Layer-by-layer assembly of titania nanoparticles based ionic networks†‡
Bernhard
Basnar
a,
Marco
Litschauer
b,
Stefan
Abermann
a,
Emmerich
Bertagnolli
a,
Gottfried
Strasser
a and
Marie-Alexandra
Neouze
*b
aInstitute of Solid Stated Electronics, Vienna University of Technology, Vienna, Austria. E-mail: basnar@tuwien.ac.at
bInstitute of Materials Chemistry, Vienna University of Technology, Vienna, Austria. E-mail: mneouze@mail.zserv.tuwien.ac.at
First published on 23rd August 2010
Abstract
Recently nanoparticles ionic networks were reported in the frame of the remarkable development of new inorganic–organic hybrid materials based on nanoparticles assembly. In this article a layer-by-layer deposition method for the formation of imidazolium-based assemblies of photocatalytic titania nanoparticles is presented. This provides a new route for the controlled processing of this promising class of materials.
Recently, the material chemistry community led great efforts on the design of controlled nanoparticles assembly.1–6 The obtained hybrid materials demonstrated highly interesting properties for electron transfer-based applications,7,8 optoelectronics9 or bio-medical applications.10,11 One of the methods towards such hybrid materials uses imidazolium moieties as linker to bridge the nanoparticles.12–16 The presence of such linkers was shown to be advantageous for the material properties. The imidazolium linker delivers high ionic content, remarkable solubilization properties to the material, whilst maintaining a high thermal stability for the material.16
However, at this early stage, very few studies have been devoted to the processing of those new hybrid materials, even though the processing is a crucial step on the way to concrete applications for a material. In this frame, the processing into thin films is very advantageous as thin films are suitable for numerous applications, like membranes or electrolytes.6 For nanoparticles assemblies, layer-by-layer deposition methods have proven to be very efficient, providing homogeneous coatings with good control on the thickness.15,17–19
In this paper, we describe a simple layer-by-layer deposition method for the newly developed ionic nanoparticles assemblies, based on titania nanoparticles bridged with imidazolium linkers.
Fig. 1 depicts the fabrication process for the formation of nanoparticle monolayers. The N-(3-propyltrimethoxysilane)imidazole (1) linker was synthesized according to the literature.12Titania nanoparticles were fabricated in an analogous fashion to a published procedure (see supporting information for exact details and reference). The titania nanoparticles possess a very narrow size distribution with an average diameter of 8 nm (see ESI). The 3-chloropropyl phosphonic acid (2) was synthesized according to an established route20 and used to functionalize the titania nanoparticles surface.
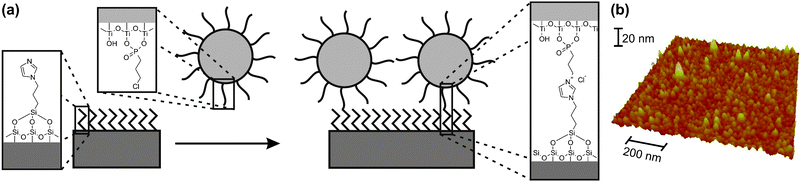 | ||
| Fig. 1 (a) Scheme for the preparation of TiO2-nanoparticle monolayers on silicon substrates with a native or thermal oxide layer. (b) AFM image of a nanoparticle monolayer on a silicon substrate. | ||
Washed and UV/ozone activated silicon wafer substrates were immersed into a 100 mmolar solution of 1 in ethanol for varying times. After cleaning of the samples (sonication in isopropanol and blow-drying), ellipsometric characterization of 1-modified surfaces showed an increase in the surface coverage with increasing deposition time, reaching saturation within 30 min. The measured thickness of 0.29 ± 0.05 nm is in agreement with the expected value for a complete monolayer of this molecule.
Deposition of the titania nanoparticles onto the 1-modified silicon wafers was undertaken by immersion of the slides into a 0.01 mg mL−1 solution of titania nanoparticles capped with 2, followed by the same cleaning procedure as above. The covalent linking of the particles to the modified surface occurred through a nucleophilic substitution reaction between the imidazole and chloroalkyl groups (Fig. 1a).12 This reaction proceeded at a much lower rate than the initial modification of the slides, requiring several hours to reach saturation. Ellipsometry showed thickness values of 2.63 ± 0.25 nm for overnight deposition. This value seems very low when keeping in mind the diameter of the nanoparticles. This comes from the fact that ellipsometry yields only the equivalent height for a dense layer, whereas the particle layer contains voids. Therefore, the ellipsometric thickness is equivalent to about 55% of a hexagonal closed packing of 8 nm diameter nanoparticles. Thus the obtained height is close to the random close packing limit for a nanoparticles-based system.
Formation of a TiO2 nanoparticles monolayer was further confirmed by atomic force microscopy (AFM, Fig. 1b). The AFM image shows a homogeneous particle layer with a fully covered surface, i.e. no silicon substrate is visible.
Also X-ray photoelectron spectroscopy (XPS) was employed for the characterization of the monolayers (see ESI for spectrum). Peaks for nitrogen at 400 eV, corresponding to the presence of 1, phosphor (2s at 191 eV and 2p at 133 eV) and chlorine (2s at 270 eV and 2p at 200 eV) corresponding to the presence of 2, as well as silicon (substrate and 1), titanium (nanoparticles), carbon (1, 2, and remnant gas in the XPS system) and oxygen (1, 2, substrate, and nanoparticles) could be identified.
Taking advantage of the high homogeneity and surface coverage of the monolayer films, structured monolayer formation was performed. To this end, silicon wafers with a native oxide layer were passivated by deposition of an octadecyltrichlorosilane (3) monolayer.21 This self-assembled monolayer was locally oxidized using AFM with an applied voltage of −7 V between tip and sample. Only small changes in the height (up to 1 nm) due to oxidation and concurrent swelling of the underlying silicon substrate were observed.
The patterned samples were subsequently modified with functionalized titania nanoparticles in the same way as mentioned above, i.e. immersion into the linker solution (1) for 30 min, rinsing, and immersion into the modified-nanoparticle solution for 16 h (overnight). AFM imaging (Fig. 2) showed high pattern fidelity with dense monolayer formation on the oxidized patterns. Next to the particles, only a small degree of unspecific adsorption was observed.
 | ||
| Fig. 2 AFM images of a structured nanoparticle monolayer deposited onto a pre-patterned surface; (a) shows the full pattern and (b) shows part of this structure at a higher resolution, resolving individual nanoparticles. | ||
The height of the titania pattern, measured by AFM, was 6 nm. This value is smaller than the diameter of the nanoparticles. However, it is well known that AFM is prone to produce small height deviations (in the nanometre range) if areas with strongly varying surface properties are measured.21 This is the case for our nanostructured surface, where 3 is soft and hydrophobic, whereas the titania nanoparticles are hard and hydrophilic.
The dense titania monolayer designed by our approach lends itself also to the fabrication of multilayer structure with high control on the number of layers. To form such multilayer samples, repeated immersions into the linker and nanoparticle solutions, respectively, were performed.
Fig. 3a shows the thickness for each individual layer measured by ellipsometry. As can be seen, the first layer (1) and the second layer (2-nanoparticles) exhibit the thickness values found for an individual layer, i.e. 0.29 ± 0.05 and 2.63 ± 0.25 nm respectively. The third layer (again 1), however, shows about twice the value of the first layer, namely 0.71 ± 0.09 nm, albeit the deposition time needed for saturation was much higher (several hours). The higher coverage can be explained by the higher specific surface of the nanoparticle layer as compared to the flat silicon substrate and the longer deposition time can be accounted for by a lower diffusion rate of 1 between the particles. The next particle layer (layer 4) exhibits the same thickness as the previous particle layer (layer 2), which indicates the formation of a second, full monolayer of titania nanoparticles.
 | ||
| Fig. 3 Fabrication of titania nanoparticle multilayers: (a) shows the ellipsometrically determined height for each individual layer, and (b) depicts an AFM image of the surface topography after 3 layers. | ||
The fifth layer (1) has the same thickness as layer 3, which confirms the fact that the amounts of nanoparticles in the layers 1 and 2 are identical. However, layer 6 (2-nanoparticles) shows a 40% higher value for the thickness, 3.74 ± 0.27 nm. This indicates that the surface corrugation increased with the individual steps, leading to a higher surface area and, thus, more anchoring possibilities for the particles.
This was further investigated by AFM. As can be seen in Fig. 3b, the surface roughness for three layers is, indeed, higher than what is seen in Fig. 1b for a single layer.
To investigate the stability of these layers additional tests were undertaken. Firstly, the layer is not degraded by sonication in isopropanol for prolonged periods (minimum up to 10 min) at maximum power of our sonicator. Secondly, lateral force microscopy (LFM) was employed. This atomic force microscopy technique utilizes the shear forces exerted onto the sample during scanning in contact mode. Changing the pressure of the cantilever also changes the shear force and, thus, the mechanical stability can be evaluated. It was found that for low pressures (contact forces up to 24 nN), no material is physically removed from the surface whereas forces of 36 nN or above lead to lateral removal and trench formation. This is not surprising as the number of anchoring points between particles is low, yielding a low number of covalent bonds for the stabilisation of the layered structure.
Although there is a general consensus that the Si–O–Ti bonds are not stable,22,23 our findings for stable monolayer formation based on these bonds are supported by other experimental work.24–27 Nevertheless further investigations utilizing phosphonic acid-based linkers as binding elements between the particle layers were undertaken for comparison, as the P–O–Ti bond is known to be stable.23
To this purpose, a multilayered sample with identical deposition conditions was prepared using imidazolylpropyl phosphonic acid (3) as the linking molecule between the nanoparticle layers, instead of the alkoxysilane-based 2. Due to the lower solubility of 3 in ethanol, however, a solution with lower concentration had to be prepared.
We found that, for identical deposition times as used before (21 h), the ellipsometrically determined surface coverage for both 3 and a subsequent particle layer was about 30% that of the layers formed using 2 as linker. Increasing the deposition time to several days led to a higher coverage similar to that found using 2. This indicates that the linking process is governed by diffusion. These values, i.e. 0.21 ± 0.09 nm for the linker layer (3) and 0.65 ± 0.07 nm for the 2-nanoparticle layer after 21 h of deposition, where repeatedly obtained for subsequent layers.
The stability of these layered structures was also investigated. As for the siloxane-based layered system, prolonged sonication in isopropanol at high powers led to no degradation of the surface film. This already evidenced a high stability for both multilayered structures. Nevertheless, LFM investigations showed a slightly higher stability toward mechanical wear when the titania nanoparticles are linked by means of a phosphonic acid-based ligand. Forces of 48 nN or above were required for the formation of trenches.
Although the system is very interesting for the controlled formation of 3D nanoparticle structures with predetermined surface functionalisation, bare titania nanoparticle layers can also be obtained via this approach. Towards this end, we investigated the influence of UV and oxygen plasma treatment on deposited mulitlayer structures.
Exposure to 254 nm UV light led to a significant reduction in the ellipsometrically determined layer thickness, from 8.97 to 7.42 nm. This is equivalent to the removal of about 60% of the organic material in the film. XPS measurements showed the elimination of the chlorine peaks. Similarly, the nitrogen peaks around 400 eV and the carbon peak at 387 eV were greatly reduced, whilst the phosphor peaks were shifted by about 1 eV. Concurrently, an increased oxygen peak at 533 eV is visible. These changes in the XPS spectrum of the layered material are attributed to photocatalytic degradation of the ligands through illumination of the photoactive titania nanoparticles. The remaining structures show similar mechanical stability towards sonication as well as towards LFM. This is either caused by a crosslinking of free titanium hydroxide molecules on the surface of neighbouring nanoparticles or via the remaining phosphonic acid groups.
In conclusion, we have demonstrated a novel method for the fabrication of three-dimensional nanoparticle scaffolds with full control over the lateral dimensions and the number of nanoparticle layers. This approach allows the processing of the newly reported hybrid materials based on imidazolium linked nanoparticles, which synthesis method was extended to titania based hybrid materials. A remarkable observation was made on the designed hybrid materials, indicating that both phosphonic acid–titanate and siloxane–titanate bonds show sufficient stability for the three-dimensional nanoparticle assemblies on surfaces. If necessary, removal of the organic linker through oxidation provides a means for the formation of highly porous, inorganic structures with photocatalytic activity.
ML and MAN thank the Austrian Science Foundation (Project P21190-N17) for financial support. BB acknowledges funding by FFG/BMVIT through the Nanoinitiative project PLATON.
Notes and references
- G. Evans, G. V. Duong, M. J. Ingleson, Z. Xu, J. T. A. Jones, Y. Z. Khimyak, J. B. Claridge and M. J. Rosseinsky, Adv. Funct. Mater., 2010, 20, 231 CrossRef CAS.
- D. Janczewski, N. Tomczak, S. Liu, M.-Y. Han and G. J. Vancso, Chem. Commun., 2010, 46, 3253 RSC.
- X. Liu, H. Liu, W. Zhou, H. Zheng, X. Yin, Y. Li, Y. Guo, M. Zhu, C. Ouyang, D. Zhu and A. Xia, Langmuir, 2010, 26, 3179 CrossRef CAS.
- M. N. Tchoul, S. P. Fillery, H. Koerner, L. F. Drummy, F. T. Oyerokun, P. A. Mirau, M. F. Durstock and R. A. Vaia, Chem. Mater., 2010, 22, 1749 CrossRef CAS.
- L. Wang, J. Luo, M. J. Schadt and C.-J. Zhong, Langmuir, 2010, 26, 618–632 CrossRef CAS.
- S. K. Das and A. J. Bhattacharyya, J. Phys. Chem. B, 2010, 114, 6830 CrossRef CAS.
- K. K. Haldar, T. Sen and A. Patra, J. Phys. Chem. C, 2010, 114, 4869 CrossRef CAS.
- M. Wu, P. Mukherjee, D. N. Lamont and D. H. Waldeck, J. Phys. Chem. C, 2010, 114, 5751 CrossRef CAS.
- N. Gaponik, J. Mater. Chem., 2010, 20, 5174 RSC.
- T.-C. Lee and O. A. Scherman, Chem. Commun., 2010, 46, 2438 RSC.
- R. Liu, J. Aw, W. Teo, P. Padmanabhan and B. Xing, New J. Chem., 2010, 34, 594 RSC.
- M. Litschauer and M.-A. Neouze, J. Mater. Chem., 2008, 18, 640 RSC.
- M. Litschauer, H. Peterlik and M.-A. Neouze, J. Phys. Chem. C, 2009, 113, 6547 CrossRef CAS.
- M. Litschauer, M. Puchberger, H. Peterlik and M.-A. Neouze, J. Mater. Chem., 2010, 20, 1269 RSC.
- C. Zhu, S. Guo, Y. Zhai and S. Dong, Langmuir, 2010, 26, 7614 CrossRef CAS.
- M.-A. Neouze, J. Mater. Chem., 2010 10.1039/c0jm00616e.
- S. Dey, K. Mohanta and A. J. Pal, Langmuir, 2010, 26, 9627 CrossRef CAS.
- X. Jiang, G. Zhang, R. Narain and S. Liu, Soft Matter, 2009, 5, 1530 RSC.
- S.-z. Tan, S. Long, J.-y. Xia, Z. Cao, L. Zhang and F.-c. Gong, J. Cent. South Univ. Technol., 2009, 16, 212 Search PubMed.
- J. T. Kley, C. Unger and U. Massing, Monatsh. Chem., 1998, 129, 173 CAS.
- B. Basnar, G. Friedbacher, H. Brunner, T. Vallant, U. Mayer and H. Hoffmann, Appl. Surf. Sci., 2001, 171, 213 CrossRef CAS.
- L. Crouzet, D. Leclercq, P. H. Mutin and A. Vioux, Chem. Mater., 2003, 15, 1530 CrossRef CAS.
- M.-A. Neouze and U. Schubert, Monatsh. Chem., 2008, 139, 183 CrossRef CAS.
- R. Fandos, B. Gallego, A. Otero, A. Rodriguez, M. J. Ruiz and P. Terreros, Dalton Trans., 2007, 871 RSC.
- C. Gervais, F. Babonneau and M. E. Smith, J. Phys. Chem. B, 2001, 105, 1971 CrossRef CAS.
- K. Kakiage, M. Yamamura, E. Fujimura, T. Kyomen, M. Unno and M. Hanaya, Chem. Lett., 2010, 39, 260 CrossRef CAS.
- W. B. Kim, S. H. Choi and J. S. Lee, J. Phys. Chem. B, 2000, 104, 8670 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental procedures. DLS graph for TiO2 nanoparticles. XPS spectra. See DOI: 10.1039/c0cc02243h |
| This journal is © The Royal Society of Chemistry 2011 |
