Disulfide formation via sulfenamides†‡
Jia
Pan
and
Ming
Xian
*
Department of Chemistry, Washington State University, Pullman, WA 99164, USA. E-mail: mxian@wsu.edu; Fax: 509-335-8867; Tel: 509-335-6073
First published on 23rd August 2010
Abstract
A phosphine-mediated one-step disulfide formation from S-nitrosothiols has been developed. This reaction can convert unstable S-nitrosothiols to stable disulfidesviasulfenamide intermediates under very mild conditions. It has the potential to be used for the detection ofS-nitrosothiols.
As an important post-translational modification, S-nitrosation converts protein cysteine residues (SH) to S-nitrosothiols (SNO). This protein modification leads to modulation of structure, inhibition of active site cysteines, disruption of allosteric interactions with other molecules, etc.1 The formation of S-nitrosothiols in proteins may be directed by peptide sequences surrounding sensitive cysteine residues, by exogenous versus endogenous nitric oxide (NO) sources or by compartmentalization of nitric oxide synthases. Although the importance of S-nitrosation has been well recognized, the detection ofS-nitrosation is still a challenge.2 This is primarily due to the lability of S-nitrosothiols and the lack of reliable methods for their detection. From a chemistry point-of-view, SNO is a unique functional group; it might have some distinct reactivity from other biological functional groups. If specific reactions which only target SNO can be developed, such reactions may be very useful for the detection ofS-nitrosation.
Our group recently initiated a program to study phosphine-based reactions of S-nitrosothiols. In 2008, a fast reductive ligation of SNO was developed (Scheme 1).3 This reaction selectively converts unstable SNO to stable sulfenamide products via a Staudinger ligation-like mechanism.3,4 However, we also noticed that sulfenamides generated from cysteine derivatives could be over-reduced by the excess of phosphine ligation reagents. This might be a problem for directly applying reductive ligation in SNO detection.
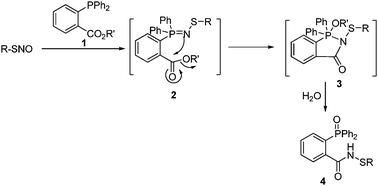 | ||
| Scheme 1 Reductive ligation of SNO. | ||
To solve this problem, we developed a bis-ligation of SNO in 2009 (Scheme 2).5 This reaction can convert SNO to stable disulfide-iminophosphorane conjugates in one step. Previous studies have revealed that disulfides are stable towards excess triarylphosphines such as the phosphine reagents used in our reaction.3–5
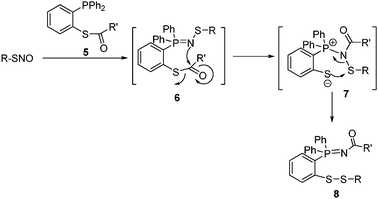 | ||
| Scheme 2 Bis-ligation of SNO. | ||
Given the high reactivity of the sulfenamide intermediates towards the thiolate observed in bis-ligation, we propose another one-step reaction to convert unstable SNO to stable products (Scheme 3). As such, SNO will be treated with regular reductive ligation reagent 1. As the reductive ligation is a very fast reaction, sulfenamide products should be formed in minutes. Then, if a nucleophile is added into the reaction mixture, it may convert sulfenamide 4 to a stable final product 9 and liberate the phosphine oxide 10. The formation of simple adducts, without the bulky triarylphosphine-oxide, would be attractive for applications in protein systems. We realized that if this one-pot reaction is used in the detection of protein SNO, one critical concern is that the nucleophile should only react with sulfenamides, not with protein disulfides.
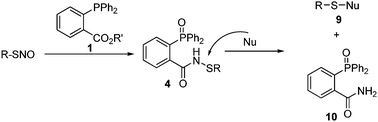 | ||
| Scheme 3 Proposed one-pot reaction to convert SNO to stable products. | ||
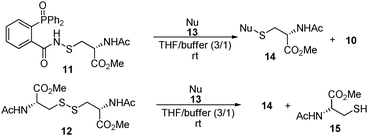
To test this idea, sulfenamide 11 was prepared and used as a model compound to test the reactivity of a series of mild nucleophiles. The reaction was carried out in a mixture of THF and PBS buffer (pH 7.4) solution. At the same time, compound 12 was used to examine the reactivity of the nucleophiles towards disulfides under the same conditions.
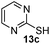
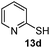
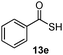
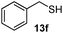
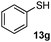
As shown in Table 1, two thiol nucleophiles (13a and 13b) showed good selectivity for the sulfenamide, as no reaction was observed when they were treated with disulfide 12. Other thiols (13c–13g) reacted with both the sulfenamide and disulfide. Tertiary thiols like 13h, however, showed no reactivity to either 8 or 9, probably due to steric hindrance. We also tested carbon nucleophiles 13i and 13j in our reactions. 13i was previously reported to react with cyclic sulfenamides.6 However, both 13i and dimedone 13j failed to show any reactivity to sulfenamide 11 even under refluxing conditions.
| Nu | Reaction time/h | Reaction with 11 | Reaction with 12 |
|---|---|---|---|
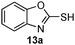
|
2 |
14a
78% |
NR |
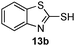
|
0.5 |
14b
90% |
NR |
|
0.5 |
14c
70% |
14c
15% |
|
0.5 |
14d
92% |
14d
14% |
|
0.5 |
14e
63% |
14e
40% |
|
0.5 |
14f
85% |
14f
90% |
|
0.5 |
14g
60% |
14g
72% |
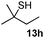
|
2 | NR | NR |
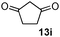
|
2 | NR | NR |
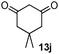
|
2 | NR | NR |
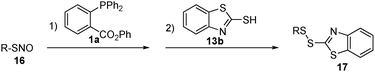
With these results in hand, we turned to the one-step disulfide formation from S-nitrosothiols using 13b, the best substrate found in Table 1. A series of SNO substrates were tested (Table 2). The reaction was carried out as follows: freshly prepared SNO substrates were treated with phosphine 1a in a mixture of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 THF/PBS buffer (pH 7.4) at room temperature. After 10 min, 13b was added and the reaction mixture was stirred for an additional 30 min to afford the desired product. As shown in Table 2, all of the SNO substrates exhibited good reactivity in the reaction and the desired disulfide products were obtained in good yields.
1 THF/PBS buffer (pH 7.4) at room temperature. After 10 min, 13b was added and the reaction mixture was stirred for an additional 30 min to afford the desired product. As shown in Table 2, all of the SNO substrates exhibited good reactivity in the reaction and the desired disulfide products were obtained in good yields.
| RSNO | Product | Yield (%) |
|---|---|---|
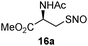
|
17a | 85 |
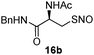
|
17b | 86 |
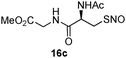
|
17c | 43 |
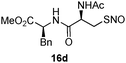
|
17d | 55 |
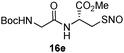
|
17e | 65 |
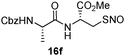
|
17f | 82 |
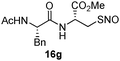
|
17g | 75 |
In order to confirm that the reaction is specific only to SNO, while not affecting disulfides, we performed a crossover experiment. As shown in Scheme 4, the one-step disulfide formation using 16b was carried out in the presence of disulfide 12. In this reaction, 17b was the only product and no crossover product was observed. Disulfide 12 was completely recovered.
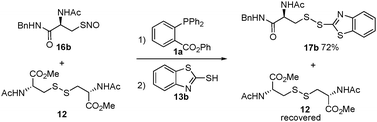 | ||
| Scheme 4 Crossover experiment. | ||
In conclusion, we have developed a one-pot disulfide formation from S-nitrosothiols under very mild conditions. This reaction does not affect disulfide bonds. We believe this reaction has the potential to be used in the detection ofS-nitrosothiols.
We acknowledge financial support by the American Heart Association (0930120N) and NSF CAREER award (0844931).
Notes and references
- For selected reviews, see: (a) J. R. Lancaster, Jr., Nitric Oxide, 2008, 19, 68 CrossRef; (b) M. W. Foster, T. J. McMahon and J. S. Stamler, Trends Mol. Med., 2003, 9, 160 CrossRef CAS; (c) Y. Zhang and N. Hogg, Free Radical Biol. Med., 2005, 38, 831 CrossRef CAS.
- For recent reviews on RSNO detection, see: (a) A. Gow, A. Doctor, J. Mannick and B. Gaston, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 851, 140 CrossRef CAS; (b) N. J. Kettenhofen, K. A. Broniowska, A. Keszler, Y. Zhang and N. Hogg, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 851, 152 CrossRef CAS; (c) P. H. MacArthur, S. Shiva and M. T. Gladwin, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 851, 93 CrossRef CAS; (d) D. Giustarini, A. Milzani, I. Dalle-Donne and R. Rossi, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2007, 851, 124 CrossRef CAS.
- H. Wang and M. Xian, Angew. Chem., Int. Ed., 2008, 47, 6598 CrossRef CAS.
- (a) E. Saxon and C. R. Bertozzi, Science, 2000, 287, 2007 CrossRef CAS; (b) F. L. Lin, H. M. Hoyt, H. van Halbeek, R. G. Bergman and C. R. Bertozzi, J. Am. Chem. Soc., 2005, 127, 2686 CrossRef CAS.
- J. Zhang, H. Wang and M. Xian, J. Am. Chem. Soc., 2009, 131, 3854 CrossRef CAS.
- T. P. Shiau, D. A. Erlanson and E. M. Gordon, Org. Lett., 2006, 8, 5697 CrossRef CAS.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0cc02076a |
| This journal is © The Royal Society of Chemistry 2011 |