Selective and sensitive detection of melamine by intra/inter liposomal interaction of polydiacetylene liposomes†‡
Jiseok
Lee
a,
Eun
Jeong Jeong
b and
Jinsang
Kim
*abc
aMacromolecular Science and Engineering, University of Michigan, 2300 Hayward St., Ann Arbor, MI 48109-2136, USA. E-mail: jinsang@umich.edu; Fax: +1 734-763-4788; Tel: +1 734-936-4681
bMaterials Science and Engineering, University of Michigan, MI 48109-2136, USA
cChemical Engineering, Biomedical Engineering, University of Michigan, MI 48109-2136, USA
First published on 14th September 2010
Abstract
We report a convenient melamine detection system based on polydiacetylene (PDA) liposomes having rapid, selective, and sensitive detection, and dual signal capabilities. The detection limit of the sensory PDA liposome is 1 and 0.5 ppm in the colorimetric and the fluorescence detection schemes, respectively, satisfying the world regulation level.
Melamine is an organic compound that is often combined with formaldehyde to make a synthetic polymer having both excellent thermal resistance and heat tolerance. The melamine resin is a versatile material having a highly stable structure and is considered as non-toxic plastic. However, above the safety regulation level (2.5 ppm in USA and EU and 1 ppm in China) melamine monomers can cause acute kidney failure, serious renal problems and even death.1 Unreacted melamine monomers may possibly leach out from the polymer at high temperatures. However, the misuse of melamine is a much more important issue. Melamine has high nitrogen content and this fact was abused to misleadingly fortify protein content by adding melamine to wheat gluten and infant formula. It has been reported that hundreds of pets died of renal failure in 2007 and there were an estimated 300
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 victims and 50
000 victims and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 babies that were hospitalized from the infant formula containing melamine in 2008.2
000 babies that were hospitalized from the infant formula containing melamine in 2008.2
The conventional analytical method for the detection of melamine is a mass spectrometry. However, conventional lab-scale equipment is expensive and requires specific skills for operation. Therefore, there has been much effort to devise an effective and simple detection system for melamine. Recently, various methods including electrochemical,3gas chromatography (GC),4liquid chromatography (LC),5capillary electrophoresis,6 ELISA,7 and colorimetric sensors8 have been investigated to detect melamine. In this contribution, we report a practical and convenient colorimetric detection system based on polydiacetylene (PDA) liposome having a dual signaling capability for rapid, selective and sensitive detection of melamine.
PDA sensory systems are unique in that they have the sensitive colorimetric/fluorescence dual detection capability and are prepared through a simple molecular self-assembly followed by photo-polymerization.9–11 The photo-induced topochemical polymerization converts well-assembled diacetylene monomers into conjugated PDA with a blue color (absorption λmax at 640 nm). Upon exposure to external stimuli, the absorption λmax of PDA shifts from 640 nm (blue phase) to 540 nm (red phase). Interestingly, the triggered red phase of PDA is also weakly fluorescent, so PDA can provide dual signaling capability.12,13 The colorimetric change is believed to appear from the conformational change of conjugated backbone of PDA induced by external stimuli including pH, temperature,14,15 ions,16,17 and mechanical pressure.18,19
The interaction between melamine and cyanuric acid (CA) is well known in the fact that they form a stable melamine–cyanurate complex through hydrogen bonding between imide and amino-pyridine moieties at a neutral pH.20–22 We rationally encoded the melamine/CA interaction mechanism in our CA-carrying PDA design in such a way that melamine molecules form multiple hydrogen bondings with the CA at the surface of PDA liposomes. As we presented in our previous works,16,17,23 the steric repulsion resulting from the complex formation between the probe and the target at the liposome surface induces the rearrangement of yne–ene conjugated backbone of PDA and produces the color change to red and fluorescence development. The same phenomenon is expected to occur in the colorimetric/fluorescent PDA liposome system for melamine detection. The degree of induced color change by melamine should depend on how target melamine molecules form hydrogen bonds with CA at the PDA liposome surface. When densely packed CA probes on the liposome surface recognize melamine by forming hydrogen bonding, two types of CA–melamine–CA hydrogen bonding can be defined. First, a melamine molecule can form hydrogen bonding with CA molecules of the same PDA liposome. That can be called an intra-liposomal CA–melamine–CA hydrogen bonding. Alternatively, a melamine molecule can form inter-liposomal CA–melamine–CA hydrogen bonding with CA molecules from difference PDA liposomes. The melamine/CA hydrogen bonding will induce both intra-liposomal repulsion and inter-liposomal aggregation strain to the PDA liposomes resulting in the conformational change of conjugated PDA backbone as schematically illustrated in Scheme 1B. The resulting perturbation of the yne–ene backbone of PDA will produce rapid, selective and sensitive colorimetric/fluorescent dual signal in the presence of melamine.
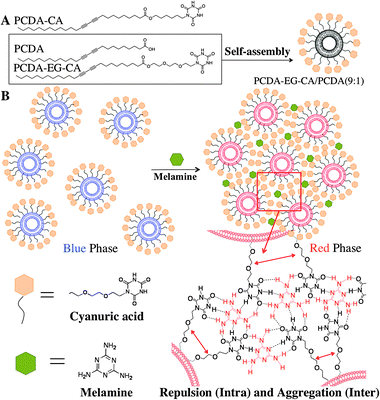 | ||
| Scheme 1 (A) Chemical structure of the investigated diacetylene monomers, PCDA, PCDA-CA and PCDA-EG-CA. (B) Schematic illustration of melamine and CA derived PDA liposome by intra/inter molecular hydrogen bond and resulting steric aggregation and repulsion. | ||
We synthesized two types of CA-carrying PDA monomers (PCDA-CA and PCDA-EG-CA) and examined the sensitivity for melamine detection (Scheme 1A). The PDA liposome solutions were prepared through sonication of the CA-carrying PCDA (PCDA-CA or PCDA-EG-CA) and PCDA (10,12-pentacosadiynoic acid) mixture having various mole ratios (final concentration: 0.2 mM) in 5 mM HEPES buffer, pH 7.0, at 80 °C, followed by cooling for 6 hours and irradiation of 254 nm UV light for 30 s. We first conducted melamine detection tests with PCDA-CA/PCDA liposomes. We could confirm that the PCDA-CA/PCDA liposome (4/1 mole ratio) was able to detect melamine. However, the liposome solution produced a rather weak blue color after the photopolymerization and the detectable range of the optical color change by naked eye was 10 ppm level as shown in Fig. S1 (ESI†). The rather weak blue color means that the quality of the PDA liposome formation is not good. We reasoned that we could improve the quality of the PDA liposome formation and the ensuing sensitivity of the system by improving the ordered structure of the self-assembled mixed PDA liposomes.
As one can see in Scheme 2, there is spatial mismatch between the hydrophilic and hydrophobic parts of PCDA-CA and PCDA. The weak blue color after the polymerization can be explained by the amphiphilic mismatch and imbalance. Accordingly, we designed PCDA-EG-CA having hydrophilic and flexible linker to achieve improved ordering of the self-assembled liposome. PCDA-EG-CA has the ethylene glycol linker to match the length of its hydrophobic part with that of PCDA and also has a more balanced amphiphilic structure. As expected, PCDA-EG-CA/PCDA liposomes produced intense blue color upon polymerization and as shown in Fig. S2 (ESI†), 100% of PCDA-EG-CA liposome showed 5 ppm level of detection limit by the naked eye. In the case of 100% of PCDA-CA liposome solution, on the contrary, the blue color intensity was very weak and the colorimetric change was not noticeable by the naked eye upon addition of the same amount of melamine.
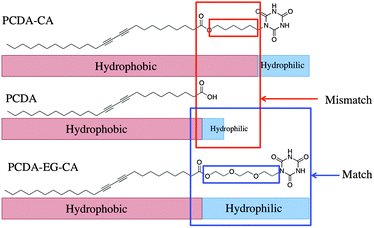 | ||
| Scheme 2 Schematic illustration of amphiphilic matching of PCDA, PCDA-CA and PCDA-EG-CA molecules. | ||
We further optimized the PDA sensory system to achieve better detection limit and sensitivity by investigating various mixing ratios between PCDA and PCDA-EG-CA at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, 1
9, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 1
6, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 3
1, 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 6
1, 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 9
1 and 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∶
∶![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S3A, ESI†). As the amount of PCEA-EG-CA increases the sensitivity also increases. Fig. 1C shows the color change of the best performing PCDA-EG-CA/PCDA (9/1 mole ratio) liposome solutions upon addition of various concentrations of melamine from 1 ppm to 40 ppm. 1 ppm level of melamine triggered the color change of the PDA liposome solution and this detection limit is suitable to detect melamine at the world regulation level. Even though it takes 5 min until the liposome solution shows a saturated color change from blue to red, we could observe a noticeable color change within 30 s after the addition of melamine. Prolonged incubation caused aggregation and precipitation of the liposome as shown in Fig. S3B (ESI†).
1 (Fig. S3A, ESI†). As the amount of PCEA-EG-CA increases the sensitivity also increases. Fig. 1C shows the color change of the best performing PCDA-EG-CA/PCDA (9/1 mole ratio) liposome solutions upon addition of various concentrations of melamine from 1 ppm to 40 ppm. 1 ppm level of melamine triggered the color change of the PDA liposome solution and this detection limit is suitable to detect melamine at the world regulation level. Even though it takes 5 min until the liposome solution shows a saturated color change from blue to red, we could observe a noticeable color change within 30 s after the addition of melamine. Prolonged incubation caused aggregation and precipitation of the liposome as shown in Fig. S3B (ESI†).
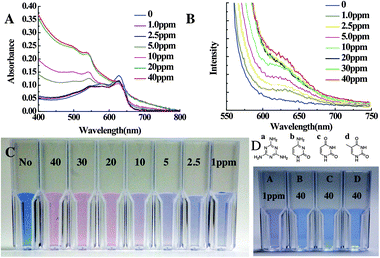 | ||
| Fig. 1 (A) UV-vis spectra and (B) PL spectra of the PCDA-EG-CA/PCDA (9/1) liposome solution (final concentration: 0.2 mM) upon the addition of various concentrations of melamine after 5 min incubation. (C) Optical colorimetric change of polydiacetylene liposome upon addition of melamine (40 ppm–1 ppm). (D) Colour change of PCDA-EG-CA/PCDA (9/1) liposome solution in 50 mM HEPES buffer, pH 7.0, upon the addition of 1 ppm of melamine (a), 40 ppm of thymine (b), cytosine (c) and uracil (d). | ||
We also confirmed the detection of melamine by the PDA liposome solution by means of UV-vis absorption and PL emission spectra. The absorption peak at 650 nm (blue phase) decreased and the 550 nm (red phase) absorption peak appeared and grew upon the addition of various concentrations of melamine (Fig. 1A). The fluorescence intensity of the PCDA-EG-CA/PCDA liposome also increased upon the addition of melamine (Fig. 1B). We also conducted selectivity tests by adding uracil, cytosine and thymine. These molecules have similar chemical structures with melamine but do not form proper hydrogen bonds with CA to trigger the color change. Because those molecules can form hydrogen bonds with single CA, the necessary intra-liposomal repulsion and inter-liposomal aggregation for the colorimetric/fluorescence change are not likely formed. As one can clearly see in Fig. 1D, we did not observe any detectable colorimetric change nor fluorescent development after adding even 40 ppm of cytosine, thymine and uracil.
We further developed analogous solid-state sensors and investigated the detection limit using the microarray technique. Fig. 2 shows the fluorescence microscopy images of the PDA liposome microarray and the correlation between the fluorescence intensity and the concentration of melamine. We microarrayed the PCDA-EG-CA/PCA liposome solution to an amine modified glass substrate and the physically attached PDA liposome layer was photopolymerized for 30 seconds. Various concentrations of melamine were added to the PDA liposome layer. Fig. 2B shows the red fluorescence development upon the addition of various concentrations of melamine. As the concentration of melamine increases, more CA/melamine complex will be formed, and this induces stronger perturbation to the conjugated PDA backbone, resulting in a more intense red fluorescence. Based on the correlation curve, a quantitative analysis of an unknown melamine concentration is also achievable. The observed detection limit was 0.5 ppm lower than that of the colorimetric detection by the solution system. This detection limit was obtained by our fluorescence microscope, and could be much better if more sensitive equipment is used.
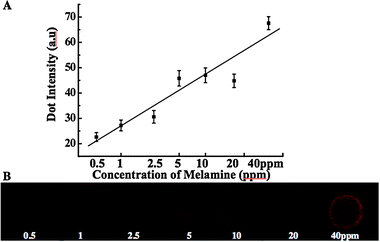 | ||
| Fig. 2 (A) Correlation curve between the fluorescence intensity and the amount of melamine (the error bar is a standard deviation and each point represents the mean value). (B) Fluorescence microscope image of the PDA microarray upon exposure to the various concentrations of melamine from 0.5 to 40 ppm after 30 min incubation at room temperature (excitation at 550 nm and a long-pass emission filter with 600 nm cutoff were used). | ||
In conclusion, we represented a rapid, sensitive, and selective PDA sensory system for melamine detection based on the multiple hydrogen bondings between cyanuric acid and melamine. The intra/inter liposomal hydrogen bonding between the target melamine and cyanuric acid receptor at the PDA liposome surface induces perturbation of the conjugated PDA backbone and results in rapid and sensitive colorimetric/fluorescence change of the PDA liposome. Detection limit of the developed PDA system is 1.0 ppm and 0.5 ppm for colorimetric PDA liposome solution and fluorescent PDA liposome microarray, respectively. The PDA liposome-based sensor assay can be conveniently prepared by simple self-assembly of rationally designed diacetylene molecules. The presented design principle of the sensing mechanism by intra/inter liposomal interaction of PDA liposome and target molecules can be readily applicable to the development of other PDA-based colorimetric/fluorescent sensory systems to detect various biomolecules and chemicals selectively and sensitively.
We gratefully acknowledge financial supports from the National Science Foundation (DMR CAREER 0644864).
Notes and references
- L. Zhu, G. Gamez, H. Chen, K. Chingin and R. Zenobi, Chem. Commun., 2009, 559–561 RSC.
- http://en.wikipedia.org/wiki/2008_Chinese_milk_scandal .
- H. Zhu, S. Zhang, M. Li, Y. Shao and Z. Zhu, Chem. Commun., 2010, 46, 2259–2261 RSC.
- J. P. Toth and P. C. Bardalaye, J. Chromatogr., A, 1987, 408, 335–340 CrossRef CAS.
- M. S. Filigenzi, E. R. Tor, R. H. Poppenga, L. A. Aston and B. Puschner, Rapid Commun. Mass Spectrom., 2007, 21, 4027–4032 CrossRef CAS.
- H. A. Cook, C. W. Klampfl and W. Buchberger, Electrophoresis, 2005, 26, 1576–1583 CrossRef CAS.
- E. A. E. Garber, J. Food Prot., 2008, 71, 509.
- K. Ai, Y. Liu and L. Lu, J. Am. Chem. Soc., 2009, 131, 9496–9497 CrossRef CAS.
- D. J. Ahn, S. Lee and J.-M. Kim, Adv. Funct. Mater., 2009, 19, 1483–1496 CrossRef CAS.
- D. J. Ahn and J.-M. Kim, Acc. Chem. Res., 2008, 41, 805–816 CrossRef CAS.
- K. Lee, L. K. Povlich and J. Kim, Analyst, 2010, 135, 2179–2189 RSC.
- R. R. Chance, Macromolecules, 1980, 13, 396–398 CrossRef CAS.
- J.-M. Kim, J.-S. Lee, H. Choi, D. Sohn and D. J. Ahn, Macromolecules, 2005, 38, 9366–9376 CrossRef CAS.
- S. Lee and J.-M. Kim, Macromolecules, 2007, 40, 9201–9204 CrossRef CAS.
- J. Pang, L. Yang, B. F. McCaughey, H. Peng, H. S. Ashbaugh, C. J. Brinker and Y. Lu, J. Phys. Chem. B, 2006, 110, 7221–7225 CrossRef CAS.
- J. Lee, H. Jun and J. Kim, Adv. Mater., 2009, 21, 3674–3677 CrossRef CAS.
- J. Lee, H.-J. Kim and J. Kim, J. Am. Chem. Soc., 2008, 130, 5010–5011 CrossRef CAS.
- K. Tashiro, H. Nishimura and M. Kobayashi, Macromolecules, 1996, 29, 8188–8196 CrossRef CAS.
- R. W. Carpick, D. Y. Sasaki and A. R. Burns, Langmuir, 2000, 16, 1270–1278 CrossRef CAS.
- J. M. Lehn, M. Mascal, A. Decian and J. Fischer, J. Chem. Soc., Chem. Commun., 1990, 479–481 RSC.
- F. J. Hoeben, J. Zhang, C. C. Lee, M. J. Pouderoijen, M. Wolffs, F. Würthner, A. P. Schenning, E. Meijer and S. De Feyter, Chem.–Eur. J., 2008, 14, 8579–8589 CrossRef.
- S. Yagai, S. Hamamura, H. Wang, V. Stepanenko, T. Seki, K. Unoike, Y. Kikkawa, T. Karatsu, A. Kitamura and F. Wurthner, Org. Biomol. Chem., 2009, 7, 3926–3929 RSC.
- D. Seo and J. Kim, Adv. Funct. Mater., 2010, 20, 1397–1403 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details for the synthesis, sensor preparation protocol and additional figures. See DOI: 10.1039/c0cc02183k |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| This journal is © The Royal Society of Chemistry 2011 |
