A pyridinium cation–π interaction sensor for the fluorescent detection of alkyl halides†‡
Wenbo
Chen
a,
Souad A.
Elfeky
a,
Ysé
Nonne
ab,
Louise
Male
a,
Kabir
Ahmed
a,
Claire
Amiable
bc,
Philip
Axe
c,
Shinji
Yamada
d,
Tony D.
James
c,
Steven D.
Bull
*c and
John S.
Fossey
*ae
aSchool of Chemistry, University of Birmingham, Edgbaston, Birmingham, West Midlands, B15 2TT, UK. E-mail: j.s.fossey@bham.ac.uk; Web: www.johnfossey.com
bESCOM (Ecole Supérieur de Chimie Organique et Minérale), 1, Allée du réseau Jean-Marie Buckmaster, 60200 Compiègne, France
cDepartment of Chemistry, University of Bath, Claverton Down, Bath, BA2 7AY, UK. E-mail: s.d.bull@bath.ac.uk
dDepartment of Chemistry, Ochanomizu University, 2-1-1 Otsuka, Bunkyo-ku, Tokyo 112-8610, Japan
eDepartment of Chemistry, Ochanomizu University, 2-1-1 Otsuka, Bunkyo-ku, Tokyo 112-8610, Japan
First published on 5th July 2010
Abstract
N-Alkylation of a novel pyridine sensor results in pyridinium salts whose conformations are stabilised by pyridinium cation–π interactions resulting in a fluorescent response that can be used to sense the presence of alkylating agents in solution at low concentration.
Potent alkylating agents such as methyl iodide or benzyl bromide are extremely useful reagents for organic synthesis that have been used widely for the synthesis of many drug molecules. However, their inherent reactivity means they are potentially toxic/mutagenic due to their ability to react with numerous biological nucleophiles present within the human body.1 Consequently, their use requires rigorous detection regimes to ensure that no alkylating agent is present in any pharmaceutical agent that is used for human consumption.2Alkylating agents are also present in trace amounts in the environment due to their use as soil sterilizers,3 cancer drugs,4 chemical reagents and chemical weapon agents.5 Therefore, there is significant demand for the development of reliable assays that allow the presence of alkylating agents to be detected in a wide range of scenarios.6
Cation–π interactions have been widely exploited in supramolecular chemistry, where they play pivotal roles in the design of host–guest assemblies7 and synthetic receptors.8 These type of interactions have also proven useful for synthesis,9 where their presence has been invoked to explain the selectivity of nucleophilic catalysts,10 cyclopropanations,11cycloadditions12 and various nucleophilic additions.13 In the biological arena cation–π interactions have been implicated in many biomolecular recognition events,14 whilst they also play an important role in stabilising the secondary and tertiary structures of proteins.15 We have previously used NOEsy and fluorescence spectroscopy to show that conversion of conformationally flexible 4-(phenylpropyl)-pyridine 1 into its corresponding N-methyl-pyridinium iodide 2a (Scheme 1) results in a large change in fluorescence response, due to the presence of intramolecular cation–π interactions in 2a.16 Consequently, we now report that the increased fluorescent response of pyridinium salts 2 may be exploited to enable pyridine 1 to be used to sense the presence of low concentrations of alkylating agents in solution (Scheme 2).17,18
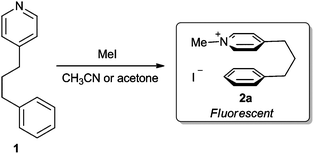 | ||
| Scheme 1 Alkylation of pyridine 1 with methyl iodide forms pyridinium 2 which exhibits characteristic cation–π fluorescence. | ||
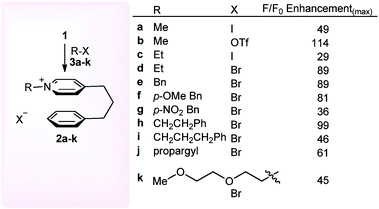 | ||
| Scheme 2 Screening of fluorescent response of N-alkyl-pyridinium salts 2a–k derived from different alkylating agents. | ||
We reasoned that the contrasting fluorescent properties of pyridine 1 and its corresponding N-alkyl-pyridinium salts 2 might enable it to be used as a sensor to detect the presence of alkylating agents in solution. In order to test this hypothesis, aliquots of methyl iodide (0–0.6 mM) were sequentially added to a solution of pyridine 1 in CH2Cl2 (1.0 mM) and the fluorescent spectra of the solution recorded 5 minutes after each addition. This revealed an eleven fold fluorescence enhancement (F/Fo) over the concentration range (0–0.6 mM) of methyl iodide assayed (see Fig. 1).19 In order to determine the detection limits of this sensor we reduced the concentration of pyridine 1 to 0.01 mM in CH2Cl2 and assayed its fluorescent response to the presence of low concentrations of methyl iodide (0–400 nM). This resulted in a three fold increase in fluorescence over a concentration range of 0–400 nM, with a lower detection limit for methyl iodide of 1 nM (see Fig. 2 and ESI†).
![Fluorescence increase on addition of aliquots of MeI (0 to 0.6 mM) to a solution of pyridine 1 (1.0 mM) in CH2Cl2 (λex = 260 nm); F/F0versus wavelength (nm) for increasing [MeI].](/image/article/2011/CC/c0cc01420f/c0cc01420f-f1.gif) | ||
| Fig. 1 Fluorescence increase on addition of aliquots of MeI (0 to 0.6 mM) to a solution of pyridine 1 (1.0 mM) in CH2Cl2 (λex = 260 nm); F/F0versus wavelength (nm) for increasing [MeI]. | ||
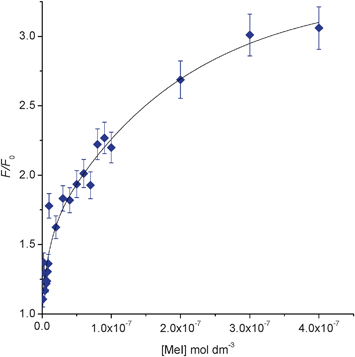 | ||
| Fig. 2 Fluorescence F/F0 at 400 nm for addition of MeI (0–400 nM) to pyridine 1 (0.01 mM) in CH2Cl2 (λex = 260 nm). | ||
In order to demonstrate the potential of this cation–π stacking sensor towards other types of alkylating agent, we then treated pyridine 1 (1.0 mM, CH2Cl2) with a range of commonly used electrophiles 3a–k (0.6 mM) under reflux for 3 h to ensure complete consumption of electrophile, and determined the maximum fluorescence of each solution. In each case a fluorescence enhancement was observed (29–114 fold increase), indicating that pyridine 1 has the potential to be used as a sensor for a wide range of alkylating agents. It is noteworthy that the fluorescent response of the N-alkyl-pyridinium triflate salt 2b was 2.3 fold greater than the corresponding iodide salt 2a, which we ascribed to the known heavy atom quenching effect of the iodide counter ion.19 Whilst alkylation of pyridine 1 with the potent triflate/iodide alkylating agents 3a–c gave an almost instantaneous increase in fluorescence (seconds), use of alkyl bromides 3d–k required a longer time (hours) to react before solutions with stable fluorescence maxima were obtained. The corresponding control experiments where pyridine and benzene with and without added methyl iodide had previously confirmed an intramolecular interaction is responsible for the observed fluorescence.16a
A range of N-alkyl-pyridinium salts 2a–k were then prepared in order to confirm that these species were responsible for the fluorescent response observed in these assays. Isolated yields of each fluorescent salt were generally high (>90%).20 The structure of compound 2i was determined by X-ray crystallography (see Fig. 3),21 which revealed that electrostatic cation–anion interactions dominate in the solid state, with no intramolecular cation–π stacking interactions being observed.16b
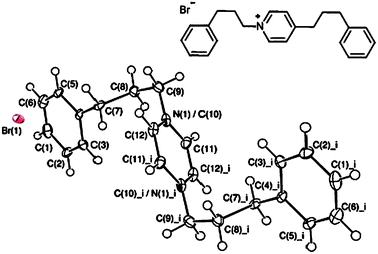 | ||
| Fig. 3 ORTEP plot of 2i with ellipsoids drawn at the 50% probability level. Atoms N(1) and C(10) lie on the same site at 50% occupancy each. Selected bond lengths (Å) and angles (°): C(9)–C(10)/N(1) = 1.490 (9), C(10)/N(1)–C(11) = 1.371 (8), C(10)/N(1)–C(12) = 1.379 (8), C(4)–C(7)–C(8) = 111.9 (5), C(7)–C(8)–C(9) = 112.5 (5), C(8)–C(9)–C(10)/N(1) = 111.5 (5), C(11)–C(10)/N(1)–C(12) = 118.9 (6), C(4)–C(7)–C(8)–C(9) = −171.0 (5), and C(7)–C(8)–C(9)–C(10) = 62.2 (7). | ||
In conclusion, we have reported an operationally simple, robust and cheap pyridine sensor 1 for the fluorescent detection of a range of commonly employed alkylating agents. We are currently working on the design of new pyridine architectures with higher fluorescent output and improved response time that we envisage will enable even lower concentrations of alkylating agent to be detected.
The Leverhulme Trust, Royal Society, Universities of Birmingham and Bath, EPSRC, Birmingham AWM II and the JSPS are thanked for support. SAE acknowledges Cairo University (NILES-LAMPA). The EPSRC National Crystallography Service is thanked for the collection of X-ray diffraction data on 2i.
Notes and references
- (a) C. R. Kirman, L. M. Sweeney, M. L. Gargas and J. H. Kinzell, Inhalation Toxicol., 2009, 21, 537–551 Search PubMed; (b) E. W. Vogel and M. J. M. Nivard, Mutat. Res., 1994, 305, 13–32 CrossRef CAS; (c) H. M. Bolt, R. J. Laib, H. Peter and H. Ottenwalder, J. Cancer Res. Clin. Oncol., 1986, 112, 92–96 CrossRef CAS.
- D. P. Elder, A. M. Lipczynski and A. Teasdale, J. Pharm. Biomed. Anal., 2008, 48, 497–507 CrossRef CAS.
- D. G. Karpouzas, E. Karanasios, I. O. Giannakou, A. Georgiadou and U. Menkissoglu-Spiroudi, Soil Biol. Biochem., 2005, 37, 541–550 CrossRef CAS.
- L. H. Hurley, Nat. Rev. Cancer, 2002, 2, 188–200 CrossRef CAS.
- R. Burke, Counter-Terrorism for Emergency Responders, Taylor & Francis, Oxford, 2nd edn, 2007, p. 68 Search PubMed.
- (a) D. Q. Liu, M. Sun and A. S. Kord, J. Pharm. Biomed. Anal., 2010, 51, 999–1014 CrossRef CAS; (b) C. Hermouet, R. Garnier, M.-L. Efthymiou and P.-E. Fournier, Am. J. Ind. Med., 1996, 30, 759–764 CrossRef CAS; (c) J. J. Lee and B. D. Smith, Chem. Commun., 2009, 1962–1963 RSC; (d) J. J. Lee, B. C. Noll and B. D. Smith, Org. Lett., 2008, 10, 1735–1738 CrossRef CAS.
- (a) W. J. Belcher, M. Fabre, T. Farhan and J. W. Steed, Org. Biomol. Chem., 2006, 4, 781–786 RSC; (b) J. M. Heemstra and J. S. Moore, Chem. Commun., 2004, 1480–1481 RSC; (c) H.-J. Schneider, Angew. Chem., Int. Ed., 2009, 48, 3924–3977 CrossRef CAS; (d) V. Balzani, M. Gomez-Lopez and J. F. Stoddart, Acc. Chem. Res., 1998, 31, 405–414 CrossRef CAS; (e) C. A. Hunter, C. M. R. Low, C. Rotger, J. G. Vinter and C. Zonta, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 4873–4876 CrossRef CAS.
- (a) S. Mecozzi, A. P. West and D. A. Dougherty, Proc. Natl. Acad. Sci. U. S. A., 1996, 93, 10566–10571 CrossRef CAS; (b) J. C. Ma and D. A. Dougherty, Chem. Rev., 1997, 97, 1303–1324 CrossRef CAS; (c) L. Zeng, W. Liu, X. Zhuang, J. Wu, P. Wang and W. Zhang, Chem. Commun., 2010, 46, 2435–2437 RSC.
- S. Yamada, Org. Biomol. Chem., 2007, 5, 2903–2912 RSC.
- (a) S. Yamada and K. Yamashita, Tetrahedron Lett., 2008, 49, 32–35 CrossRef CAS; (b) S. Yamada, T. Misono and Y. Iwai, Tetrahedron Lett., 2005, 46, 2239–2242 CrossRef CAS; (c) T. Kawabata, R. Stragies, T. Fukaya and K. Fuji, Chirality, 2003, 15, 71–76 CrossRef CAS.
- S. Yamada, J. Yamamoto and E. Ohta, Tetrahedron Lett., 2007, 48, 855–858 CrossRef CAS.
- (a) S. Yamada and Y. Tokugawa, J. Am. Chem. Soc., 2009, 131, 2098–2099 CrossRef CAS; (b) S. Yamada, Y. Nojiri and M. Sugawara, Tetrahedron Lett., 2010, 51, 2533–2535 CrossRef CAS.
- (a) S. Yamada and C. Morita, J. Am. Chem. Soc., 2002, 124, 8184–8185 CrossRef CAS; (b) S. Yamada, A. Toshimitsu and Y. Takahashi, Tetrahedron, 2009, 65, 2329–2333 CrossRef CAS; (c) S. Yamada and M. Inoue, Org. Lett., 2007, 9, 1477–1480 CrossRef CAS.
- (a) K. M. Guckian, B. A. Schweitzer, R. X. F. Ren, C. J. Sheils, D. C. Tahmassebi and E. T. Kool, J. Am. Chem. Soc., 2000, 122, 2213–2222 CrossRef CAS; (b) G. B. McGaughey, M. Gagne and A. K. Rappe, J. Biol. Chem., 1998, 273, 15458–15463 CrossRef CAS; (c) N. Zacharias and D. A. Dougherty, Trends Pharmacol. Sci., 2002, 23, 281–287 CrossRef CAS.
- T. S. Kang and R. M. Kini, Cell. Mol. Life Sci., 2009, 66, 2341–2361 CrossRef CAS.
- (a) I. Richter, J. Minari, P. Axe, J. P. Lowe, T. D. James, K. Sakurai, S. D. Bull and J. S. Fossey, Chem. Commun., 2008, 1082–1084 RSC; (b) I. Richter, M. R. Warren, J. Minari, S. A. Elfeky, W. Chen, M. F. Mahon, P. R. Raithby, T. D. James, K. Sakurai, S. J. Teat, S. D. Bull and J. S. Fossey, Chem.–Asian J., 2009, 4, 194–198 CrossRef CAS.
- For other pyridine based sensors of alkylating agents see: (a) E. Olejnik, C. Herzog-Ronen, Y. Eichen and E. Ehrenfreund, Synth. Met., 2009, 159, 1024–1027 CrossRef CAS; (b) Y. Gannot, C. Hertzog-Ronen, N. Tessler and Y. Eichen, Adv. Funct. Mater., 2010, 20, 105–110 CrossRef CAS; (c) C. Hertzog-Ronen, E. Borzin, Y. Gerchikov, N. Tessler and Y. Eichen, Chem.–Eur. J., 2009, 15, 10380–10386 CrossRef CAS.
- Also see: S. Tal, H. Salman, Y. Abraham, M. Botoshansky and Y. Eichen, Chem.–Eur. J., 2006, 12, 4858–4864 Search PubMed.
- M. Okamoto and H. Teranishi, J. Phys. Chem., 1984, 88, 5644–5646 CrossRef CAS.
- Pyridinium salt 2j was not isolated as a single compound, rather as a mixture of 2j and its corresponding allene, Dr Richard Grainger (University of Birmingham) is thanked for helpful discussion.
- X-Ray crystal structure data for 2i: single crystals of 2i were recrystallised from acetone, mounted in inert oil and transferred to the cold gas stream of the diffractometer. C23H26N·Br, M = 396.36, orthorhombic, a = 7.3748 (2) Å, b = 10.0622 (2) Å, c = 25.6743 (8) Å, U = 1905.21 (9) Å3, T = 120 (2) K, space groupPbca (no. 61), Z = 4, 22
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 489 reflections measured, 2183 unique (Rint = 0.0468) which were used in all calculations. The final R-indices were: R1 = 0.0950 (observed data) and wR2=0.2020 (all data). CCDC 775629.
489 reflections measured, 2183 unique (Rint = 0.0468) which were used in all calculations. The final R-indices were: R1 = 0.0950 (observed data) and wR2=0.2020 (all data). CCDC 775629.
Footnotes |
| † Electronic supplementary information (ESI) available: A representative synthetic procedure, corresponding analysis and X-ray crystallographic data. CCDC 775629. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0cc01420f |
| ‡ This article is part of the ‘Emerging Investigators’ themed issue for ChemComm. |
| This journal is © The Royal Society of Chemistry 2011 |
