DOI:
10.1039/C0AY00619J
(Paper)
Anal. Methods, 2011,
3, 653-658
Sensitive determination of phenols from water samples by temperature-controlled ionic liquid dispersive liquid-phase microextraction
Received
15th October 2010
, Accepted 6th January 2011
First published on 3rd February 2011
Abstract
This paper established a new determination method for phenols using temperature-controlled ionic liquid dispersive liquid-phase microextraction prior to high-performance liquid chromatography. In this experiment, 1-octyl-3-methylimidazolium hexafluorophosphate ([C8MIM][PF6]) was employed as the extraction solvent for the enrichment of 2-chlorophenol, 2-naphthol, 2,4-dinitrophenol, and 2,4-dichlorophenol. Parameters that may affect the extraction efficiency including the volume of [C8MIM][PF6], dissoluble temperature, extraction time, sample pH, amount of ethanol, centrifugation time and salting-out effect have been investigated in detail. Under the optimal conditions, they have good linear relationships over the concentration range of 1.0-100 ng mL−1 for 2-chlorophenol, 2-naphthol, 2,4-dinitrophenol, and 1.5-150 ng mL−1 for 2,4-dichlorophenol, and excellent detection sensitivity with limits of detection (LOD, S/N = 3) in the range of 0.27–0.68 μg L−1. Intra day and inter day precisions of the proposed method (RSDs, n = 6) were 2.1–3.7% and 5.1–7.2%, respectively. The proposed method has been successfully applied to analyze real water samples spiked with two different concentrations and good spiked recoveries over the range of 85.8–117.0% were obtained. These results indicated that the proposed method would be competitive in the analysis of phenols in the future.
1. Introduction
Phenolic compounds are important precursors for the manufacture of many dyes, drugs, perfumes, insecticides, and surfactants.1 Many investigations had confirmed the presence of chlorophenols in many ecosystems: surface and ground waters, bottom sediments, atmospheric air and soils. Possible routes of human exposure to chlorophenols are inhalation, ingestion and eye and dermal contact.2 Owing to their high toxicity, persistence in the environment and potential carcinogenicity, the US Environmental Protect Agency (US EPA) and European Community (EC) have included some phenols, mainly nitrophenols and chlorophenols in their lists of priority pollutants.3
Analytical procedures have been developed for the separation and preconcentration of the contaminants due to their low concentration or complicated matrices in environmental and biological samples, such as liquid–liquid extraction (LLE),4,5 liquid-phase miroextraction (LPME),6,7 headspace liquid-phase miroextraction (HS-LPME),8 solid-phase extraction (SPE),9–11 solid-phase microextraction (SPME),12–14 headspace solid-phase microextraction (HS-SPME)15–19 and single-drop microextraction (SDME).20–24
Recently, Assadi and co-workers reported a novel microextraction technique, termed dispersive liquid–liquid microextraction (DLLME).25 It has the advantages of simplicity, rapidity, low sample volume, low cost, high recovery, and a high enrichment factor,26 and has been widely used for the pretreatment of organic pollutants and heavy metal pollution.27 In a DLLME method, it is considerably important to select an extraction solvent with higher density than water, high extraction capability of compounds of interest and good chromatographic behavior.28 Toxic solvents such as chlorobenzene, carbon tetrachloride, tetrachloroethylene and carbon disulfide have been often used as extraction solvents. In order to reduce the effect of toxic solvent on the environment, environmental friendly solvents are expected for use.29 Room temperature ionic liquids (RTILs), known as a new and novel generation of solvents, have been widely applied in separation and many other fields. They have many properties include low volatility, chemical and thermal stability, and good solubility for both organic and inorganic molecules.30 Moreover, by fine-tuning the structure, these properties can be designed to match the specific application requirements. The main reason that made them useful in analytical chemistry is the negligible vapor pressure of most RTILs. Ionic liquids have been used in the development of DLLME for the enrichment and determination of heavy metals,31, 32 and our research group has developed a new liquid phase microextraction technique named as temperature-controlled ionic liquid dispersive liquid-phase microextraction based on the similar principle of DLLME using ionic liquids as the extraction solvents.33
The goal of the present study is to develop a new method for powerful preconcentration and sensitive detection of four phenols in water samples using temperature-controlled ionic liquid dispersive liquid-phase microextraction method. The effects of various experimental parameters such as the volume of room temperature ionic liquids, temperature, extraction time, sample pH, centrifugation time, and salting-out effect were optimized.
2. Experimental
2.1 Instrumentation
A high performance liquid chromatography system, which consisted of two LC-10 ATvp pumps and an SPD-10 Avp, ultraviolet detector (Shimadzu, Kyoto, Japan) was used for the analysis and separation. A reversed-phase SunFire C18 column (150 mm × 4.6 mm, particle size 5 μm) was used for separation at ambient temperature and Chromato Solution Light Chemstation for LC system was employed to acquire and process chromatographic data. The mobile phase was a mixture of methanol and ultrapure water (containing 1% acetic acid) (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, v/v). The mobile phase flow-rate was set at 0.8 mL min−1, the injection volume and the detection wavelength were set at 20 μL and 275 nm, respectively. An Anke TDL80-2B (Shanghai, China) centrifuge was used for phase separation.
45, v/v). The mobile phase flow-rate was set at 0.8 mL min−1, the injection volume and the detection wavelength were set at 20 μL and 275 nm, respectively. An Anke TDL80-2B (Shanghai, China) centrifuge was used for phase separation.
2.2 Reagents
2,4-Dichlorophenol and 2-chlorophenol (purity, 99%) were achieved from Acros organics (New Jersey, USA). 2-Naphthol (Analytical grade) and 2,4-dinitrophenol (Guarantee Reagent) were obtained from Shanghai Chemical Cooperation (Shanghai, China). Stock solutions at 500 mgL−1 were prepared by dissolving suitable amounts of them each in methanol and stored at 4 °C in the refrigerator. The stock solutions were further diluted to yield the appropriate working solutions with methanol.
1-Octyl-3-methylimidazolium tetrafluoroborate ([C8MIM][PF6]) was synthesized in our laboratory. HPLC grade methanol and acetonitrile were obtained from Huaiyin Guoda Chemical Reagent Co., Ltd. (Huaian, China). Ultrapure water was obtained from a Milli-Q water purification system (Millipore, Bedford, MA, USA). The aqueous solutions were prepared daily by diluting the standard mixture with ultra-pure water. All the other solvents were analytical grade unless stated. 3 mol L−1 of sodium hydroxide were used for adjusting the pH value of the water samples. All glassware used in the experiments was cleaned with ultrapure water, then soaked in 6 mol L−1 nitric acid for 24 h and rinsed five times with ultrapure water before use.
In the temperature-controlled ionic liquid dispersive liquid-phase microextraction procedure, 10 mL ultra-pure water or sample was added. This solution was spiked with a concentration of 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, and 40 μg L−1 for 2,4-dichlorophenol. 50 μL 1-octyl-3-methylimidazolium hexafluorophosphate [C8MIM][PF6], 60 μL 1 mol L−1 hydrochloric acid and 700 μL ethanol were added into a 10 mL conical tube. Then the conical tubes were heated in the water bath with the temperature controlled at 60 °C. [C8MIM][PF6] was then completely dissolved in the aqueous solution and mixed with the solution entirely. The analytes would be transferred into the IL phase based on the higher solubility of analytes in IL. The tube was thereafter cooled with icewater and a cloudy solution was formed. The tube was kept for 20 min to enhance the migration of phenols from the sample solution into the tiny droplets of [C8MIM][PF6]. Then the water–ethanol–[C8MIM][PF6] mixture was centrifuged for 20 min at 4000 rpm. The upper aqueous phase was removed with a syringe, and the residue was dissolved in 200 μL methanol and 20 μL was injected into the HPLC system for analysis.
2.4 Water samples
Four real water samples were collected for validation of the proposed method. Melted water was obtained from Henan Normal University in Xinxiang City, Henan province. Lake waters were collected from Donghu Lake, Xinxiang City, Henan province, China and Shouxihu Lake, Yangzhou City, Jiangsu province, China. Wastewater sample was taken from the exit of a factory, Xinxiang city, Henan province, China. Before use, all the water samples were filtered through 0.45 μm micro-pore membranes and stored in brown glass containers at the temperature of 4 °C.
3. Results and discussion
The extraction efficiency of temperature-controlled ionic liquid dispersive liquid-phase microextraction procedure depends on some important experimental parameters, such as the amount of IL, temperature, extraction time, sample pH, the addition of organic solvent, centrifugation time, and ionic strength, and they were investigated in detail. In order to calculate the enrichment factors and recoveries, eqn (1) and (2) were used.
EF, Csed and C0 are the enrichment factor, the concentration of analytes in the sedimented ionic liquid phase and the initial concentration of analytes in the aqueous samples, respectively.
| | | R% = (CsedVsed)/(C0Vaq) × 100 = EF × Vsed/Vaq × 100 | (2) |
where
R%,
Vsed,
Vaq, are the
extraction recovery, the volume of the sedimented phase and the volume of the aqueous sample, respectively.
3.1 Effect of the volume of ionic liquid
The volume of extraction solvent was a crucial parameter which seriously had an important impact on the extraction performance in liquid phase microextraction. Theoretically, a larger volume of exaction solvent resulted in a higher extraction efficiency. Solutions containing different volumes (40, 45, 50, 55 and 60 μL) of [C8MIM][PF6] were subjected to the same temperature-controlled ionic liquid dispersive liquid-phase microextraction procedure, and the results are shown in Fig. 1. The results indicated that the peak areas of phenols increased along with the added volume of [C8MIM][PF6] from 40 to 50 μL and reached the maximum at 50 μL. Maybe the amount of ionic liquid exceeded the target dispersed amount about 50 μL, and excess IL was adsorbed on to the wall of the tube, and led to a few loss of analytes. Meanwhile, the more IL was used, the more sedimented IL and the concentrations of analytes would be diluted. Therefore, 50 μL ionic liquid was employed for further use.
![Effect of ionic liquid volume. Conditions: ionic liquid, [C8MIM][PF6]; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH,6; temperature, 60 °C; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f1.gif) |
| | Fig. 1 Effect of ionic liquid volume. Conditions: ionic liquid, [C8MIM][PF6]; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH,6; temperature, 60 °C; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol. | |
3.2 Effect of dissolving temperature
In this experiment, temperature is the driving force to make [C8MIM][PF6] dispersed into the sample solution completely. Further the analytes have the best chance and largest contact area and migrate into the IL phase. A series of experiments were designed for the optimization of the effect of temperature ranging from 40 to 80 °C. From Fig. 2, we can see that 60 °C was the optimal temperaure for obtaining the best extraction performance. The reason was that the mass transfer coefficients were enhanced along with the temperature, but at a higher temperature, the extraction performance would decrease due to the volatilization. So 60 °C was used in the further experiments.
![Effect of temperature. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH, 6; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f2.gif) |
| | Fig. 2 Effect of temperature. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH, 6; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol. | |
3.4 Effect of sample pH
The sample pH is also an important factor in the enrichment process, and which can affect the extraction efficiencies of analytes. The sample pH was investigated in the range of pH 1–9. The results are exhibited in Fig. 3. From Fig. 3, it was found that the peak areas of all the phenols increased gradually from pH 1–3 and deceased from pH 4–6. Only 2-naphthol was detected when the sample pH was at pH 8 and pH 10. The reason is that phenols are weak acidic compounds and exist as a molecular form at acidic conditions, and an ionic form at strong alkali conditions. However, phenols will also exist as addition of a [H+] when the acidity of sample solution was too low, which was helpless to the enrichment process. Due to these facts, pH 3 was used in the following experiments.
![Effect of sample pH. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f3.gif) |
| | Fig. 3 Effect of sample pH. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol. | |
3.5 Effect of organic solvents addition
Ref. 34 and 35 indicated that organic solvents could help enhance the extraction efficiency of analytes, and which was mainly due to the reduction of the adsorption onto the tube walls of analytes. A series of experiments were performed to investigate the effect of addition of organic solvents such as methanol, acetonitrile, ethanol and acetone. The results are demonstrated in Fig. 4. From Fig. 4, we can see that the largest peak areas were obtained with adding ethanol. This is identical with the principle of like dissolving like. The amount of ethanol was investigated over the range of 0 ∼ 11% (v/v). The results are shown in Fig. 5. It was found that the largest peak areas of phenols were obtained at the concentration of 7%. Too large an amount of ethanol would reduce the IL phase because it could dissolve IL and be soluble in water in any proportion. Therefore, 7% ethanol (v/v) was adopted.
![Effect of adding of organic solvents. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; concentration of acetone/methanol–ethanol–acetonitrile, 7%; sample pH, 3. (B) 2-chlorophenol; (C) 2-naphthol; (D) 2, 4-dinitrophenol; (E) 2, 4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f4.gif) |
| | Fig. 4 Effect of adding of organic solvents. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; concentration of acetone/methanol–ethanol–acetonitrile, 7%; sample pH, 3. (B) 2-chlorophenol; (C) 2-naphthol; (D) 2, 4-dinitrophenol; (E) 2, 4-dichlorophenol. | |
![Effect of the ethanol addition. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; methanol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f5.gif) |
| | Fig. 5 Effect of the ethanol addition. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; methanol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol. | |
3.7 Salting-out effect
In general, addition of a certain amount of salt can decrease the solubility of analytes in the aqueous phase and enhance their partitioning into the organic phase. However, much more salt can change the physical properties of the Nernst diffusion film, which reduced the rate of diffusion of the analytes into the microdrop.24 In order to investigate the effect of salt addition, a series of experiments over the NaCl concentration range of 5 ∼ 25% (w/v) were performed while keeping the other parameters constant. The experimental data are shown in Fig. 6. It can be seen that the peak area increased with the increase of the amount of NaCl and reached its largest at 15% (w/v), and then decreased when the NaCl concentration was over 15% (w/v). This result was very like the results in the literature.7,36 So 15% NaCl was used for achieving a better extraction performance.
![Salting-out effect. Conditions: volume of [C8MIM] [PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; ethanol addition, 7%; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f6.gif) |
| | Fig. 6 Salting-out effect. Conditions: volume of [C8MIM] [PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; ethanol addition, 7%; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol. | |
3.8 Analytical performance
As far as the analytical method is concerned, linear ranges, precisions and detection limits are very important. Under optimal experimental conditions, a series of experiments were performed for investigating such parameters. The experimental results showed that they had good linear relationships over the concentration ranges of 1.0 ∼ 100 μg L−1 for 2-chlorophenol 2,4-dinitrophenol, and 2-naphthol, and 1.5 ∼ 150 μg L−1 for 2,4-dichlorophenol. The precisions were obtained by six reduplicate extractions. The experimental results are summarized in Table 1. The results indicated that this method was excellent with good linearity with correlation coefficients over the range of 0.9995 ∼ 0.9998, the limits of detection (LODs), were in the range of 0.27 ∼ 0.68 μg L−1 (S/N = 3) and the intra day precisions were in the range of 2.1 ∼ 3.7% and inter day precisions were in the range of 5.1 ∼ 7.2% (RSD, n = 6). These merits indicated that the proposed method would be a creative development in analytical and environmental fields. Enrichment factors are important parameters for the extraction and preconcentration method, and which detemines the sensitivity and merits of the developed method. The EF of the proposed method was calculated and listed in Table 1. It can seen that the EFs were very good and the sensitivity of proposed method was satisfied.
| Compounds |
Linear range (μg L−1) |
R
2
|
Precision (RSD%, n = 6, intra day) |
Precision(RSD%, n = 6, inter day) |
Limits of detection (μg L−1) |
EFa |
|
EF = Csed/C0.
|
| 2-chlorophenol |
1–100 |
0.9998 |
3.7 |
5.1 |
0.36 |
339 |
| 2-naphthol |
1–100 |
0.9998 |
2.1 |
6.2 |
0.27 |
334 |
| 2,4-dinitrophenol |
1–100 |
0.9997 |
3.1 |
6.3 |
0.49 |
357 |
| 2,4-dichlorophenol |
1.5–150 |
0.9995 |
2.8 |
7.2 |
0.68 |
371 |
3.9 Real water sample analysis
The present method was evaluated by determining the concentration of phenols in four real water samples. These samples were directly analyzed and no target analytes were found. In order to validate the applicability of the proposed method, the samples were spiked with 5, 10, and 100 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 7.5, 15, 150 μg L−1 for 2,4-dichlorophenol, respectively. The results are exhibited in Table 2 and the typical chromatogram of spiked real water sample is shown in Fig. 7. The spiked recoveries were satisfied in the range of 85.8 ∼ 117%. Our research group had developed a headspace liquid-phase microextraction based on a bell mouthed device with a 3 mm PTFE tube.37 In the extraction procedure, 10 μL [C4MIM][PF6] was suspended for extraction, a 10 mL aliquot of the sample solution containing 35% NaCl was placed in a 45 mL vial immersed in the recirculating water bath at a temperature of 80 °C, the magnetic stirrer was turned on at 1000 rpm, and extraction time was 40 min. The results demonstrated that the limits of detection for 2-nitrophenol, 4-chlorophenol, 2-naphthol, and 2,4-dichlorophenol were 0.5, 0.5, 0.3 and 0.3 μg L−1 and the pricisions were in the range of 5.4–8.9% (RSD, n = 6). In this procedure, the ionic liquid phase was completely injected into HPLC for analysis, and the extraction time was obviously longer than that of proposed method. However, the proposed method was better than this method and provided a comparatively low detection limit (0.27–0.68 µg L−1).
Table 2 Spiked recoveries obtained in samples by the proposed methoda,b
| Compounds |
Concentrations (μg L−1) |
Melted snow water |
Donghu Lake water |
Shouxihu lake water |
Water from Xinfei factory |
|
N.D.: not detected.
Spiked recovery, mean ± standard deviation (%).
|
| 2-chlorophenol |
0 |
N.D. |
N.D. |
N.D. |
N.D. |
| 5 |
105.7 ± 1.6 |
93.5 ± 5.9 |
97.3 ± 3.3 |
108.5 ± 1.5 |
| 10 |
102.3 ± 3.4 |
94.8 ± 2.9 |
104.3 ± 4.3 |
101.7 ± 4.1 |
| 100 |
88.6 ± 5.1 |
83.2 ± 6.2 |
87.2 ± 6.5 |
86.2 ± 6.8 |
| 2-naphthol |
0 |
N.D. |
N.D. |
N.D. |
N.D. |
| 5 |
98.6 ± 2.2 |
85.8 ± 5.6 |
91.6 ± 1.1 |
100.9 ± 6.6 |
| 10 |
109.8 ± 1.5 |
93.7 ± 2.8 |
103.8 ± 2.8 |
101.4 ± 3.1 |
| 100 |
86.7 ± 6.5 |
84.5 ± 5.9 |
84.9 ± 5.4 |
83.9 ± 7.5 |
| 2,4-dinitrophenol |
0 |
N.D. |
N.D. |
N.D. |
N.D. |
| 5 |
111.5 ± 2.6 |
97.9 ± 4.3 |
109.5 ± 5.2 |
114.6 ± 6.0 |
| 10 |
110.6 ± 1.9 |
100.1 ± 3.7 |
99.42 ± 1.2 |
102.4 ± 3.4 |
| 100 |
87.1 ± 7.2 |
84.7 ± 4.8 |
84.2 ± 3.9 |
85.1 ± 6.7 |
| 2,4-dichlorophenol |
0 |
N.D. |
N.D. |
N.D. |
N.D. |
| 7.5 |
109.7 ± 2.8 |
99.7 ± 8.1 |
109.4 ± 3.4 |
117.0 ± 7.5 |
| 15 |
112.0 ± 2.4 |
103.9 ± 3.2 |
107.5 ± 2.7 |
109.9 ± 3.4 |
| 150 |
87.5 ± 5.7 |
83.8 ± 5.4 |
87.8 ± 6.8 |
87.4 ± 7.8 |
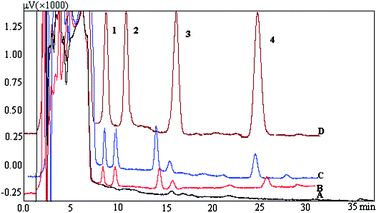 |
| | Fig. 7 Typical water sample: Melted snow water: A: Blank; B: 5 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 7.5 μg L−1 for 2,4-dichlorophenol; C: 10 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 15 μg L−1 for 2,4-dichlorophenol; D: 100 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 150 μg L−1 for 2,4-dichlorophenol. | |
4. Conclusions
In this study, a new convenient, sensitive, simple determination method based on temperature-controlled ionic liquid dispersive liquid-phase microextraction prior to high performance liquid phase chromatography was developed. The proposed method has satisfied LODs and precisions which were in the range of 0.27 ∼ 0.68 μg L−1, precisions were in the range of 2.1 ∼ 3.7% (intra day, RSD, n = 6) and 5.1 ∼ 7.2% (inter day, RSD, n = 6). The proposed method was also applied for the analysis of phenols in real water samples and the spiked recoveries were in the range of 85.8 ∼ 117%. All these results indicated that the proposed method had advantages such as good sensitivity, simplicity, easy to operate, limited chance of exposure to the toxic solvent, high enrichment factor which could be tuned by changing the volume of ILs in a relatively wide range, etc., however, the toxicity of ionic liquid has also been studied and paid more attention. In order to give reasonable results, the used ionic liquid should be regenerated and reused for reducing the possible secondary pollution. In other words, the developed method was a good alternative and would be very competitive in the analysis of phenols in the future.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (20877022), the Personal Innovation Foundation of Universities in Henan Province ([2005]126), the Natural Science Foundation of Henan Province (No. 082102350022), and the funds from the Henan Key Laboratory for environmental pollution control.
References
-
M. Howe-Grant. Kirk–Othmer encyclopedia of chemical technology. 4th edn Wiley–InterscienceNew York, 1991 Search PubMed.
- M. Czaplicka, Sci. Total. Environ., 2004, 322, 21 CrossRef CAS.
- H. Bagheri, A. Saber and S. R. Mousavi, J. Chromatogr. A, 2004, 1046, 27 CrossRef CAS.
- S. Kjellstrőm and O. N. Jensen, Anal. Chem., 2003, 75, 2362 CrossRef CAS.
- S. X. Peng, C. Henson, M. J. Strojnowski, A. Golebiowski and S. R. Klopfenstein, Anal. Chem., 2000, 72, 261 CrossRef CAS.
- L. W. Chung and M. R. Lee, Talanta, 2008, 76, 154 CrossRef CAS.
- X. Wang, L. Luo, G. Ouyang, L. Lin, N. F. Y. Tam, C. Lan and T. Luan, J. Chromatogr. A, 2009, 1216, 6267 CrossRef CAS.
- H. Xu, Y. Liao and J. Yao, J. Chromatogr. A, 2007, 1167, 1 CrossRef CAS.
- Y. Cai, Y. Cai, S. Mou and Y. Lu, J. Chromatogr. A, 2005, 1081, 245 CrossRef CAS.
- M. Nichkova and M. P. Marco, Anal. Chim. Acta, 2005, 533, 67 CrossRef CAS.
- S. Insa, V. Salvadó and E. Anticó, J. Chromatogr. A, 2004, 1047, 15 CrossRef CAS.
- M. N. Sarrioó, F. J. Santos and M. T. Galceran, J. Chromatogr. A, 2002, 947, 155 CrossRef CAS.
- M. R. Lee, Y. C. Yeh, W. S. Hsiang and B. H. Hwang, J. Chromatogr. A, 1998, 806, 317 CrossRef CAS.
- L. Wennrich, P. Popp and M. Mő1der, Anal. Chem., 2000, 72, 546 CrossRef CAS.
- H. P. Ho, R. J. Lee and M. R. Lee, J. Chromatogr. A, 2008, 1213, 245 CrossRef CAS.
- Y. A. Shi, M. Z. Chen, S. Muniraj and J. F. Jen, J. Chromatogr. A, 2008, 1207, 130 CrossRef CAS.
- A. Martínez-Uruñuela, J. M. González-Sáiz and C. Pizarro, J. Chromatogr., A, 2004, 1048, 141 CrossRef CAS.
- J. Regueiro, E. Becerril, C. Garcia-Jares and M. Llompart, J. Chromatogr., A, 2009, 1216, 4693 CrossRef CAS.
- L. M. Chiesa, S. Panseri, S. Soncin, L. Vallone and I. Dragoni, Vet. Res. Commun., 2010, 34(Suppl 1), S167.
- K. Choi, S. J. Kim, Y. G. Jin, Y. O. Jang, J. S. Kim and D. S. Chung, Anal. Chem., 2009, 81, 225 CrossRef CAS.
- E. Aguilera-Herrador, R. Lucena, S. Cárdenas and M. Valcárcel, Anal. Chem., 2008, 80, 793 CrossRef CAS.
- L. Xia, B. Hu, Z. Jiang, Y. Wu and Y. Liang, Anal. Chem., 2004, 76, 2910 CrossRef CAS.
- H. Chen, R. Chen, R. Feng and S. Li, Chromatographia, 2009, 70, 165 CrossRef CAS.
- M. Palit, D. Pardasani, A. K. Gupta and D. K. Dubey, Anal. Chem., 2005, 77, 711 CrossRef CAS.
- S. Berijani, Y. Assadi, M. Anbia, M. R. M. Hosseini and E. Aghaee, J. Chromatogr., A, 2006, 1123, 1 CrossRef CAS.
- S. Li, S. Cai, W. Hu, H. Chen and H. Liu, Spectrochim. Acta, Part B, 2009, 64, 666 CrossRef.
- M. Gharehbaghi, F. Shemirani and M. Baghdadi, Int. J. Environ. Anal. Chem., 2009, 89, 21–33 CrossRef CAS.
- L. He, X. Luo, H. Xie, C. Wang, X. Jiang and K. Lu, Anal. Chim. Acta, 2009, 655, 52 CrossRef.
- H. Bai, Q. Zhou, G. Xie and J. Xiao, Anal. Chim. Acta, 2009, 651, 64 CrossRef CAS.
- Y. Liu, E. Zhao, W. Zhu, H. Gao and Z. Zhou, J. Chromatogr., A, 2009, 1216, 885 CrossRef CAS.
- S. R. Yousefi and F. Shemirani, J. Chromatogr. A, 2010, 669, 25 CAS.
- P. Berton, E. M. Martinis and R. G. Wuilloud, J. Hazard. Mater., 2010, 176, 721 CrossRef CAS.
- Q. X. Zhou, H. H. Bai and J. P. Xiao, J. Chromatogr., A, 2008, 1177, 43 CrossRef CAS.
- S. Insa, V. Salvadó and E. Anticó, J. Chromatogr., A, 2004, 1047, 15 CrossRef CAS.
- J. F. Liu, G. B. Jiang, Y. G. Chi, Y. Q. Cai, Q. X. Zhou and J. T. Hu, Anal. Chem., 2003, 75, 5870 CrossRef CAS.
- C. Y. Lin and S. D. Huang, J. Chromatogr., A, 2008, 1193, 79 CrossRef CAS.
- C. L. Ye, Q. X. Zhou, X. M. Wang and J. P. Xiao, J. Sep. Sci., 2007, 30, 42 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45, v/v). The mobile phase flow-rate was set at 0.8 mL min−1, the injection volume and the detection wavelength were set at 20 μL and 275 nm, respectively. An Anke TDL80-2B (Shanghai, China) centrifuge was used for phase separation.
45, v/v). The mobile phase flow-rate was set at 0.8 mL min−1, the injection volume and the detection wavelength were set at 20 μL and 275 nm, respectively. An Anke TDL80-2B (Shanghai, China) centrifuge was used for phase separation.
![Effect of ionic liquid volume. Conditions: ionic liquid, [C8MIM][PF6]; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH,6; temperature, 60 °C; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f1.gif)
![Effect of temperature. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; sample pH, 6; extraction time, 30 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f2.gif)
![Effect of sample pH. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f3.gif)
![Effect of adding of organic solvents. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; extraction time, 20 min; centrifugation time, 20 min; concentration of acetone/methanol–ethanol–acetonitrile, 7%; sample pH, 3. (B) 2-chlorophenol; (C) 2-naphthol; (D) 2, 4-dinitrophenol; (E) 2, 4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f4.gif)
![Effect of the ethanol addition. Conditions: volume of [C8MIM][PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; methanol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f5.gif)
![Salting-out effect. Conditions: volume of [C8MIM] [PF6], 50 μL; sample volume, 10 mL; spiked concentration, 20 μg L−1 for 2-chlorophenol, 2-naphthol, and 2,4-dinitrophenol, 30 μg L−1 for 2,4-dichlorophenol; temperature, 60 °C; extraction time, 20 min; centrifugation time, 20 min; ethanol addition, 7%; sample pH, 3; (■) 2-chlorophenol; (●) 2-naphthol; (▲) 2,4-dinitrophenol; (▼) 2,4-dichlorophenol.](/image/article/2011/AY/c0ay00619j/c0ay00619j-f6.gif)

