).18 The log KZn value of the single zinc binding site is close to those of the fully zinc-loaded isoforms (Table 1); in contrast, the average affinity of the remaining six binding sites towards Cd2+ is 0.8–1.1 log units larger than the average determined for Cd7–CeMT-1. Using the average binding constants for Cd2+ in Cd7–CeMT-1 and Cd6Zn–CeMT-1, a log KCd value of 7.6 ± 1.1 for the single binding site can be estimated. Considering that the affinity for Cd2+ at this single binding site is three to five orders of magnitude lower than that for Zn2+ binding (log KZn = 11.6), it is not surprising that Cd2+ cannot displace Zn2+ from this site. Furthermore, the magnitude of the stability constants strongly suggests that the seventh site is an isolated CysHis3 site, as comparisons with literature data17 reveal that the affinity for Cd2+ at zinc finger sites reduces in the order Cys4 > Cys3His > Cys2His2, with log KCd values of ca. 13 > 11 > 9, respectively. If this trend is extrapolated to a CysHis3 site, this would give a log KCd value in the order of 7, a value comparable with that estimated for the single binding site in CeMT-1. In contrast, the affinities for Zn2+ were only slightly affected by Cys-to-His replacements in zinc finger peptides and the small difference in log KZn for the seventh site in CeMT-1 is also consistent with this observation. Importantly, the finding that the average log KCd over the Cd6-sites in CeMT-1 is still significantly lower than for the Cd6-sites in CeMT-2 suggests that the seventh site is not solely responsible for the differences in Cd2+ affinity.
The new and published stability constants (Table 1) allowed estimation of the theoretical distributions of Zn2+ and Cd2+ between the two MT isoforms at different Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratios. A comparison (Table S3, ESI‡) revealed that Cd
Zn ratios. A comparison (Table S3, ESI‡) revealed that Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratios observed by ESI-MS and those calculated using log K values were very similar at all titration points. The method for the determination of log K values is based on competition for a single metal ion between the chelator 5F-BAPTA and a single protein, whilst ESI-MS provides a direct method involving two proteins and two metal ions; therefore, the remarkable agreement between these very different approaches validates both experiments.
Zn ratios observed by ESI-MS and those calculated using log K values were very similar at all titration points. The method for the determination of log K values is based on competition for a single metal ion between the chelator 5F-BAPTA and a single protein, whilst ESI-MS provides a direct method involving two proteins and two metal ions; therefore, the remarkable agreement between these very different approaches validates both experiments.
The isoform-specific partitioning of metal ions observed in our ESI-MS experiment provides direct insight into a molecular mechanism that could allow C. elegans to deal with Cd2+ without compromising Zn2+ homeostasis; namely, through the simultaneous induction of CeMT-1 and CeMT-2 expression upon exposure to Cd2+. The discrimination between different metal ions in organisms can be understood as a series of selectivity filters. The facts of coordination chemistry preclude, in many cases, a filter with 100% efficiency, as a certain binding strength is required for effective metal transport proteins. The Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratios employed in our experiments are probably higher than those encountered in vivo, but even at these levels, total Cd2+ was divided between CeMT-1 and CeMT-2 in a distribution approximating 17%
Zn ratios employed in our experiments are probably higher than those encountered in vivo, but even at these levels, total Cd2+ was divided between CeMT-1 and CeMT-2 in a distribution approximating 17%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 83% (using the numbers for the 0.22
83% (using the numbers for the 0.22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Cd
1 Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratio). It can be calculated that at lower Cd
Zn ratio). It can be calculated that at lower Cd![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn ratios, e.g. 0.1
Zn ratios, e.g. 0.1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, the partitioning is even more effective. Thus, the two MTs provide a highly efficient selectivity filter in C. elegans, with subsequent steps likely to contribute to further metal sorting.
1, the partitioning is even more effective. Thus, the two MTs provide a highly efficient selectivity filter in C. elegans, with subsequent steps likely to contribute to further metal sorting.
We thank the BBSRC (grant BBE025099), the Altajir Trust, Advantage West Midlands and the European Regional Development Fund (Birmingham Science City) for support.
Notes and references
- (a) Y. Chen, R. S. Simmonds, G. L. Sloan and R. Timkovich, J. Biol. Inorg. Chem., 2008, 13, 855–860 CrossRef CAS; (b) C. A. Blindauer, M. D. Harrison, J. A. Parkinson, N. J. Robinson and P. J. Sadler, in 10th Int. Symp. on Metal Ions in Biology and Medicine, ed. P. Collery, I. Maymard, T. Theophanides, L. Khassanova and T. Collery, John Libby Eurotext, 2008, vol. 10, pp. 167–173 Search PubMed.
- (a) O. I. Leszczyszyn and C. A. Blindauer, Mol. BioSyst., 2010, 6, 1592–1603 RSC; (b) P. Palumaa, O. Njunkova, L. Pokras, E. Eriste, H. Jörnvall and R. Sillard, FEBS Lett., 2002, 527, 76–80 CrossRef CAS.
- R. Kothinti, A. Blodgett, N. M. Tabatabai and D. H. Petering, Chem. Res. Toxicol., 2010, 23, 405–412 CrossRef CAS.
- F. Thevenod, Toxicol. Appl. Pharmacol., 2009, 238, 221–239 CrossRef CAS.
- M. l. Viaua, J. Gastaldo, Z. Bencokova, A. Joubert and N. Foray, Mutat. Res., 2008, 365, 13–21.
- N. M. Tabatabai, S. S. Blumenthal and D. H. Petering, Toxicology, 2005, 207, 369–382 CrossRef CAS.
- K. Balamurugan and W. Schaffner, Met. Ions Life Sci., 2009, 5, 31–49 Search PubMed.
- M. Nordberg and G. F. Nordberg, Met. Ions Life Sci., 2009, 5, 1–29 Search PubMed.
- D. Egli, A. Selvaraj, H. Yepiskoposyan, B. Zhang, E. Hafen, O. Georgiev and W. Schaffner, EMBO J., 2003, 22, 100–108 CrossRef CAS.
- R. Bofill, M. Capdevila and S. Atrian, Metallomics, 2009, 1, 229–234 RSC.
- J. H. Freedman, L. W. Slice, D. Dixon, A. Fire and C. S. Rubin, J. Biol. Chem., 1993, 268, 554–564.
- S. C. Swain, K. Keusekotten, R. Baumeister and S. R. Stürzenbaum, J. Mol. Biol., 2004, 341, 951–959 CrossRef CAS.
- S. Zeitoun-Ghandour, J. M. Charnock, M. E. Hodson, O. I. Leszczyszyn, C. A. Blindauer and S. R. Stürzenbaum, FEBS J., 2010, 277, 2531–2542 CrossRef CAS.
- P. Palumaa, I. Tammiste, K. Kruusel, L. Kangur, H. Jörnvall and R. Sillard, Biochim. Biophys. Acta, Proteins Proteomics, 2005, 1747, 205–211 CrossRef CAS.
- R. Bofill, R. Orihuela, M. Romagosa, J. Domenech, S. Atrian and M. Capdevila, FEBS J., 2009, 276, 7040–7069 CrossRef CAS.
- C. A. Blindauer, N. C. Polfer, S. E. Keiper, M. D. Harrison, N. J. Robinson, P. R. R. Langridge-Smith and P. J. Sadler, J. Am. Chem. Soc., 2003, 125, 3226–3227 CrossRef CAS.
- B. A. Krizek, D. L. Merkle and J. M. Berg, Inorg. Chem., 1993, 32, 937–940 CrossRef CAS.
- D. W. Hasler, L. T. Jensen, O. Zerbe, D. R. Winge and M. Vašák, Biochemistry, 2000, 39, 14567–14575 CrossRef CAS.
Footnotes |
| † This manuscript is part of an Emerging Investigators issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental methods, Tables S1–S3 and Fig. S1–S3. See DOI: 10.1039/c0cc02188a |
| This journal is © The Royal Society of Chemistry 2011 |
