Sub-100 nm TiO2 mesocrystalline assemblies with mesopores: preparation, characterization, enzyme immobilization and photocatalytic properties†‡
Pedro
Tartaj
*
Instituto de Ciencia de Materiales de Madrid (CSIC), Cantoblanco, 28049, Madrid, Spain. E-mail: ptartaj@icmm.csic.es
First published on 9th July 2010
Abstract
The in situ formation of sub-100 nm solid frameworks stabilized against dissolution by the addition of nanoseeds allows the facile and controllable synthesis of TiO2 (anatase) mesocrystalline structures with spherical shape, mesoporosity and sizes between 50 and 70 nm. As an example of their multifunctionality, these structures show good capabilities for enzyme immobilization and adequate photocatalytic properties.
Titanium dioxide (TiO2) can be considered as a paradigm of multifunctionality.1 It can be used as a pigment, in water remediation, photovoltaics and H2 generation.1,2 It is considered as an alternative to carbonaceous anodes in Li-ion batteries and it was recently used in memristive switches.3 Some of these applications require mesoporous structures. Mesoporous TiO2 materials are typically produced as bulk powders and films. Indeed, these arrays are adequate for applications such as photovoltaics or Li-batteries. However, other applications involving catalysis in a liquid-phase or eradication of malignancy require the preparation of colloidal suspensions.4 For these applications, mesoporous TiO2 particles with sizes below 100 nm and good crystallinity are preferred. Good crystallinity minimizes the degradation of properties associated with surfaces. Furthermore, if we develop mesocrystallinity in these structures, a lower impact in properties degradation should be expected. Mesocrystallinity involves an oriented structure of mesosize crystals (it includes nanocrystals).5 Nanocasting routes could be promising routes to obtain these type of TiO2 particles but the production yield is limited for sub-100 nm sizes and mesocrystallinity is difficult to reach.6 Other routes lead to non-mesocrystalline and non-uniform porous aggregates,7 to mesocrystals of 1–2 µm with limited porosity,8 to non-porous particles,1a or to non-mesocrystalline porous spheres with sizes above 100 nm.9
It is established that the thermal destabilization of microemulsions leads to biphasic systems. In fact, we have recently exploited this concept for the preparation of monoclinic zirconia and β-FeOOH nanorods by a modified hydrothermal method.10 Rather than mixing all the reactants at ambient pressure, some of them are added in situ by fast destabilization of inverse microemulsions. We have recently established, however, that conditions exist at which the thermal destabilization of inverse microemulsions takes place through the formation of kinetically stable self-assembled nanomicellar structures with sizes below 100 nm.11 A cation can be hydrolysed by reaction with NH3 vapours to produce amorphous mesoporous nanostructures with sizes below 100 nm. A fundamental drawback of the method is that crystallinity in these structures is not generated in situ, so that it must be developed by heating in N2 atmosphere at relatively high temperatures (600 °C). This thermal treatment, however, is not able to generate structures with mesocommensurate crystallinity. Partially inspired by these results and partially inspired by the concept of seeding-assisted chemistry (i.e. nucleation energy barriers are lowered by the presence of isostructural phases), we here report that relatively uniform (polydispersity indexes around 10–15%) mesocrystalline structures with spherical shape, mesoporosity and sizes below 100 nm can be obtained from inverse microemulsions.
In order to produce sub-100 nm mesocrystalline nanostructuresvia inverse microemulsions the following conditions must be established: (a) setting a two-stage temperature program; (b) absence of any hydrolysing agent (NH4OH, KOH or NH3 vapours) and (c) addition of some anatase nanoseeds. A schematic representation of the synthesis route is displayed in Fig. 1. The two-stage temperature program consists of setting first a temperature (60 °C), at which the thermal destabilization of inverse microemulsions takes place through the formation of kinetically stable self-assembled nanomicellar structures with sizes below 100 nm. In the second stage the temperature is increased to favour crystallization. The second condition (absence of any hydrolysing agent) assures a slow hydrolysis process only assisted by temperature and pressure (we work in closed systems). Thus, during the first stage of the temperature program (60 °C) only a fraction of TiOSO4 is hydrolysed (20–30 wt% expressed as TiO2). In this way, a relatively stable sub-100 nm spherical TiO2 framework is made via replica of the self-assembled nanomicellar structures. This inorganic framework acts as a template for the formation of mesocrystalline arrays at higher temperatures. As aforementioned inverse microemulsions at high temperatures (80 °C or higher) destabilize very fast (i.e. nanomicellar structures do not form) and so this inorganic framework is needed. Furthermore, the absence of any hydrolysing agent assures that a significant amount of cations remain in solution to favour crystallization at higher temperatures. If a hydrolysing agent is present the TiOSO4 is completely hydrolysed at 60 °C and amorphous structures are formed.11 Finally, anatase seeds are needed to lower the nucleation barrier for the formation of anatase avoiding the dissolution of the TiO2 spherical framework at high temperatures.
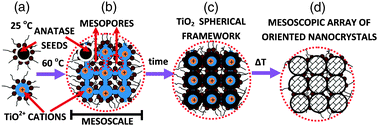 | ||
| Fig. 1 (A) Reverse nanomicelles containing TiOSO4 are produced at 25 °C. (B) and (C) At 60 °C these nanomicelles can self-assemble to form sub-100 nm structures that by partial hydrolysis of TiOSO4 produces a sub-100 nm TiO2 spherical framework. (D) Increasing temperature produces sub-100 nm mesocrystalline structures with interstitial porosity partially filled with surfactants. Mesoporosity is fully generated after removing the interstitial surfactant. As we describe in the main text the presence of anatase nanoseeds is essential for the stability of the solid framework. | ||
We here present the results for 4 samples (C80, C120, D80 and D120) prepared with the methodology and conditions described in the Experimental section (see ESI‡). C and D stand for concentrated and diluted (higher or lower reactants concentration). 80 and 120 stand for the final temperature (80 or 120 °C). X-Ray diffraction (XRD) patterns of samples prepared at different conditions are shown in Fig. 2. All samples consist of anatase whose crystallite size increases with temperature but is not dependent on reactants concentration (crystals of 6–7 nm for C80 and D80 and 9–10 nm for C120 and D120). The textural properties of samples are also shown in Fig. 2. N2 isotherms for samples C80 and D80 are similar and show a relatively low irreversibility (large fraction of accessible pores). For samples C120 and D120 the irreversibility is higher (smaller fraction of accessible pores). Values for the BET specific surface area are about 250 m2 g−1 for C80 and D80 and ∼200 m2 g−1 for C120 and D120. Pore size distributions show a mesopore population centred about 2.5 and 4 nm for samples prepared at 80 and 120 °C, respectively. TEM pictures (Fig. 3 and Fig. S2 in ESI‡) show that all the samples consist of spherical assemblies whose size is only dependent on reactants concentration (50 nm for D80 and D120 and 65 nm for C80 and C120). Polydispersity indexes (standard deviation/mean size) in all samples were similar and close to a value of 10–15%. More detailed structural information of the materials was revealed by high resolution transmission electron microscopy (HR-TEM) and selected-area electron diffraction (SAED). Fig. 3 shows a typical HR-TEM picture of the spherical assemblies and its corresponding SAED pattern. We clearly show the presence of mesocrystallites which grow all oriented in the same direction. In our particular case, the nanostructures are dominated by the most stable energetically (101) surfaces.12 It is worthy to mention that the porosity of the spherical assemblies is disordered. However, their sizes (60–70 nm) are very small so that diffusion distances to cover the entire interstitial volume are very short (i.e. no need for a highly ordered mesoporosity as it is the case of massive porous materials). Band gap measurements of all of the samples were similar to those of bulk anatase (3.2 eV). This result seems to confirm previous results that in absence of defects or impurities, size quantization effects for anatase crystals are not present at least for crystal sizes above 2 nm.13
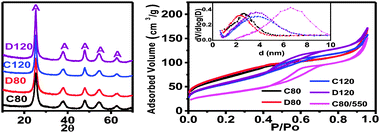 | ||
| Fig. 2 (left) XRD patterns and (right) N2 isotherms (inset pore size distributions) of samples (A stands for Anatase). The isotherm and pore size distribution for C80 heated at 550 °C/N2–300 °C/air is also included. As mentioned in ESI‡ all samples were heated at 300 °C/air to eliminate the residual interstitial surfactant (experimental evidence was confirmed by the reduction of textural properties at lower temperatures (Fig. S1)). | ||
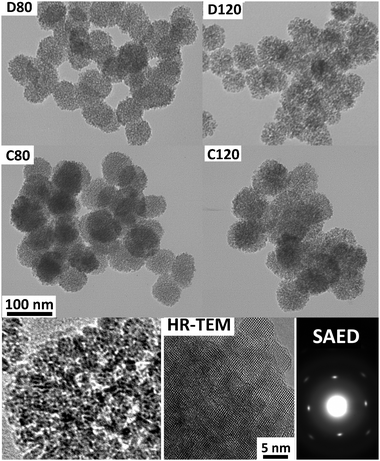 | ||
| Fig. 3 (upper and middle parts) TEM pictures of samples (magnification is shown in the C80 picture). We should remark that TEM pictures are shown at a relatively high magnification to better visualize the internal microstructure (spherical assemblies of small units) and the differences in sizes of D and C samples (50 vs. 65 nm). See size histograms and TEM pictures at lower magnification in Fig. S2 of ESI‡ for reliable size statistics. (Lower part) TEM magnification showing pores. HR-TEM picture showing that the particles are formed by an assembly of anatase nanocrystals (in this case mesocrystallites around 7 nm) which grow all oriented in the same direction ((101) surfaces). In accordance, the SAED picture of an entire spherical assembly corresponds to a single crystal. | ||
The information derived from the different techniques deserves a detailed discussion. The results clearly show the formation of anatase mesocrystallites which grow all oriented in the same direction and form relatively uniform mesoscopic structures with spherical shape and mesoporosity. When compared to the typical mesocrystals,5 it seems clear that the spherical shape, the size (below 100 nm) and the good textural properties (accessible porosity and high BET surface area) are the consequence of the shape, size and porosity directing agent (in situ obtained sub-100 nm spherical TiO2 frameworks). In fact, according to TEM (Fig. 3 and Fig. S2 in ESI‡) the size of the assemblies is only a function of reactants concentration (not temperature). The size of the TiO2 framework depends on the size of the self-assembled nanomicellar structures formed at 60 °C, through the effect that the reactants concentration has on the size of nanomicellar structures.11 The phase nucleating agent (anatase nanoseeds) is also essential for the formation of these structures. In its absence, the nanostructures were not formed and only some rutile was obtained. Likely, without nanoseeds the framework is dissolved when increasing the temperature and crystallization takes place in the absence of the inorganic template. Finally, the results derived from TEM indicate that independently of reactants concentration and final temperature the anatase nanocrystals are assembled in spherical arrays. Thus, the observed increase in pore size with temperature (2.5 to 4 nm) reflects the increase in the anatase crystal size observed by XRD (6–7 to 9–10 nm). For a particular array (spherical in this case) basic packing arguments dictate that pore sizes increase with the size of the building blocks (keep in mind that the surfactant concentration was the same in all samples).
Simple enzyme immobilization and photocatalysis experiments were carried out only with the purpose of showing the potential capabilities of the nanostructures here obtained (sample C80 was selected for these proof-of-concept experiments). Lysozyme was selected for immobilization studies because it is widely used in mesoporous materials.14 The immobilization study was carried out in sample C80 and sample C80 heated at 550 °C/N2–300 °C/air. In a typical temperature-assisted sintering process, pore coalescence (larger pores grow at the expense of smaller ones) occurs prior to complete elimination of pores. Thus, even though this process simultaneously involves a reduction in specific surface area, for some conditions we can reach an increase in pore size while still preserving high surface area. The textural properties of the heated sample are shown in Fig. 2. The average pore size increased from ∼2–3 to ∼6–7 nm (anatase crystallite size increased from 7 to 13 nm). The BET specific surface area is indeed lower (135 vs. 250 m2 g−1) but it still presents an adequate value, specially considering that in particles of about 60–70 nm diffusion distances to cover the interstitial volume are very short. Fig. 4 shows the capabilities for lysozyme immobilization of non-treated and thermally treated sample C80. A pH of 10 was selected for this study because at this pH lysozyme shows a maximum in its capabilities for being adsorbed on negatively charged substrates.14cLysozyme is a globular protein with a size of 4.5 × 3.0 × 3.0 nm so it is normal that the thermally treated sample (pore size 6–7 nm vs. 2–3 nm for the non-treated) showed significantly better capabilities for lysozyme immobilization (740 vs. 260 mg g−1). In fact, the thermally treated sample shows lysozyme immobilization capabilities close to the best reported for mesoporous silica and carbon.14
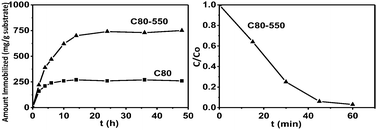 | ||
| Fig. 4 (left) Lysozyme adsorbed as a function of time for samples C80 and C80 heated at 550 °C in N2. (right) UV photocatalytic degradation of methylene blue in the presence of the sample C80 treated at 550 °C in N2. | ||
Finally, simple photocatalytic experiments (Fig. 4) were carried out in the sample with the best immobilization capabilities for big molecules (C80 heated at 550 °C in N2). It is clear that this sample is able to photocatalytically degrade organic compounds under the action of UV radiation.
In summary, we have shown that the in situ formation of sub-100 nm porous frameworks stabilized against dissolution by the addition of nanoseeds allows the synthesis of anatase mesocrystalline structures with spherical shape, mesoporosity and sizes between 50 and 70 nm. These small structures can immobilize enzymes and photocatalytically degrade organic compounds. Thus, they are promising candidates for catalysis in a liquid-phase or bioapplications such as photoeradication of malignancy (drug delivery plus photorelease or phototherapy).
Financial support from the Spanish Ministerio de Ciencia e Innovacion through MAT2008-03224/NAN is acknowledged.
Notes and references
- (a) X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891 CrossRef CAS; (b) A. Fujishima, X. Zhang and D. A. Tryk, Surf. Sci. Rep., 2008, 63, 515 CrossRef CAS.
- A. Fujishima and K. Honda, Nature, 1972, 37, 238.
- (a) Z. Yang, D. Choi, S. Kerisit, K. M. Rosso, D. Wang, J. Zhang, G. Graff and J. Liu, J. Power Sources, 2009, 192, 588 CrossRef CAS; (b) J. Borghetti, G. S. Snider, P. J. Kuekes, J. Joshua-Yang, D. R. Stewart and R. Stanley, Nature, 2010, 464, 873 CrossRef CAS.
- A.-H. Lu, E. L. Salabas and F. Schuth, Angew. Chem., Int. Ed., 2007, 46, 1222 CrossRef CAS.
- R.-Q. Song and H. Colfen, Adv. Mater., 2010, 22, 1301 CrossRef CAS.
- A.-H. Lu and F. Schuth, Adv. Mater., 2006, 18, 1793 CrossRef CAS.
- S. K. Das, M. K. Bhunia and A. Bhaumik, Dalton Trans., 2010, 39, 4382 RSC.
- L. Zhou, D. S. Boyle and P. O'Brien, Chem. Commun., 2007, 144 RSC.
- W.-G. Yang, F.-R. Wang, Q.-W. Chen, J.-J. Li and D.-S. Xu, J. Mater. Chem., 2010, 20, 2870 RSC.
- (a) P. Tartaj, O. Bomatí-Miguel, A. F. Rebolledo and T. Valdes-Solis, J. Mater. Chem., 2007, 17, 1958 RSC; (b) O. Bomatí-Miguel, A. F. Rebolledo and P. Tartaj, Chem. Commun., 2008, 4168 RSC.
- (a) P. Tartaj, Chem. Commun., 2009, 3228 RSC; (b) P. Tartaj, Small, 2010, 6, 880 CrossRef CAS.
- (a) U. Diebold, Surf. Sci. Rep., 2003, 48, 53 CrossRef CAS; (b) M. Liu, L. Piao, L. Zhao, S. Ju, Z. Yan, T. He, C. Zhou and W. Wang, Chem. Commun., 2010, 46, 1664 RSC.
- N. Serpone, D. Lawless and R. Khairutdinov, J. Phys. Chem., 1995, 99, 16646 CrossRef CAS.
- (a) A. Vinu, M. Miyahara, V. Sivamurugan, T. Mori and K. Ariga, J. Mater. Chem., 2005, 15, 5122 RSC; (b) Y. Wang and F. Caruso, Chem. Mater., 2005, 17, 953 CrossRef CAS; (c) T. Valdes-Solis, A. F. Rebolledo, M. Sevilla, P. Valle-Vigon, O. Bomatí-Miguel, A. B. Fuertes and P. Tartaj, Chem. Mater., 2009, 21, 1806 CrossRef.
Footnotes |
| † This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| ‡ Electronic supplementary information (ESI) available: Experimental section, TEM pictures, size histograms and PSD. See DOI: 10.1039/c0cc01540g |
| This journal is © The Royal Society of Chemistry 2011 |
