Domino gold-catalyzed rearrangement and fluorination of propargyl acetates†
Teresa
de Haro
and
Cristina
Nevado
*
Organic Chemistry Institute, University of Zürich, Winterthurerstrasse 190, Switzerland. E-mail: nevado@oci.uzh.ch; Tel: +41 4463 53975
First published on 29th June 2010
Abstract
A combination of IPrAuNTf2 as catalyst in the presence of Selectfluor has been successfully used for the high yielding synthesis of α-fluoroenones via 1,3-acyloxy rearrangement of propargyl acetates followed by Csp2–F bond formation, likely involving a redox Au(I)/Au(III) catalytic cycle.
The efficient formation of C–F bonds represents a major challenge in organic and organometallic chemistry.1 In recent years catalytic methods have been developed, usually involving electrophilic fluorinating reagents and/or specific directing groups in the substrate with Pd as catalyst.2 Gold-complexes have also been used in catalytic fluorinations, as independently reported by the groups of Sadighi and Governeur. While Sadighi’s group has focused on the hydrofluorination of alkynes,3 Governeur has used the fluorodeauration of vinyl gold species to create new Csp2–F bonds, although the competitive formation of Csp2–H bonds could not be avoided.4 Additionally, vinyl-gold species have proved to efficiently form Csp2–Br and Csp2–I bonds in a catalytic fashion.5 Based on our previous experience in the gold-catalyzed rearrangement of propargyl acetates,6 we decided to explore the reactivity of these motifs in the presence of different fluorinating agents combining the “alkynophilicity” of Au(I) complexes with a redox Au(I)/Au(III) catalytic cycle to establish a new C–F bond upon acyloxy migration to give α-fluoroenones. Despite their synthetic potential as Michael acceptors or as precursors of peptidomimetics,7a only a few methods to prepare α-fluoroenones are known.7b–cAcetate 1a was prepared and used as the benchmark substrate. After some optimization,8 we found that the ligand bound to gold plays a key role in the formation of the undesired homocoupling (3a)9 and/or protonated products (4a). In contrast to Ph3P, the use of a bulky N-heterocyclic ligand such as 1,3-bis(2,6-diiso-propylphenyl)-imidazol-2-ylide, afforded the fluorinated product 2a with complete conversion and excellent selectivity (eqn (1)).
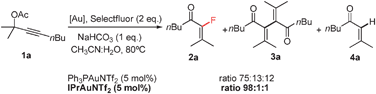 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E
1 mixture of E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
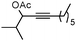
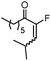
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z isomers (Table 1, entry 2).8 Substrates derived from cyclic ketones such as 1c, 1d and 1e reacted smoothly in the presence of IPrAuNTf2 delivering the correponding fluorinated products (2c–e) in 82, 71 and 87% yield, respectively (Table 1, entries 3–5). We also explored the substitution pattern in the alkyne moiety. A longer alkylic chain (1f) was well accommodated under these conditions (Table 1, entry 6). The presence of a cyclopropyl group (1g) was also tolerated, although partial decomposition of 2g could not be avoided during the purification process (Table 1, entry 7). Phenyl substituted alkyne 1h smoothly reacted to give fluorinated ketone 2h in 81% yield (Table 1, entry 8). Secondary acetates (5a, 5b) reacted efficiently by increasing the concentration and the reaction time (Table 1, entries 9 and 10). In these cases, the E isomer was the major product detected in the reaction mixtures. Interestingly, p-methoxyphenyl substituted propargylacetate 5c afforded not only the isomeric α-fluoroenones 6c (2
Z isomers (Table 1, entry 2).8 Substrates derived from cyclic ketones such as 1c, 1d and 1e reacted smoothly in the presence of IPrAuNTf2 delivering the correponding fluorinated products (2c–e) in 82, 71 and 87% yield, respectively (Table 1, entries 3–5). We also explored the substitution pattern in the alkyne moiety. A longer alkylic chain (1f) was well accommodated under these conditions (Table 1, entry 6). The presence of a cyclopropyl group (1g) was also tolerated, although partial decomposition of 2g could not be avoided during the purification process (Table 1, entry 7). Phenyl substituted alkyne 1h smoothly reacted to give fluorinated ketone 2h in 81% yield (Table 1, entry 8). Secondary acetates (5a, 5b) reacted efficiently by increasing the concentration and the reaction time (Table 1, entries 9 and 10). In these cases, the E isomer was the major product detected in the reaction mixtures. Interestingly, p-methoxyphenyl substituted propargylacetate 5c afforded not only the isomeric α-fluoroenones 6c (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Z
1 Z![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) E ratio), but also bis-fluorinated compound 7 isolated in 26% yield (eqn (2)).4,10 Reactivation of the double bond in 6c by Au(I) or Au(III) present in the reaction mixture could trigger the nucleophilic attack of water (I), followed by fluorination at the Cα of the ketone (II) through the Csp3–Au bond.
E ratio), but also bis-fluorinated compound 7 isolated in 26% yield (eqn (2)).4,10 Reactivation of the double bond in 6c by Au(I) or Au(III) present in the reaction mixture could trigger the nucleophilic attack of water (I), followed by fluorination at the Cα of the ketone (II) through the Csp3–Au bond.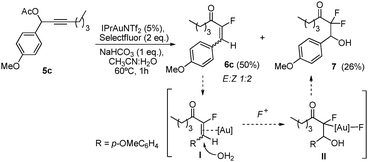 | (2) |
| Entry | Substrate | Product | Yield(%)b | ||
|---|---|---|---|---|---|
a Standard reaction conditions: IPrAuNTf2 (5 mol%), Selectfluor (2 eq.), 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CH3CN–H2O as solvent (substrate concentration 0.02 M), 80 °C, 20 min.
b Isolated yield after column chromatography.
c 1.5 1 CH3CN–H2O as solvent (substrate concentration 0.02 M), 80 °C, 20 min.
b Isolated yield after column chromatography.
c 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E 1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio.
d As standard conditions but stirred for 2 h, substrate concentration 0.1 M.
e 4 Z ratio.
d As standard conditions but stirred for 2 h, substrate concentration 0.1 M.
e 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E 1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratio. Z ratio.
|
|||||
| 1 |
|
1a |
|
2a | 77 |
| 2 |
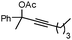
|
1b |
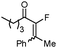
|
2b | 84c |
| 3 |
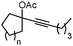
|
1c (n = 1) |
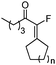
|
2c (n = 1) | 82 |
| 4 | 1d (n = 2) | 2d (n = 2) | 71 | ||
| 5 | 1e (n = 3) | 2e (n = 3) | 87 | ||
| 6 |
|
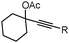
1f (R = nHex) |
|
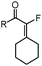
2f (R = nHex) | 79 |
| 7 | 1g (R = 1-cyclopropyl) | 2g (R = 1-cyclopropyl) | 57 | ||
| 8 | 1h (R = Ph) | 2h (R = Ph) | 81 | ||
| 9 |
|
5a |
|
6a | 71d,e |
| 10 |
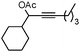
|
5b |
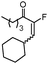
|
6b | 72d,e |
Our mechanistic proposal for the formation of α-fluoroenones has been outlined in Scheme 1. Upon gold coordination, the complex III undergoes a [3,3]-sigmatropic rearrangement of the acyloxy moiety affording alleneIV, which according to previously reported DFT calculations, features gold coordinated to the external double bond of the allene.11Hydrolysis of the acetoxy moiety in the aqueous media will deliver vinyl-gold(I) species V while releasing acetic acid, which can be neutralized in the presence of base (NaHCO3). In the presence of a strong oxidant such as Selectfluor, oxidation of Au(I) to Au(III) can occur, affording complex VI.12 Reductive elimination in VI will deliver the new Csp2–F bond and the observed α-fluorenones VII.13 As competing pathways, protonolysis of V could deliver α,β-unsaturated ketones (VIII), or transmetallation between V and VI could afford the homocoupling products IX (Scheme 1, green pathways).9 Under the standard reaction conditions, E-6a,6b did not isomerize to the thermodynamically more stable Z-isomers,14 thus indicating that the step defining the stereochemistry of the enone must be under kinetic control.
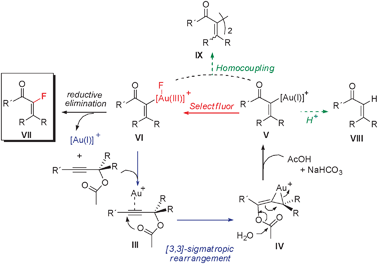 | ||
| Scheme 1 Proposed mechanistic pathway. | ||
In summary, we present here a novel and efficient method to synthesize α-fluoroenones based on a domino gold-catalyzed process starting from easily available propargyl acetates. The known tendency of Au(I)-complexes to activate alkynes triggers the 1,3-migration of the acyloxy moiety onto the triple bond delivering vinyl-Au(I) intermediates, which can be oxidized in the presence of Selectfluor to form Au(III) species prone to reductive elimination to give Csp2–F bonds. Further studies on the reaction mechanism and applications of this method to other substrates are currently ongoing.
Notes and references
- (a) Modern Fluoroorganic Chemistry: Synthesis Reactivity Applications, Wiley-VCH, Weinheim, 2004 Search PubMed; (b) Organo-Fluorine Compounds. Methods of Organic Chemistry, Houben-Weyl, E10, Thieme Verlag, Stuttgart, 1999 Search PubMed.
- (a) K. L. Hull, W. Q. Anani and M. S. Sanford, J. Am. Chem. Soc., 2006, 128, 7134 CrossRef CAS; (b) T. Furuya, H. M. Kaiser and T. Ritter, Angew. Chem., Int. Ed., 2008, 47, 5993 CAS; (c) D. A. Watson, M. Su, G. Teverovskiy, Y. Zhang, J. García-Fortanet, T. Kinzel and S. L. Buchwald, Science, 2009, 325, 1661 CrossRef CAS.
- J. A. Akana, K. X. Bhattacharyya, P. Müller and J. P. Sadighi, J. Am. Chem. Soc., 2007, 129, 7736 CrossRef CAS.
- M. Schuler, F. Silva, C. Bobbio, A. Tessier and V. Gouverneur, Angew. Chem., Int. Ed., 2008, 47, 7927 CrossRef CAS.
- (a) S. F. Kirsch, J. T. Binder, B. Crone, A. Duschek, T. T. Haug, C. Liébert and H. Menz, Angew. Chem., Int. Ed., 2007, 46, 2310 CrossRef CAS; (b) A. Buzas and F. Gagosz, Org. Lett., 2006, 8, 515 CrossRef CAS; (c) M. Yu, G. Zhang and L. Zhang, Org. Lett., 2007, 9, 2147 CrossRef CAS; (d) M. Yu, G. Zhang and L. Zhang, Tetrahedron, 2009, 65, 1846 CrossRef CAS; (e) Z. Shi and C. He, J. Am. Chem. Soc., 2004, 126, 13596 CrossRef CAS.
- (a) X. Huang, T. De Haro and C. Nevado, Chem.–Eur. J., 2009, 15, 5904 CrossRef CAS; (b) Y. Zou, D. Garayalde, Q. Wang, C. Nevado and A. Goeke, Angew. Chem., Int. Ed., 2008, 47, 10110 CrossRef CAS.
- (a) G. Dutheuil, S. Couve-Bonnaire and X. Pannecoucke, Angew. Chem., Int. Ed., 2007, 46, 1290 CrossRef CAS; (b) Dutheuil, S. Couve-Bonnaire and X. Pannecoucke, Tetrahedron, 2009, 65, 6034 CrossRef and references therein; (c) A. K. Ghosh, S. Banerjee, S. Sinha, S. B. Kang and B. Zajc, J. Org. Chem., 2009, 74, 3689 CrossRef CAS.
- For full experimental details, see the ESI†.
- L. Cui, G. Zhang and L. Zhang, Bioorg. Med. Chem. Lett., 2009, 19, 3884 CrossRef CAS.
- Under the standard reaction conditions 6c was quantitatively transformed in 7.
- For an in depth computational study on similar intermediates, see: (a) A. Correa, N. Marion, L. Fensterbank, M. Malacria, S. P. Nolan and L. Cavallo, Angew. Chem., Int. Ed., 2008, 47, 718 CrossRef CAS; (b) V. Gandon, G. Lemière, A. Hours, L. Fensterbank and M. Malacria, Angew. Chem., Int. Ed., 2008, 47, 7534 CrossRef CAS; (c) D. Garayalde, E. Gómez-Bengoa, X. Huang, A. Göeke and C. Nevado, J. Am. Chem. Soc., 2010, 132, 4720 CrossRef CAS.
- (a) H. A. Wegner, S. Ahles and M. Neuenburg, Chem.–Eur. J., 2008, 14, 11310 CrossRef CAS; (b) G. Zhang, Y. Peng, L. Cui and L. Zhang, Angew. Chem., Int. Ed., 2009, 48, 3112 CrossRef CAS; (c) G. Zhang, L. Cui, Y. Wang and L. Zhang, J. Am. Chem. Soc., 2010, 132, 1474 CrossRef CAS; (d) A. Iglesias and K. Muñiz, Chem.–Eur. J., 2009, 48, 9346.
- Fluorodeauration of the vinylic Csp2–Au(I) bond in the presence of “F+” can not be ruled out.
- Semiempirical calculations (PM3 level), showed an energy difference between the E and Z isomers ≈ 5 kcal mol−1, with the Z isomer being the thermodynamically more stable.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures and characterization of products. See DOI: 10.1039/c002679d |
| This journal is © The Royal Society of Chemistry 2011 |

