Identification and semi-quantitative analysis of parabens and UV filters in cosmetic products by direct-analysis-in-real-time mass spectrometry and gas chromatography with mass spectrometric detection
Manuela
Haunschmidt
*a,
Wolfgang
Buchberger
a,
Christian W.
Klampfl
a and
Robert
Hertsens
b
aInstitute of Analytical Chemistry, Johannes Kepler University Linz, Altenbergerstrasse 69, A-4040, Linz, Austria. E-mail: manuela.haunschmidt@jku.at; Fax: +43 732 2468 8679; Tel: +43 732 2468 8720
bJEOL (Europe) BV, Leuvensesteenweg 542, B-1930, Zaventem, Belgium
First published on 7th December 2010
Abstract
A method based on direct-analysis-in-real-time mass spectrometry (DART-MS) for the qualitative and semi-quantitative analysis of eight organic UV filters and four parabens in twelve cosmetic products with substantially different formulations (as cream, milk, lotion, oil, lipstick) was developed. All tested substances could be identified unambiguously in the investigated samples without any sample pre-treatment. Direct analysis of cosmetic products allows semi-quantitative determination of parabens. For UV filters no satisfactory results were obtained by direct analysis but all analytes could be quantified by simply dissolving the samples in methanol, addition of an internal standard and subsequent measurement of the solution by DART-MS without further pre-treatment. The results obtained using DART-MS were confirmed by a more established method namely gas chromatography with mass spectrometric detection (GC-MS).
Introduction
During the last decade, the popularity of cosmetics has increased rapidly. While in earlier times cosmetics were originally developed to add colour or make a fashion statement, modern products also aim to repair damaged skin, reverse visual signs of aging or to provide ultraviolet protection. Cosmetics and skin care products are generally safe and well tolerated. Because of intensive testing of the products before release in the market, most cosmetics do not cause any unwanted reactions in most people. However, some substances which are added to provide preservation or to protect the skin from UV radiation can trigger allergic reactions1,2 or influence the human body in an adverse way.3UV filters, mainly organic substances, are often classified as derivatives of cinnamate, anthranilates, benzophenones, camphors, p-aminobenzoates, salicylates and dibenzoylmethanes. These substances can absorb UV light and protect the skin against sunburn, hyperplasia, solar keratosis, photocarcinogenesis and photoaging. Unfortunately, some UV filters can provoke undesirable dermatological side effects like urticaria or allergic reactions of photocontact origin.1
Parabens, esters of p-hydroxybenzoic acid, have a broad antimicrobial spectrum, relatively low toxicity, good stability and low-volatility and are therefore used as preservatives in cosmetic products. The toxicity of the parabens is still under discussion. They are suspected to act as endocrine disrupting substances and provoke breast cancer.4,5
For all these reasons, the analysis of cosmetic products is an important necessity, especially with respect to safety and health issues. Accordingly the main focus lies on the development of rapid and simple analytical methods and screening techniques for this purpose.
In the past, high performance thin layer chromatography (HPTLC) was a widely used method6–8 for the determination of UV filters in cosmetic products. Over the last few years, several methods based on gas chromatography (GC) and liquid chromatography have been implemented. Although UV filters have relatively high boiling points, making them less suited for GC analysis, several successful examples for their identification9–13 and quantitation12,13 using this technique have been described. GC was also found to be suited for the determination of parabens (after derivatisation) in cosmetic products.14–16 However, high performance liquid chromatography (HPLC) is usually the preferred method for quantitative analysis of these two groups of substances, whereby UV-vis detection is commonly used for both UV filters17–20 and parabens.15,21–23
Focusing on alternative analytical techniques, some electroseparation methods such as micellar electrokinetic chromatography24 and microemulsion electrokinetic chromatography25,26 have also been employed for this purpose.
Despite the fact that these methods provide quantitative results they are affected with one main drawback: they are commonly quite time- and labour-consuming. Sample preparation steps are necessary and the separation often takes more than 30 minutes per run. Often e.g. for the screening of cosmetics a qualitative or at least semi-quantitative analysis of UV filters and parabens would be enough. For this reason methods that can provide the requested information with a reduced effort regarding time and labour would be highly desirable.
Direct-analysis-in-real-time mass spectrometry (DART-MS) is a relatively new analytical technique allowing the measurement of solid, liquid and gaseous samples directly without further sample pre-treatment.27 Coupled with a mass spectrometer providing sufficient resolution (like a time-of-flight instrument), spectra with exact masses are obtained within a few seconds, allowing the unambiguous identification of the analytes in samples with different matrices. Up to now DART-MS has been employed for the determination of a variety of substances in diverse types of samples. Focusing on the classes of compounds discussed in the present paper, DART-MS was found useful for the determination of UV filters in environmental water samples after an enrichment procedure by stir bar sorptive extraction.28
Main aim of the present work is to introduce a rapid and simple method for direct analysis of a wide range of different cosmetic products (like lotion, cream, suntan oil, make-up and lip care product) with respect to the presence of eight widely used UV-filters and four parabens. A major advantage of this approach employing DART-MS over previously described methods for the analysis of cosmetic products is the fact that even for semi-quantitative determination no (for parabens) or only very little (for UV filters) sample pretreatment is required.
Experimental
Standards and samples
The names and the structures of the tested UV filters and parabens are summarised in Fig. 1. All UV filters, the two internal standards benzyl cinnamate (BC) and 4-hydroxybenzoic acid (4-HBA) and methanol (of chromatographic analysis grade) were obtained from Merck (Darmstadt, Germany). The parabens were purchased from Sigma-Aldrich (Steinheim, Germany). All used chemicals were of analysis grade. Two stock solutions were prepared in methanol: one containing UV filters at a concentration level of 1 mg mL−1 and a second one containing parabens at a level of 0.1 mg mL−1. These stock solutions were stored at 4 °C and protected from sunlight. The standard solutions were prepared daily directly before use by dilution of the stock solutions with methanol to the final working concentrations. Internal standard solutions were prepared by dissolving 50 mg BC in 50 mL methanol, or 5 mg 4-HBA in 50 mL methanol. | ||
| Fig. 1 Structure of the UV filters, parabens and the internal standards included in this study. | ||
To demonstrate the suitability of DART-MS for the direct detection of UV filters and parabens in sun protection products, model samples were prepared by weighing specified amounts of the tested substances to a commercially available cream (blank cream), which did not contain any of the analytes (this was previously checked by GC-MS). The compositions of these samples were: sample I (HMS, EHS, OD-PABA, OC), sample II (BP-3, BM-DBM, 4-MBC, EHC) and sample III (methyl-, ethyl-, propyl-, butylparaben) with each UV filter added to a concentration of about 5% (250 mg to 5 g cream) and with each paraben added to a concentration of 0.3% (1.5 mg to 5 g cream). After homogenisation the model samples were directly measured by DART-MS. For establishing calibration curves, again three different types of model samples corresponding to I, II and III; (see above) with concentration ranges of 1.0 to 10% UV filters and 0.05 to 5.0% parabens (representing the relevant range for real samples) were prepared. As internal standards 5% BC and 0.5% 4-HBA were added.
In addition to the model samples, real samples such as suntan cream, lotion, milk, oil, make-up and lipstick were analysed. For the qualitative and semi-quantitative analysis by DART-MS the samples were just spiked with the internal standards (5% for BC and 0.5% for 4-HBA) and directly measured without further pre-treatment.
For analysis of dissolved samples by DART-MS and GC-MS 100 mg of the sample were treated with 5 mL methanol by positioning the flask in an ultrasonic bath for 10 min. Not all components of the sun protection products were soluble in methanol (e.g.titanium dioxide, other pigments). Therefore, samples had to be filtrated through a 0.45 µm membrane filter before GC-MS measurements, whereas for DART-MS measurements no filtration was needed. For quantification 50 µL of internal standard were added to 100 µL of the sample solution and filled up to 1 mL with methanol. The solution was then ready for analysis.
Instrumentation
DART-MS
All DART-MS measurements were performed with an ion source from IonSense, Inc. (Saugus, MA, USA) coupled to a JMS T100 LP (AccuTOF-LC-plus) time-of-flight mass spectrometer (JEOL, Peabody, MA, USA). The analysis of the UV filters was performed in the positive ion mode and the following voltages were applied to the ion source: orifice 1, 10 V; orifice 2, 5 V; and ring electrode, 10 V. For the parabens the negative ion mode and the same voltages as for the UV filters were used. Orifice 1 was always operated at a temperature of 120 °C to avoid contamination and the ion guide potential was set to 600 V. For operation of the DART source helium gas (4.6) was employed, whereas nitrogen gas was used in the standby mode.Optimum alignment of the DART orifice and the MS orifice was achieved by monitoring the signal intensity for the water cluster (m/z 37.0290). Mass calibration in the positive ion mode was carried out employing polyethylene glycol 600 (PEG 600) from Baker (Deventer, The Netherlands) as a solution of 50 µL PEG in 10 mL methanol/dichloromethane, 50/50. In the negative ion mode a mass calibration mix containing the following substances was used: benzoic acid (m/z 121.0295), 2,4-dihydroxybenzoic acid (m/z 153.0193), veratric acid (m/z 181.0506), and 1,3,5-benzenetricarbonic acid (m/z 209.0092) (all purchased from Sigma Aldrich) dissolved in methanol. Measurements were performed by dipping a Dip-IT glass rod (IonSense) into the respective solution and placing it into the DART source. Data acquisition was performed for both ion modes in a range from 100 to 400 m/z for all analytes. To achieve reproducible positioning of the glass rods in the ion source a lab-made modified Dip-IT holder was used.
GC-MS
The GC-MS measurements were carried out with a 6890N Network gas chromatograph coupled to a 5973 Network MSD mass spectrometer from Agilent (Waldbronn, Germany). The injection was performed in the splitless mode for the UV filters and in split mode (split: 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) for the parabens. The separation was carried out on an HP-5ms fused silica column (30 m × 0.25 mm i.d.; 0.25 µm film thickness, Agilent) in constant flow mode. The temperature program of the oven started at 70 °C (held for 2 min), then the temperature was increased in a first step to 160 °C at 20 °C min−1 and in a second step to 280 °C at 8 °C min−1. Helium was used as a carrier gas with a column flow rate of 1 mL min−1. The mass spectrometer was operated in the selected ion monitoring (SIM) mode at the following m/z for the UV filters: 120.00, 138.00, 181.00, 251.00 (0 to 11.5 min); 120.00, 138.00, 261.00 (11.5 to 12.5 min); 131.00, 151.00, 227.00, 254.00 (12.5 to 15.5 min); 148.00, 165.00, 277.00 (15.5 to 17.0 min); and 204.00, 249.00, 360.00 (17.0 min to end of the run). The following m/z were selected for the parabens: 93.00, 121.00, 138.00, 152.00, 166.00 (5 to 10.0 min); 93.00, 121.00, 138.00, 180.00, 194.00 (10.0 to 15.0 min); and 131.00, 191.00, 238.00 (15.0 to end of the run). The bold numbers given above indicate the m/z ratios used for quantitative analysis.
20) for the parabens. The separation was carried out on an HP-5ms fused silica column (30 m × 0.25 mm i.d.; 0.25 µm film thickness, Agilent) in constant flow mode. The temperature program of the oven started at 70 °C (held for 2 min), then the temperature was increased in a first step to 160 °C at 20 °C min−1 and in a second step to 280 °C at 8 °C min−1. Helium was used as a carrier gas with a column flow rate of 1 mL min−1. The mass spectrometer was operated in the selected ion monitoring (SIM) mode at the following m/z for the UV filters: 120.00, 138.00, 181.00, 251.00 (0 to 11.5 min); 120.00, 138.00, 261.00 (11.5 to 12.5 min); 131.00, 151.00, 227.00, 254.00 (12.5 to 15.5 min); 148.00, 165.00, 277.00 (15.5 to 17.0 min); and 204.00, 249.00, 360.00 (17.0 min to end of the run). The following m/z were selected for the parabens: 93.00, 121.00, 138.00, 152.00, 166.00 (5 to 10.0 min); 93.00, 121.00, 138.00, 180.00, 194.00 (10.0 to 15.0 min); and 131.00, 191.00, 238.00 (15.0 to end of the run). The bold numbers given above indicate the m/z ratios used for quantitative analysis.
Results and discussion
Optimisation of DART-MS parameters for the analysis of standard solutions and model samples
To find the best DART parameters for the determination of UV filters and parabens, solutions of the analytes were prepared in methanol and directly measured by dipping a Dip-IT glass rod into the solution and subsequently positioning it into the helium gas stream of the ion source. The following parameters were investigated in order to achieve the highest signal intensity: temperature of the gas heater on the ceramic tube (50–450 °C in 50 °C steps); helium gas flow; needle voltage (2000–4000 V in 500 V steps); discharge electrode and grid electrode voltages (50–300 V in 50 V steps). Optimum parameters were: needle voltage 3500 V, discharge electrode and grid electrode voltages 100 V and 50 V respectively. The optimum heater temperature was 200 °C. Due to their different chemical structures, UV filters provided higher intensities in the positive ion mode (detecting [M + H]+) whereas for parabens the negative ion mode (detecting [M − H]−) was favored.The conditions elaborated with solutions were also rechecked for the UV filters and the parabens spiked into a cosmetic cream. Comparing results from these experiments for UV filters and parabens respectively indicate that the optimal conditions for DART-MS are the same for both groups; only the ionisation mode has to be changed from positive to negative.
A list of all analytes together with measured exact masses in the positive/negative ion mode is provided in Table 1.
| Analyte | Empirical formula | [M + H]+ | Δ | |
|---|---|---|---|---|
| Calculated mass | Measured mass | |||
| EHS | C15H22O3 | 251.1642 | 251.1652 | 0.0010 |
| HMS | C16H22O3 | 263.1612 | 263.1627 | 0.0015 |
| BP-3 | C14H12O | 229.0895 | 229.0877 | −0.0018 |
| 4-MBC | C18H22O | 255.1685 | 255.1714 | 0.0029 |
| OD-PABA | C17H17NO2 | 278.2148 | 278.2131 | −0.0016 |
| OC | C24H27NO2 | 362.2214 | 362.2165 | −0.0050 |
| BM-DBM | C20H22O3 | 311.1653 | 311.1648 | −0.0006 |
| EHC | C18H26O3 | 291.1975 | 291.1965 | −0.0010 |
Direct analysis of model samples and real samples
After the optimisation of the DART parameters the suitability of this novel technique for direct identification of UV filters and parabens in real samples was checked by measuring model samples I–III (see Experimental section). For this purpose, a Dip-IT glass rod was dipped into the model sample, gently wiped off with a lint-free cloth and directly placed into the DART ion source. All tested substances could be detected in the cream and no interferences from the matrix occurred.In the next step twelve sun protection products of different formulations like suntan oil, suntan cream, milk, lotion, lipstick and make-up were measured directly. The measurement was performed in the same way as done for the model samples. By obtaining exact masses for the components, an unambiguous identification was possible for all tested analytes. A DART-MS spectrum of suntan oil #2 is depicted in Fig. 2 (UV filters, detected in the positive ion mode) and for the suntan lotion in Fig. 3 (parabens, detected in the negative ion mode). As can be seen from these figures, the selected cosmetic products could be analysed directly and all UV filters and parabens present in these samples were unambiguously identified based on the exact masses. The results were confirmed by GC-MS measurements, whereby pre-treatment of the samples was necessary hereby (dissolving and filtration of the sample).
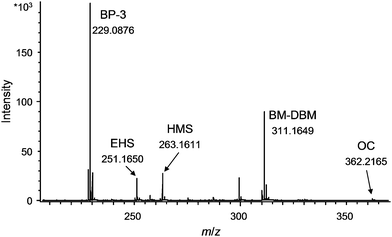 | ||
| Fig. 2 DART-MS spectrum of suntan oil #2 measured directly without further sample pre-treatment (positive ion mode). For signal assignments see Fig. 1 and conditions see Experimental. | ||
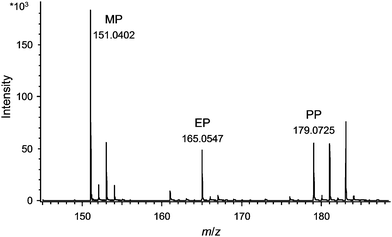 | ||
| Fig. 3 DART-MS spectrum of sun milk measured directly without further sample pre-treatment (negative ion mode). For signal assignments see Fig. 1 and conditions see Experimental. | ||
To investigate the applicability of DART-MS also for the analysis of samples with rather low content in UV-filters and parabens, the lowest detectable concentrations of these compounds in cosmetic products were determined. They were found to be between 2.5 µg g−1 (BP-3) and 460 µg g−1 (OC) for UV filters and between 0.14 µg g−1 (methylparaben) and 0.3 µg g−1 (butylparaben) for the parabens. This can be seen as sufficient with respect to concentrations expected in real samples.29
Furthermore investigations on the possibility of semi-quantitative analysis of cosmetic products with respect to their content in UV-filters and parabens were performed. For reproducibility of results, the positioning of the Dip-IT glass rod in the DART source is the most crucial point. Unfortunately, no autosampler was available for this work and the use of a Dip-IT holder did not allow a sufficiently exact positioning. Therefore an internal standard was used for area correction. Best choice as an internal standard for UV filter determination was BC, being well ionised in the positive ion mode; for parabens 4-HBA, which is accessible in the negative ion mode, was used. Spiked samples with concentration ranges from 1 to 10% for UV filters (plus 5% BC as internal standard) and from 0.05 µg mg−1 to 5 µg mg−1 for parabens (plus 0.5 µg mg−14-HBA as internal standard) were prepared. These samples were directly measured as described above. For the parabens quite good correlation coefficients (R > 0.98) were achieved with an acceptable repeatability better than 40% relative standard deviation (n = 5). These results indicate that although DART-MS without a dedicated autosampler (that might improve reproducibility) cannot provide quantitative results at least semi-quantitative analysis is possible. Due to their low volatility, UV filters are much slower desorbed from the glass rod and ionised than parabens. Whereas parabens usually give a very sharp signal immediately upon placing the rod in the ion source (indicating a quantitative desorption from the glass rod), UV filters yielded a broader and smaller peak, making quantitation less reliable. For this reason only qualitative results could be obtained for UV filters. For parabens, the concentrations found by DART-MS were in the same order of magnitude as those from GC-MS measurements.
Analysis of dissolved samples
In order to improve the quality of the (semi-) quantitative determination of the selected compounds in cosmetic products, a simple sample pre-treatment procedure, namely solution of the products in methanol, was tested. As in this case standard solutions of the UV-filters and parabens prepared in pure methanol are used for comparison, eventual suppression effects caused by the matrix of the cosmetic products investigated had to be evaluated first. Therefore, standard solutions at concentrations of 100 µg mL−1 (for UV filter) and 10 µg mL−1 (for parabens) were prepared by diluting the standard stock solution. Subsequently, 500 µL of each standard solution were mixed with 50 µL internal standard (BC and 4-HBA respectively) and 0, 100, 200, 300, 400 or 500 µL of a solution of cosmetic cream (100 mg blank cream in 5 mL methanol) and finally filled up with methanol to 1 mL. The results showed that peak areas were independent on the content of cream, so matrix effects could be regarded negligible.In the next step the calibration for the UV filters was performed at concentrations of 10, 25, 50, 75, 100 µg mL−1 of each UV filter (with 50 µg mL−1 of BC as internal standard) and 0.1, 0.5, 1.0, 2.5, 5.0, and 10 µg mL−1 of each paraben (with 5 µg mL−1 of 4-HBA as internal standard). Each concentration level was measured four times. The calibration curves were calculated by plotting the ratio of the analyte signal area/internal standard signal area versus concentration. Correlation coefficients R > 0.975 (for UV filters) and R > 0.995 (for parabens) were achieved, which seems to be sufficient for semi-quantitative analysis.
The repeatability (n = 10) was determined for a cosmetic cream containing 5% of each UV filter and 0.05% of each paraben; relative standard deviation ranged from 4% (for EHS) to 30% (for HMS) for UV filters and from 8% (for butylparaben) to 15% (for methylparaben) for parabens.
Subsequently recoveries were determined by analysing the model samples I–III; recoveries ranged from 71% (for OD-PABA) to 120% (for EHS) for the UV filters and from 73% (for methylparaben) to 86% (for ethylparaben) for the parabens.
As mentioned above, GC-MS measurements were performed in order to confirm the results achieved by DART-MS. For calibration of GC-MS, the same standard solutions as for DART-MS were used. Correlation coefficients of the calibration curves were R > 0.994 for UV filters and R > 0.999 for parabens. For GC-MS the samples were dissolved in methanol and filtrated before injection. It has to be mentioned that such minimised sample pre-treatment is unusual for the analysis of complex and oily matrices like cosmetic products. Generally, isolation of the analytes from the sample matrix is performed to protect the GC system from contamination. In the present case the simple sample preparation did not lead to problems with GC-MS, besides the necessity of a frequent change of the injection liner.
Analysis of real samples
Finally, twelve cosmetic products (all providing sun protection) with six completely different formulations were analysed (after dilution with methanol) and their content of UV filters and parabens determined. The concentrations of the UV filters in all tested samples obtained by DART-MS and GC-MS are summarised in Table 2 and those of the parabens in Table 3. Data given in these tables are based on four replicate measurements for DART-MS and single measurements for GC-MS. As can be seen from these tables comparable results were achieved by DART-MS and GC-MS whereby some minor discrepancy was observed for cosmetic products with a high amount of inorganic pigments like make-up samples.| Sample | DART-MS (%)/GC-MS (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| EHS | HMS | BP-3 | 4-MBC | OD-PABA | OC | BM-DBM | EHC | |
| a — not present in the sample. | ||||||||
| Sun cream 1 | —a | — | — | — | — | — | 2.3/2.9 | 10.1/7.5 |
| Sun cream 2 | — | — | — | — | — | 7.5/10.0 | 1.2/2.3 | — |
| Sun milk | — | — | — | — | — | 8.0/8.3 | 2.5/2.4 | — |
| Sun cream 3 | — | — | — | — | — | 8.0/8.9 | 1.4/1.5 | — |
| Sun lotion | — | — | — | — | — | 3.3/3.7 | 3.4/3.8 | — |
| Suntan oil 1 | — | — | — | — | — | 1.9/2.3 | 3.2/3.9 | 16.7/10.0 |
| Suntan oil 2 | 2.4/3.0 | 6.9/7.1 | 5.4/4.2 | — | — | 2.2/1.9 | 2.8/2.1 | — |
| Face cream 1 | 2.1/2.4 | — | — | — | — | — | — | 8.9/6.1 |
| Face cream 2 | 5.0/4.6 | — | — | — | — | — | — | — |
| Make-up 1 | — | — | — | — | — | — | — | 5.5/2.7 |
| Make-up 2 | — | — | — | — | — | — | — | 6.8/4.1 |
| Lipstick | — | — | — | 3.7/2.4 | — | — | — | 13.9/7.8 |
| Sample | (DART-MS/µg mg−1)/(GC-MS/µg mg−1) | |||
|---|---|---|---|---|
| MP | EP | PP | BP | |
| a — not present in the sample. b n.q. not quantified. | ||||
| Sun cream 1 | —a | — | — | — |
| Sun cream 2 | 1.8/1.9 | — | 1.0/1.2 | — |
| Sun milk | 1.3/1.6 | 0.9/0.9 | 0.5/0.8 | 1.0/1.0 |
| Sun cream 3 | 1.0/1.4 | n.q.b/0.6 | 0.4/0.7 | 0.8/0.9 |
| Sun lotion | 2.6/2.6 | 0.9/1.1 | 0.9/1.2 | — |
| Suntan oil 1 | — | — | — | — |
| Suntan oil 2 | — | — | — | — |
| Face cream 1 | — | — | — | — |
| Face cream 2 | — | — | — | — |
| Make-up 1 | — | — | — | — |
| Make-up 2 | — | — | — | — |
| Lipstick | — | — | — | — |
Conclusion
DART-MS has been successfully applied for the detection of UV filters and parabens in various cosmetic products. All eight UV filters and four parabens under investigation could be identified unambiguously in cosmetic matrices by direct measurement without any previous sample treatment. For the parabens also semi-quantitative determination was possible directly from the sample; this could not be realised for UV filters. A simple dissolution of the cosmetic product in methanol and addition of an internal standard also allowed the semi-quantitative analysis of UV filters. The results obtained by DART-MS were comparable to those achieved with GC-MS. As can be seen from this work DART-MS is a suitable tool for the fast and simple characterisation of cosmetic products with respect to their semi-quantitative content in two major classes of ingredients namely UV filters and parabens.Acknowledgements
The authors gratefully acknowledge JEOL (Europe) BV, Zaventem, Belgium for providing the DART-AccuTOF.References
- B. Berne and A.-M. Ros, Contact Dermatitis, 1998, 38, 61–64 CrossRef CAS.
- S. Schauder and H. Ippen, Contact Dermatitis, 1997, 37, 221–232 CrossRef CAS.
- F. P. Gasparro, M. Mitchnick and J. F. Nash, Photochem. Photobiol., 1998, 68, 243–256 CrossRef CAS.
- F. A. Andersen, Int. J. Toxicol., 2008, 27, 1–82.
- P. D. Darbre and P. W. Harvey, J. Appl. Toxicol., 2008, 28, 561–578 CrossRef CAS.
- B. Musial and J. Sherma, Acta Chromatogr., 1998, 8, 5–12 CAS.
- B. Musial and J. Sherma, J. Planar Chromatogr.–Mod. TLC, 1997, 10, 368–371 CAS.
- E. Westgate and J. Sherma, J. Liq. Chromatogr. Relat. Technol., 2000, 23, 609–615 CrossRef CAS.
- M. O. Masse, M. Herpol-Borremans, R. Grimee and S. Cleviczky, Int. J. Cosmet. Sci., 1982, 4, 235–246 CrossRef CAS.
- M. O. Masse, C. Delporte and E. Bervelt, Int. J. Cosmet. Sci., 2001, 23, 259–279 CrossRef CAS.
- G. L. Paulus, J.–Assoc. Off. Anal. Chem., 1972, 55, 47–50 CAS.
- K. Ikeda, S. Suzuki and Y. Watanabe, J. Chromatogr., 1990, 513, 321–326 CrossRef CAS.
- K. W. Ro, J. B. Choi, M. H. Lee and J. W. Kim, J. Chromatogr., A, 1994, 688, 375–382 CrossRef CAS.
- M. Saraji and S. Mirmahdieh, J. Sep. Sci., 2009, 32, 988–995 CrossRef CAS.
- H.-Y. Shen, H.-L. Jiang, H.-L. Mao, G. Pan, L. Zhou and Y.-F. Cao, J. Sep. Sci., 2007, 30, 48–54 CrossRef CAS.
- H.-B. Lee, T. E. Peart and M. L. Svoboda, J. Chromatogr., A, 2005, 1094, 122–129 CrossRef CAS.
- S. C. Rastogi and G. H. Jensen, J. Chromatogr., A, 1998, 828, 311–316 CrossRef CAS.
- A. Chisvert, M. C. Pascal-Marti and A. Salvador, J. Chromatogr., A, 2001, 921, 207–215 CrossRef CAS.
- A. Chisvert and A. Salvador, J. Chromatogr., A, 2002, 977, 277–280 CrossRef.
- A. Balaguer, S. Talamantes, E. de les Neus Duran Giner and P. C. Escamilla, LC·GC Eur., 2009, 22, 562–567 Search PubMed.
- A. Zotou, I. Sakla and P. D. Tzanayaras, J. Pharm. Biomed. Anal., 2010, 53, 785–789 CrossRef CAS.
- J. F. Garcia Jimenez, M. Carmen Valencia and L. F. Capitán-Vallvey, J. Anal. Chem., 2010, 65, 188–194 CrossRef CAS.
- M. Borremans, J. Van Loco, P. Roos and L. Goeyens, Chromatographia, 2004, 59, 47–53.
- F. P. Tomasella, T. Zuting and L. J. C. Love, J. Chromatogr., 1991, 587, 325–328 CrossRef CAS.
- C. W. Klampfl, T. Leitner and E. F. Hilder, Electrophoresis, 2002, 23, 2424–2429 CrossRef CAS.
- C. W. Klampfl and T. Leitner, J. Sep. Sci., 2003, 26, 1259–1262 CrossRef CAS.
- R. B. Cody, J. A. Laramée and H. D. Durst, Anal. Chem., 2005, 77, 2297–2302 CrossRef CAS.
- M. Haunschmidt, C. W. Klampfl, W. Buchberger and R. Hertsens, Anal. Bioanal. Chem., 2010, 397, 269–275 CrossRef CAS.
- The Rules Governing Cosmetic Products in the European Union, European Commission Enterprise Directorate-General Pharmaceuticals and cosmetics, 1999 Search PubMed.
| This journal is © The Royal Society of Chemistry 2011 |
