Dummy molecularly imprinted polymer for selective screening of trace bisphenols in river water
Junfa
Yin
a,
Zihui
Meng
b,
Yishan
Zhu
b,
Maoyong
Song
a and
Hailin
Wang
*a
aState Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China. E-mail: hlwang@rcees.ac.cn; Fax: +86 10 62849600; Tel: +86 10 62849600
bSchool of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing, 100081, China
First published on 12th November 2010
Abstract
Bisphenols (BPs) are potential endocrine-disrupting chemicals that may adversely affect human health and wildlife. The complexity of matrix encountered in real-world samples renders screening of trace BPs a formidable challenge. The present study highlighted the potential of molecularly imprinted solid-phase extraction (MISPE) for selective detection of trace bisphenols and their halogenated analogues in surface water. The template bleeding was observed at parts-per-billion levels, deteriorating the accuracy and precision of BPs quantification. To surmount this problem, a dummy MISPE strategy was proposed, in which bisphenol E (BPE) was selected as a dummy template for molecularly imprinted polymer (MIP) synthesis. Coupling this MISPE strategy with chromatographic analysis, a dummy MISPE-HPLC method was established. The linearity, precision, limit of detection (LOD) and recovery were then validated. The linearity of the calibration curve for each BP was observed over the range of 20–2000 ng L−1 (r > 0.998). LOD for each bisphenol was measured as low as 2.5–5.0 ng L−1. This technique was applied to simultaneous screening of BPs in the Qinghe River, and five bisphenols were found within the concentration range of 0–224 ng L−1 in river samples. The designed dummy MIP was superior to the commercial sorbents with regard to the selectivity, cross-reactivity, matrix removal efficiency and reusability. These merits enabled the applications of dummy MISPE for selective extraction and sensitive screening of BPs in environmental water samples. This method also provided a promising tool for monitoring the occurrence, distribution and fate of BPs in surface water.
1. Introduction
Bisphenols (BPs) are potential endocrine disrupting chemicals (EDCs) that widely used as additives in manufacturing polymers including polycarbonate plastic and epoxy resins.1,2 The global production capacity of bisphenol A (BPA) has risen to 5,200,000 tons in 2008.3 Tetrabromobisphenol A (TBBPA), currently the most utilized brominated flame retardant in the world, was estimated with an annual demand of 120,000 tons in recent years.4 Owing to their widespread use, BPs could be found far from their location of production and use, and both the levels in environment and in humans were rapidly increasing. A survey from US Geological Survey's reported that the frequency of BPA's existence was 41% in 139 US streams, with a maximum concentration of 12 μg L−1 and a medium concentration of 0.14 μg L−1.5 Other investigations revealed that the concentration of TBBPA was detected as 67 pg/g in milk samples from Norwegian mothers, 0.3–1.8 ng g−1 in human plasma, and 620 ng L−1 in landfill leachate.6–8These parts-per-billion levels of BPs were once considered as well below the safe dose, e.g. an oral reference dose (RfD) of 0.05 mg kg−1 day−1 recommended by the US-EPA.9 However, as early as in 1997, vom Saal et al. had revealed that even very low level of BPs fed to pregnant mice could enlarge the prostates of the male offspring.10 BPs were also suspected to cause acute toxicity to aquatic organisms, at a low concentration of 1–10 μg L−1.11 The subsequent studies have suggested that BPs exposure may increase chromosomal abnormalities, abnormal growth of the mammary gland ducts, rate of sexual maturation, and estrous cycles in female offspring.12−14 Epidemiology studies implied a link of BPA with breast cancer and early puberty.15 Therefore, the adverse effects in humans of even by low-level BPs are of a great concern.16 The Canadian government announced that BPA is toxic to human health and banned in the manufacture of baby bottles in April 2008. In USA, the NIH launched a $30-million stimulus grant to study the health effects of bisphenols in 2009. The US-FDA announced in January 2010 that, it had “some concerns” about its potential effects on brain development of fetuses, infants and children.
The low exposure concentration of BPs makes their monitoring in real samples more challenging, and this situation would be worse if there were high levels of interfering components in sample matrix. Therefore, highly selective sample pretreatment strategies are required for sensitive detection of BPs even by LC-MS. For this purpose, solid-phase extraction (SPE) was extensively utilized in the sample preconcentration and cleanup. ODS C18 and HLB were the most popularly used sorbents for BPs extraction.17,18 Despite of their high recovery and good reproducibility, the custom SPE sorbents suffer from a major problem, the lack of adequate selectivity to the target analytes. This is because these custom sorbents in the extraction of analytes are differentiated only by generic properties such as hydrophobic and Van der Waal forces. In order to improve the detection accuracy and precision, molecular imprinted solid-phase extraction (MISPE) has been proposed and is becoming increasedly popular, in virtue of its remarkable selectivity.19 Up to the present, BPA-imprinted polymers have been used as selective sorbents for extraction and clean-up of BPs in commercial honey, pig urine and chicken meat, milk and environmental water.20–25 The good selectivity displayed by these MIPs enabled highly sensitive detection of BPA at ppt levels in complex samples.
As MIPs are prepared using the target analytes as template, leakage of trace template from MIP will likely take place and significantly reduce the accuracy and precision of the assays of the target analytes.26,27 Surprisingly, the matter of template bleeding has seldom been comprehensively considered for BP–MIP in previous literature. Furthermore, although most of studies involved the development of MIPs for individual BP detection (in particular for BPA), the exploitation of MIPs for simultaneous screening of a group of BPs would be more meaningful in this field. Efforts to overcome these problems have been started by Haginaka et al., who pioneered an isotope template based dummy MISPE strategy, in which BPA-d16 (an isotope analogue of BPA) was involved in MIP preparation.28 Using the resultant isotope-MIP, the target BPs in samples could be separated from the bled template (BPA-d16) in the subsequent LC-MS analysis and hence reliably quantified.29 Despite of these remarkable merits, MIPs synthesized with isotope analogues also have limitations: isotope templates are very expensive, and the leakage of isotope template may produce a secondary pollution in which no data are currently available to evaluate the risks of isotopic BPs exposure. Our recent study revealed that the analogues in a group of β-lactam antibiotics (BLAs) could be employed as a pseudo (dummy) template for each other, avoiding the risks of isotopic template exposure. In this case, each BLA could be selectively trapped on the dummy MIP made with another analogue as the template.30 But as to achieve a better cross-reactivity with the higher accuracy of detection, the dummy template has to be carefully selected to produce both proper affinity and adequate recovery to the whole group, and sometimes the less toxicity.
The objective of this work is to develop a novel dummy MISPE method to improve the extraction selectivity available for trace BPs examinations. Here, bisphenol E was selected as the template for dummy MIP synthesis, in terms of its low consumption and less endocrine-disrupting effect in practice, together with the high selectivity and high recovery of BPE–MIP to the whole BP group. Coupling of MISPE strategy with chromatographic analysis, a dummy MISPE-HPLC method was established and further utilized for simultaneous screening of six bisphenols in the river water.
2. Experimental
2.1 Chemicals and reagents
Bisphenol A (BPA), bisphenol E (BPE), bisphenol F (BPF), bisphenol M (BPM), tetrachlorobisphenol A (TCBPA), tetrabromobisphenol A (TBBPA), o-nitrophenol (ONP) and 2,4,5-trichlorophenol (TCP) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethylene dimethacrylate (EDMA) and 4-vinylpridine (4-VP) were obtained from Acros (New Jersey, NJ, USA) and inhibitors were removed by an activated alumina column. The initiator 2,2′-azobisisobutyronitrile (AIBN) was supplied by J&K Chemical Ltd. (Beijing, China) and was recrystallized prior to use. HPLC grade acetonitrile and methanol was purchased from Fisher (Fair Lawn, NJ, USA). Other chemicals of analytical grade were supplied by Beijing Chemical Reagent Co. (Beijing, China). Ultra pure water (resistivity 18.2 MΩ·cm) was prepared from an ELGA system (Elga, UK) and all solutions were filtered through 0.45-μm membranes (Jinteng, Tianjin, China) prior to use.2.2 MIP preparation
BP-imprinted polymers were prepared by bulk polymerization as described elsewhere.20 Briefly, the template BP (1.0 mmol), functional monomer 4-VP (4.0 mmol), crosslinker EDMA (20 mmol) and free radical initiator AIBN (40 mg) were dissolved in 12 mL of methanol, and stood for 1 h at room temperature. Afterwards, the mixture was purged with nitrogen gas for 5 min, sealed in a conical flask, allowing the polymerization to be initiated at 60 °C in a water bath for 24 h. After the polymerization, the resultant MIP was ground into fine powders and sieved to 25–38 μm particles. These particles were packed into a 100 × 4.6 mm ID stainless steel column, washed on-line with methanol-acetic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, v/v) and then methanol until no imprinted molecule was detectable in the rinses.
20, v/v) and then methanol until no imprinted molecule was detectable in the rinses.
As a reference, non-imprinted polymer (NIP) for control experiments was prepared similarly as the MIP synthesis procedure described above except that no BP template was added in the polymerization.
2.3 Recognition selectivity and binding capacity
The imprinting factor (IF) was used to evaluate the selectivity of bisphenol-imprinted polymers, and defined as,31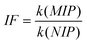 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15) as the mobile phase.
15) as the mobile phase.
To better understand the binding property of BP-imprinted polymers, rebinding experiment was carried out. BPE–MIP granules (40 mg) were immersed into a series of known concentration BP solutions (in methanol, 2.0 mL) in silanized glass containers. The containers were intermittently shaken at 25 °C for 12 h, and the mixtures were filtrated through 0.45-μm membrane. The free concentration of BPE in the filtrate was determined by HPLC- diode array detection (DAD). The amount of BPE bound to MIP, Q, was calculated by subtracting the free concentration from the initial concentration of BPE. Binding isotherms were measured in a concentration range of 0.005–2.0 mM. Data were processed according to Scatchard equation (eqn (2)) to estimate the binding properties,30,32
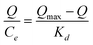 | (2) |
2.4 Dummy MISPE
BPE–MIP was chosen as the dummy MISPE sorbents for extraction and clean-up of BPs-containing water samples. MIP particles (25−38 μm, 80 mg) were packed into a short stainless steel column (10 × 4.6 mm ID). Prior to sample loading, the MISPE column was preconditioned with 4.0 mL of deionized water to generate a neutral and hydrophilic condition, preventing from the analyte losses and hence guaranteeing high recoveries of BPs. Aliquots of 250 mL water samples were pumped through the column by peristaltic pump at 5.0 mL min−1. The selective washing procedure was performed with 3.0 mL of methanol–water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) and 2.0 mL of methanol:0.05% triethylamine solution (65
90, v/v) and 2.0 mL of methanol:0.05% triethylamine solution (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) in sequence, at a flow-rate of 2.0 mL min−1. Finally, BPs were eluted with 4.0 mL of 5% HAc in methanol. All fractions were collected and evaporated to dryness under nitrogen gas stream. The residues were reconstituted in 100 μL acetonitrile and an aliquot of 20 μL was subjected to HPLC analysis.
35, v/v) in sequence, at a flow-rate of 2.0 mL min−1. Finally, BPs were eluted with 4.0 mL of 5% HAc in methanol. All fractions were collected and evaporated to dryness under nitrogen gas stream. The residues were reconstituted in 100 μL acetonitrile and an aliquot of 20 μL was subjected to HPLC analysis.
2.5 Sample collection and preparation
The river water samples (SA, SB and SC) were collected in June 2009 from three locations in the Qinghe River. Site-A lies in the upstream of the Qinghe River and is about 1.5 km from Site-B, where is nearby Qinghe Wastewater Treatment Plant (WWTP). Site-C is located in the downstream of the river, 1.5 km from Site-B. River water at Site-A (SA) was thought to have being contaminated by the drainage from an industry park nearby. The drainage of WWTP underwent hydrolyzation, acidification, anaerobic and aerobic treatments before entering the river. These water samples were filtered through 0.45-μm membrane from Jinteng (Tianjin, China) and stored at 4 °C. MISPE and HPLC analysis were carried out within 72 h after the samples collection, avoiding the degradation and hydrolysis of BPs.2.6 HPLC analysis
The detection of BPs was performed on a Hitachi L-2000 HPLC system (Tokyo, Japan), which consists of an L-2130 low-pressure gradient pump, an L-2455 diode array detector (DAD) and an L-2200 autosampler. A Capcell Pak C18 column (250 × 4.6 mm ID, 5 μm) from Shiseido (Tokyo, Japan) was employed for bisphenols separation. Two solvents (A and B) were prepared as the mobile phases for gradient elution. Solvent A consisted of 10 mM NH4Ac (pH 5.8) solution, solvent B was methanol, and the gradient program was set as: 0 − 20 min, 68% B to 90 B%; 20–25 min, 90% B; and 25–30 min, 68% B. The flow-rate was maintained at 1.0 mL min−1. Aliquots of 20 μL of the reconstituted samples was injected for HPLC analysis, and detected at UV 232 nm.3. Results and discussion
BPs are pollutants and have endocrine-disrupting effects that have been ubiquitously found in environment. It is essential to monitor the exposure levels and distribution of BPs in environmental water. A rigorous challenge in evaluation of their occurrence, distribution and fate in aquatic environment is that their concentrations are usually ultra-low. This problem could be circumvented by using a simple MISPE strategy. In this study, a dummy MISPE protocol was thus proposed for selective extraction of the target BPs in real water samples. The template bleeding and the feasibility of the dummy MISPE were firstly evaluated.3.1 Template bleeding
As MIPs are synthesized using the target BP as the template, the template may be released from the MIP network even after exhaustive washing steps. This may contaminate the samples, thereby deteriorating the accuracy and precision of the detection.26 This problem has been ignored in previous literature when dealing with MISPE. However, template bleeding is an unavoidable drawback of MISPE especially in trace analysis, and it should not be considered as a trivial matter for real applications.In this experiment, we first found that the template bleeding was affected by the type and composition of the washing and elution solvents. Percolating 50 mL of dimethyl sulfoxide (DMSO), methanol (MeOH), acetonitrile (ACN), methanol–acetic acid (MeOH/HAc= 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v), methanol–water mixture (50
10, v/v), methanol–water mixture (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, v/v), the deionized water and a blank river water through the MISPE cartridges sequentially, the effect of template-bleeding for each BP–MIP could be evaluated by measuring the eluted BP concentration in the rinses. Organic solvents including DMSO, MeOH, ACN and MeOH/HAc (90
50, v/v), the deionized water and a blank river water through the MISPE cartridges sequentially, the effect of template-bleeding for each BP–MIP could be evaluated by measuring the eluted BP concentration in the rinses. Organic solvents including DMSO, MeOH, ACN and MeOH/HAc (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, v/v), tended to cause higher template-bleeding levels than the aqueous solvent systems, including methanol–water mixture, the deionized water, and the river water (Fig. 1). The MeOH–HAc mixture showed the highest template bleeding level (7–136μg L−1), because of its strong ability in destroying the hydrogen bond and ionic interactions between the template and the binding sites on MIP.
10, v/v), tended to cause higher template-bleeding levels than the aqueous solvent systems, including methanol–water mixture, the deionized water, and the river water (Fig. 1). The MeOH–HAc mixture showed the highest template bleeding level (7–136μg L−1), because of its strong ability in destroying the hydrogen bond and ionic interactions between the template and the binding sites on MIP.
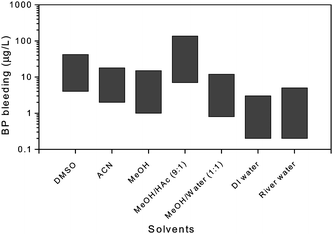 | ||
Fig. 1 Effect of washing and elution solvents on the template bleeding in MISPE. Organic solvents including DMSO, MeOH, ACN and methanol–acetic acid (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), tended to result in higher template-bleeding levels than the aqueous solvent systems, including methanol–water mixture (1 1), tended to result in higher template-bleeding levels than the aqueous solvent systems, including methanol–water mixture (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the deionized (DI) water, and the river water (blank reference; chemical oxygen demand (COD) 250 ± 60; biochemical oxygen demand (BOD) 100 ± 20; pH 7.2 ± 0.1). 1), the deionized (DI) water, and the river water (blank reference; chemical oxygen demand (COD) 250 ± 60; biochemical oxygen demand (BOD) 100 ± 20; pH 7.2 ± 0.1). | ||
To better understand this effect, we further investigated the extraction recovery of the six BPs at three concentration levels (0.05, 0.4 and 2.0 μg L−1), under the conditions described above. The results indicate that MISPE recoveries of BPs on their own MIPs are always much higher than those of their analogues (Table 1). This indicates the good selectivity of BP–MIPs towards the template BP molecules. However, all the recovery of BPs on their own MIPs are higher than 200%, in other words, the analytes determined in the sample might be much higher than the input quantities, in particular at low concentrations of 0.05 and 0.4 μg L−1. For instance, pumping 250 mL of BPs solution (0.05 μg L−1) through MISPE column, the calculated recoveries are 664% for BPA on BPA–MIP, 562% for BPE on BPE–MIP, 420% for BPF on BPF–MIP, and 309% for TBBPA on TBBPA–MIP, respectively. The excess of BPs over the input was probably generated from the undesired template bleeding from MIP sorbent. Nevertheless, when its concentration was increased to 2.0 μg L−1, MISPE recoveries dramatically decrease to a reasonable level of 80–123%, which are already acceptable for quantification.
| BPs | Concentration/μg L−1 | SPE recovery | Precision (% RSD, n = 3) b | Recovery (%, n = 3) for the method b | |||||
|---|---|---|---|---|---|---|---|---|---|
| BPA–MIP | BPE–MIP | BPF–MIP | TBBPA–MIP | Cleanert ODS C18 a | Intra-day | Inter-day | |||
| a Cleanert ODS C18 cartridges were supplied by Agela Technologies Inc. (Beijing, China). b Using BPE–MIP as the MISPE sorbent. c Not test. | |||||||||
| BPA | 0.05 | 664 ± 77 | 63 ± 4 | 47 ± 6 | 61 ± 6 | 74 ± 5 | 8.4 | 10.4 | 87 ± 4 |
| 0.4 | 309 ± 35 | 79 ± 3 | 54 ± 5 | 67 ± 2 | 81 ± 6 | 5.2 | 5.7 | 95 ± 2 | |
| 2.0 | 92 ± 14 | 89 ± 5 | 71 ± 9 | 74 ± 4 | nt c | 4.5 | 3.9 | 94 ± 2 | |
| BPE | 0.05 | 78 ± 10 | 562 ± 64 | 72 ± 2 | 51 ± 5 | 76 ± 4 | nt | nt | nt |
| 0.4 | 72 ± 3 | 224 ± 21 | 69 ± 5 | 69 ± 3 | 86 ± 2 | nt | nt | nt | |
| 2.0 | 83 ± 4 | 102 ± 12 | 84 ± 3 | 71 ± 7 | nt | nt | nt | nt | |
| BPF | 0.05 | 62 ± 8 | 62 ± 7 | 420 ± 34 | 39 ± 5 | 68 ± 6 | 11.6 | 13.9 | 81 ± 6 |
| 0.4 | 66 ± 7 | 81 ± 6 | 228 ± 56 | 56 ± 3 | 81 ± 5 | 5.8 | 6.6 | 97 ± 5 | |
| 2.0 | 69 ± 2 | 88 ± 5 | 123 ± 21 | 71 ± 8 | nt | 2.7 | 2.5 | 93 ± 4 | |
| BPM | 0.05 | 33 ± 3 | 64 ± 2 | 48 ± 4 | nt | 72 ± 4 | 5.9 | nt | 86 ± 5 |
| 0.4 | 45 ± 5 | 90 ± 7 | 59 ± 8 | nt | 79 ± 6 | 7.1 | nt | 90 ± 2 | |
| 2.0 | 65 ± 2 | 83 ± 4 | 74 ± 2 | nt | nt | 1.8 | nt | 88 ± 3 | |
| TCBPA | 0.05 | 63 ± 3 | 62 ± 7 | 52 ± 3 | 111 ± 15 | 73 ± 3 | 3.4 | 5.5 | 93 ± 5 |
| 0.4 | 68 ± 6 | 73 ± 2 | 59 ± 8 | 66 ± 4 | 86 ± 5 | 7.3 | 7.8 | 88 ± 6 | |
| 2.0 | 70 ± 5 | 80 ± 5 | 77 ± 5 | 87 ± 5 | nt | 2.6 | 4.1 | 94 ± 1 | |
| TBBPA | 0.05 | 71 ± 9 | 79 ± 7 | 67 ± 5 | 309 ± 25 | 98 ± 5 | 9.7 | 8.6 | 93 ± 8 |
| 0.4 | 67 ± 2 | 82 ± 3 | 75 ± 6 | 182 ± 25 | 90 ± 3 | 6.0 | 6.4 | 91 ± 6 | |
| 2.0 | 76 ± 5 | 89 ± 3 | 80 ± 2 | 77 ± 5 | nt | 2.5 | 3.1 | 87 ± 3 | |
These results suggest that the template bleeding from BP–MIPs remains at a sub-μg L−1 level. The concentration of BPs in real-world samples below such a level (2.0 μg L−1) would therefore be overestimated in a concentration-dependent manner. In fact, the existence of BPs in the environment is commonly below this concentration level,5,6 therefore, the template bleeding may have significant influence on the quantitative analysis of BPs in real samples.
3.2 Dummy MIPs for bisphenols
To eliminate the template bleeding, a dummy MISPE strategy was further developed by using dummy MIP prepared with a pseudo template. In this case, it does not matter if the imprinted template leaks out, because the target analyte can be separated from the leaked template in the subsequent HPLC analysis. Here, BPE was selected as the dummy template because of the high recovery and high selectivity of BPE–MIP towards the whole group of BP analogues (Table 2 and Fig. 2). In addition BPE is less used in practice, and its endocrine-disrupting effect of BPE was regarded less potent than the other bisphenols.| River samples b | BPA | BPF | BPE c | BPM | TCBPA | TBBPA |
|---|---|---|---|---|---|---|
| a HPLC conditions: the same as Fig.4. b Samples were collected in June 2009, COD 350 ± 60, BOD 160 ± 20, pH 7.2 ± 0.1, temperature 23 °C. c BPE concentration was measured by using BPF–MIP based MISPE–HPLC method. d Not detected (< LOD). | ||||||
| SA | 63.6 ± 3.4 | 31.8 ± 1.7 | nd d | nd | 143 ± 3.7 | 224 ± 11 |
| SB | 53.4 ± 1.9 | 24.4 ± 2.1 | 20.3 ± 0.6 | nd | 69.4 ± 2.0 | 54.8 ± 2.6 |
| SC | 10.7 ± 0.8 | 30.5 ± 2.6 | nd | nd | 36.5 ± 1.2 | 23.9 ± 1.9 |
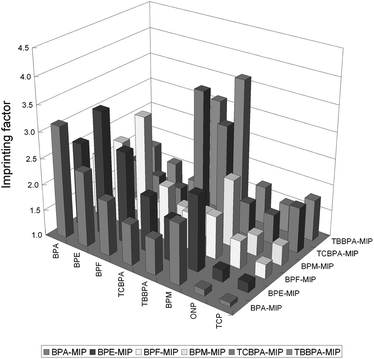 | ||
| Fig. 2 Imprinting factor (IF) for each analyte on BP–MIPs. | ||
Fig. 2 shows the imprinting factors (IFs, eqn (1)) for each bisphenol on the corresponding BP–MIPs. As expected, BP analogues achieved the largest IF on its own MIP, for instance, it was 3.1 for BPA, 3.4 for BPE, 3.2 for BPF, 2.3 for BPM, 3.5 for TCBPA and 3.7 for TBBPA. It should be noted that the dummy MIP used in this experiment, BPE–MIP, showed relatively higher selectivity towards these BPs, which is 2.6 for BPA, 2.7 for BPF, 2.4 for BPM, 2.1 for TCBPA and 1.8 for TBBPA, respectively. By contrast, two related phenolic compounds, o-nitrophenol (ONP) and 2,4,5-trichlorophenol (TCP), were neither retained on MIP nor NIP, exhibiting low IF of 1.1–1.5 for ONP, and 1.1–1.7 for TCP. This confirmed the specificity of BP–MIPs towards the bisphenols family.
BPE–MIP could selectively capture BPF, BPA, BPM, TCBPA and TBBPA, as well as the template BPE. The findings suggest that MIPs should have a broader selectivity beyond the template molecule, sometimes, also have appropriate selectivity for the closely related analogues. This kind of selectivity benefited from the cross-reactivity of BPE–MIP toward the BPs group. The cross-reactivity of MIP should not be simply regarded as a defect but as an advantage for certain applications, especially in the development of MISPE processes for the simultaneous determination of structurally related compounds.33 In this experiment, BPE–MIP exhibited some degree of cross-reactivity that could be utilized for simultaneous extraction of a group of BPs from water samples. Therefore, BPE was consequently chosen as the dummy template for MIP synthesis, and the resultant BPE–MIP as the packing sorbent for dummy MISPE protocol.
3.3 Binding capacity of the dummy MIP (BPE–MIP)
The binding properties of BPE–MIP were investigated by a rebinding experiment. Fig. 3A shows the experimental adsorption isotherms of BPE on MIP and NIP sorbent, within a BPE concentration range of 0.005–2.0 μM. MIP displayed an obviously higher affinity than NIP, and could adsorb larger amount of BPs than NIP too. Binding data were then processed with Scatchard plot (eqn (2)), and two straight lines were obtained in the plot region (Fig. 3B). The linear regression equations for the two linear lines are Q/Ce = 61.85–4.86Q (r = 0.945) and Q/Ce = 20.35–0.45Q (r = 0.991). This indicates that the binding sites in the imprinted polymer are heterogeneous in respect to BPs, which can be classified into two distinct groups: the high-affinity and the low-affinity binding sites. From the slope and intercept of Scatchard plot, the equilibrium dissociation constant (Kd) and the apparent maximum number (Qmax) were calculated as 2.06 × 10−4 mol L−1 and 12.73 μmol g−1 for the high-affinity binding sites, and 2.21 × 10−3 mol L−1 and 45.22 μmol g−1 for the low-affinity ones.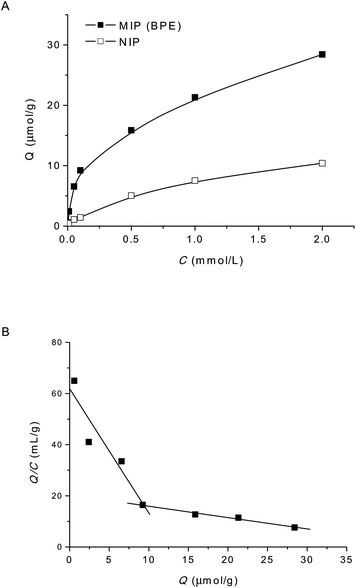 | ||
| Fig. 3 (a) Binding isotherm of BPE on BPE-MIP (■) and NIP (□); (b) Scatchard plot for BPE-MIP sorbent, Kd = 2.06 × 10−4 mol L−1 and Qmax = 12.73 μmol g−1 for the high affinity binding sites (upper line), and Kd = 2.21 × 10−3 mol L−1 and Qmax = 45.22 μmol g−1 for the low affinity binding sites (lower line). | ||
The binding capacity of MIP against BPE is approximately 58 μmol g−1 in total, including the high-affinity and low-affinity binding sites. Given BP concentration in real sample is at 10 μg L−1 level, theoretically, a MISPE column packed with 80 mg BPE–MIP could fulfil at least 80 L water sample loading, which is by far sufficient for real samples loading in this experiment.
3.4 Dummy MISPE
Dummy MISPE involves the procedures as that of custom SPE, including the routine steps of precondition, sample loading, selective washing and elution. Sample loading solvents provide the rebinding of BPs to MIP sorbent, whereas the elution solvents are chosen to fast elute them from the sorbent by destroying the interactions between BP and the binding sites. Selective washing step is carried out before the final elution in order to clean up the interfering components non-specifically retained.Prior to sample loading, MISPE column (10 × 4.6 mm ID, 80 mg MIP) was preconditioned with 4 mL of deionized water to generate a neutral and hydrophilic condition, avoiding the analyte losses and guaranteeing high BPs recovery. Afterwards, aliquots of 250 mL of river water samples were pumped through the column by peristaltic pump at 5 mL min−1. The sample loading and washing flow-rates are often considered as critical factors that affect both the analyte recovery of target analytes on MISPE sorbent and the speed of extraction procedure. We found that the use of flow-rate of 2–5 mL min−1 for sample loading and 1–2 mL min−1 for washing and elution had no obvious influence on the recovery of each bisphenol. Therefore, in the following experiments, flow-rate of 5 mL min−1 was chosen for sample loading and 2 mL min−1 for selective washing and elution, in order to save the pretreatment time.
The selective washing procedure was performed with 3 mL of methanol: water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90, v/v) and 2.0 of mL methanol: 0.05% triethylamine solution (65
90, v/v) and 2.0 of mL methanol: 0.05% triethylamine solution (65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35, v/v) in sequence. During this process, bisphenols could be strongly retained on MIP sorbent (recovery> 60%), while most of hydrophilic interferers, in particular nitrophenols and short-chain fatty acids, were rapidly eliminated.
35, v/v) in sequence. During this process, bisphenols could be strongly retained on MIP sorbent (recovery> 60%), while most of hydrophilic interferers, in particular nitrophenols and short-chain fatty acids, were rapidly eliminated.
The final elution of BPs was conducted by using 4 mL of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) acetic acid (95
acetic acid (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v). Acetic acid can destroy the hydrogen bonding between MIP matrix and the target template. In this experiment, BPs could be eluted by 5% acetic acid in methanol with MISPE recoveries of 54–80% (3 mL), 67–89% (4 mL), and 75–95% (5 mL), respectively. It is evident that the use of larger volume of the eluent allows for obtaining higher MISPE recovery. However, if the applied volume was more than 4 mL, the unidentified interferers in the sample matrix would be present. For instance, when 5 mL of the elutent was applied to the MISPE, the trace analysis of TCBPA and TBBPA could not be accomplished, because there were several strong signals appeared nearby the analyte peaks. It was found that, after the selective washing both good recovery and less interfering substances in the prepared samples could be achieved by applying 4 mL of methanol: acetic acid (95
5, v/v). Acetic acid can destroy the hydrogen bonding between MIP matrix and the target template. In this experiment, BPs could be eluted by 5% acetic acid in methanol with MISPE recoveries of 54–80% (3 mL), 67–89% (4 mL), and 75–95% (5 mL), respectively. It is evident that the use of larger volume of the eluent allows for obtaining higher MISPE recovery. However, if the applied volume was more than 4 mL, the unidentified interferers in the sample matrix would be present. For instance, when 5 mL of the elutent was applied to the MISPE, the trace analysis of TCBPA and TBBPA could not be accomplished, because there were several strong signals appeared nearby the analyte peaks. It was found that, after the selective washing both good recovery and less interfering substances in the prepared samples could be achieved by applying 4 mL of methanol: acetic acid (95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, v/v) to MISPE. Dummy MISPE demonstrates its powerful performances in decreasing the interference encountered in trace analysis of BPs in water samples. It was expected that, by using the dummy MISPE-HPLC protocol, the simultaneous detection of BPA, BPF, BPM, TCBPA, TBBPA and BPE in the river water could be achieved.
5, v/v) to MISPE. Dummy MISPE demonstrates its powerful performances in decreasing the interference encountered in trace analysis of BPs in water samples. It was expected that, by using the dummy MISPE-HPLC protocol, the simultaneous detection of BPA, BPF, BPM, TCBPA, TBBPA and BPE in the river water could be achieved.
3.5 Method validation
The dummy MISPE-HPLC method was validated with respect to linearity, precision, recovery and reproducibility. The standard calibration curves were generated for the BP standard solutions at different concentration within the range of 0.02–2.0 μg L−1. The correlation coefficients were all above 0.998. The limit of detection (LOD, at a signal-to-noise ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) was 2.5 ng L−1 (125 pg in mass) for BPA, BPE and BPF, and 5 ng L−1 (250 pg in mass) for BPM, TCBPA and TBBPA, which could properly fulfill the demand of sensitive quantification of trace BPs.
1) was 2.5 ng L−1 (125 pg in mass) for BPA, BPE and BPF, and 5 ng L−1 (250 pg in mass) for BPM, TCBPA and TBBPA, which could properly fulfill the demand of sensitive quantification of trace BPs.
Precision (represented by %RSD) was determined by HPLC using the quality control (QC) samples at 0.05, 0.4, and 2.0 μg L−1 prepared by spiking blank river water with the target BPs. Inter-day precision was determined by repetitive analysis of QC samples on three consecutive days, while intra-day precision was determined by analyzing five replicate aliquots of QC samples on the same day. The intra- and inter-day precisions are well within the limit of 15% RSD required for method validation (Table 1).
Recovery for each BP was also investigated by HPLC with the QC samples. The chromatographic peak areas of analytes were compared to those of standards at the same concentration to estimate the recovery. The results were given in Table 1. The average MISPE recoveries of BPs obtained on BPE–MIP sorbent were generally in 63–94%, by and large, which were comparable to those obtained on commercial ODS C18 sorbent (68–98%).
The reproducibility of the MISPE sorbent was assessed through the RSD of the peak area of BPA and TBBPA (0.4 μg L−1 for each analyte) in river water samples. Five batches of BPE–MIP were prepared with the same polymerization composition, and packed into five short columns (10 × 4.6 mm ID). These columns were individually used in MISPE procedure and the eluents were analyzed by HPLC with DAD detection. The RSD values were 3.8% for BPA and 8.6% for TBBPA in peak areas, all being less than 10%. This indicates that the preparation of the MISPE sorbent is universally applicable and the material can be produced industrially.
3.6 Selective screening of trace BPs in environmental water
The developed dummy MISPE-HPLC method was applied to screening of BPs in river water. BPA, BPF, TCBPA and TBBPA were all detected and quantifiable in the river samples collected at different positions (Site-A, Site-B and Site-C), with the exception of BPM (Fig. 4 and Table 2). The total BP concentrations for the 5 analytes ranged from 102 to 462 ng L−1 throughout the three sampling sites (from downsteam to upstream) in Qinghe River. They were dominated by TBBPA (23.9–224 ng L−1), TCBPA (36.5–143 ng L−1), BPA (10.7–63.6 ng L−1) and BPF (24.4–31.8 ng L−1), whereas BPE and BPM were present at very low levels, and even not detectable in some samples.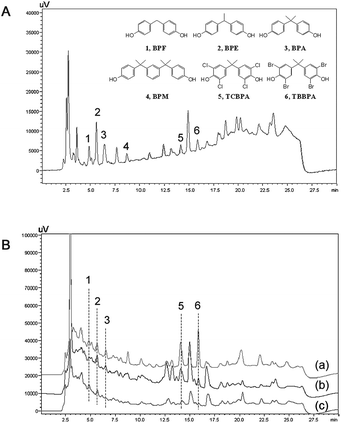 | ||
| Fig. 4 (A) The chromatogram for the blank river water spiked with 50 ng mL−1 of each BP. (B) Screening of bisphenols in river water samples SA (trace a), SB (trace b) and SC (trace c) by HPLC. HPLC conditions: column, CAPCELL PAK C18 (250 × 4.6 mm ID, SHISEIDO); mobile phase, 10 mM NH4Ac buffer (pH 5.8, A) and methanol (B), gradient process: 0–20 min, 68% B to 90 B%, 20–25 min, 90% B, and 25–30 min, 68% B; flow-rate, 1.0 mL min−1; column temperature, 25 °C; injection, 20 μL; detection wavelength, 232 nm. Peaks: 1, BPF; 2, BPE; 3, BPA; 4, BPM; 5, TCBPA; 6, TBBPA. | ||
BPA in the Qinghe River is at a relatively lower level, which is comparable to that detected in the Tama River in Japan (16.5–150.2 ng L−1 with a median of 33.2 ng L−1) and the Elbe River in Germany (8.9–77.6 ng L−1 with a median of 57 ng L−1),34,35 and much lower than those of found in the US streams ranging from n.d. to 1200 ng L−1 with a median of 140 ng L−1.5
BPF was present at a comparable level to BPA in the river water samples. This was easily explained in terms of the increasing quantities used in practice. Similar to BPA, BPF are also widely used in the production of epoxy resins and polycarbonates, and can leach out into environment. The average BPF concentration was 31.8 ng L−1 in river sample SA, 24.4 ng L−1 in SB and 30.5 ng L−1 in SC, respectively. In comparison with the results obtained from over 100 samples from Germany,36 in which BPF levels of 0.1–180 ng L−1 were found in surface water samples and 22–123 ng L−1 in sewage water samples, the concentration of BPF reported in our experiment are relatively lower.
Of the 6 BPs shown in Table 2, TCBPA and TBBPA make up about 57–80% in the river samples. They are suspected to have connection with the manufactories in the industrial park located nearby in which epoxy resin, polycarbonate and acrylonitrile butadiene styrene (ABS) polymers are widely used in the processing lines. TCBPA and TBBPA, blended as flame retardants in these polymers, are not chemically linked to other components of the polymer and can easily leach out from the polymer matrix. TBBPA is the most abundant BP in SA (54%), but became the subordinate in SB (25%) and SC (23%) (Fig. 5). The proportions of TCBPA (31–36%) in these samples are almost not changed, although its concentration descends from 143 (SA) to 36.5 ng L−1 (SC). This is probably because of the entrance and dilution of the effluent from WWTP (near SB-sampling position), in which no obvious BPs were detected. But strangely, the changes of BPF in the three river samples are not in the same way. The concentration of BPF in SC (31.8 ng L−1) is comparable to that in SA (30.5 ng L−1), and slightly higher than that in SB (24.4 ng L−1), whereas its proportion increases from 7% (in SA) to 11% (in SB) and 30% (in SC). This implies that the dilution of the up-river stream (SA, near the discharge source) may be associated with the transformation and/or degradation of TCBPA and TBBPA. Biodegradation studies have shown that TBBPA can be partly degraded to lesser brominated analogues under some certain conditions, in soil and river sediment.37 These bisphenols were found readily biodegradable in surface waters under aerobic conditions.38–40 But it is not very clear whether such degradation (such as dehalogenation) directly raised the concentration level of BPF. Actually, we did find there were several abundant components present in the chromatogram (Fig. 4), which had not yet been structurally identified in this experiment. More samples and specificity assays are necessary to confirm or deny these hypotheses.
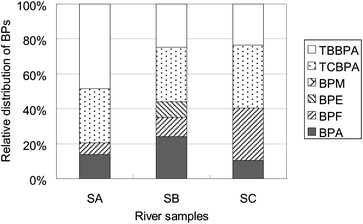 | ||
| Fig. 5 Percentage contribution of BPs in river water. | ||
BPE in the river samples, the dummy template employed in this experiment, was measured using a BPF-imprinted polymer based MISPE-HPLC method, in virtue of its good selectivity (IF = 2.6) and recovery (72–84%) towards BPE (Fig. 2 and Table 1). Consequently, BPE was only found in SB at a low concentration level of 20.3 ng L−1, and was negative in the other two river samples.
3.7 Advantages of MISPE sorbent
One major merit of MISPE sorbent is the high selectivity towards the target analytes, which originates from the specific interactions between the template and functional monomer(s) during MIP synthesis. In this experiment, most of interfering substances in sample matrix were removed by using BPE–MIP sorbent. BPs were well separated from interfering matrix components, and the target peaks could be well distinguished and reliably quantified (Fig. 4). To achieve satisfactory cleanup efficiency, no more actions that commonly involved in custom SPE are usually required, including time-consuming dilution, pH adjustment and tedious selective washing steps.Another merit of MISPE sorbents is their remarkable reusability, which has been addressed in the previous work.30,41 Due to its good robustness and good reproducibility, MIP sorbent can be repeatedly used; this is a prominent advantage over ODS C18 sorbent, which is commonly recommended to be used only once. In this experiment, the same MISPE column was reused more than 40 times without losing its extraction capability. In such cases, the recovery declines were well within 10%, and the corresponding chromatograms did not show any indication for the appearance of extra interfering matrix components nor detectable increase of the baseline.
Conclusions
We have demonstrated the utility of a dummy MISPE technique with subsequent liquid chromatography analysis for simultaneously selective screening of six bisphenols in environmental water. It was found that the template bleeding for BP-imprinted polymers synthesized with the target BP as the template generally occurred at ppb levels. The template leakage at ppb levels may significantly contaminate water samples and deteriorate the accuracy and precision of the detection. This problem was circumvented by the application of a dummy MISPE strategy, which provides a promising alternative to achieve selective extraction of the target BPs from river water samples. High precision and sensitivity, besides the appropriate recoveries, were achieved for the dummy MISPE-HPLC method, despite the ultra-low concentration of BPs (0–224 ng L−1) in the real-world samples. Compared with a custom extraction sorbent, the merits of MISPE sorbent including the robustness and reusability have also been addressed. This method provides a promising tool for monitoring the occurrence, distribution and fate of BPs in the environment.Acknowledgements
This research was supported by grants from the National Basic Research Program of China (Nos. 2007CB407305, 2010CB933502 and 2009CB421605), the National Natural Science Foundation of China (Nos. 20805057, 20877091 and 20775007), the National High Technology Research and Development Program of China (Nos. 2007AA06A407 and 2007AA10Z433) and the fund from SKLECE, RCEES, CAS.Notes and references
- U. Sellström and B. Jansson, Chemosphere, 1995, 31, 3085 CrossRef.
- C. A. de Wit, Chemosphere, 2002, 46, 583 CrossRef.
- ICIS Chemical Business, Chemical profile: bisphenol A, 13 Oct 2008. http://www.icis.com/articles/2008/10/13/9162868/Chemical-profile-bisphenol-A.html Search PubMed.
- T. Reistad, E. Mariussen, A. Ring and F. Fonnum, Toxicol. Sci., 2007, 96, 268 CAS.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202 CrossRef CAS.
- C. Thomsen, H. Leknes, E. Lundanes and G. Becher, J. Anal. Toxicol., 2002, 26, 129 CAS.
- C. Thomsen, E. Lundanes and G. Becher, J. Environ. Monit., 2001, 3, 366 RSC.
- M. Osako, Y. J. Kim and S. Sakai, Chemosphere, 2004, 57, 1571 CrossRef CAS.
- C. Leranth, T. Hajszan, K. Szigeti-Buck, J. Bober and N. J. MacLusky, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 14187 CrossRef CAS.
- F. S. vom Saal, B. G. Timms, M. M. Montano, P. Palanza, K. A. Thayer, S. C. Nagel, M. D. Dhar, V. K. Ganjam, S. Parmigiani and W. V. Welshons, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 2056 CrossRef CAS.
- H. C. Alexander, D. C. Dil, L. W. Smith, P. D. Guiney and P. Dorn, Environ. Toxicol. Chem., 1988, 7, 19 CrossRef CAS.
- J. Cohen, Science, 2003, 300, 31 CAS.
- F. S. vom Saal and W. V. Welshons, Environ. Res., 2006, 100, 50 CrossRef CAS.
- C. M. Markey, P. R. Wadia, B. S. Rubin, C. Sonnenschein and A. M. Soto, Biol. Reprod., 2005, 72, 1344 Search PubMed.
- C. Brisken, Chimia, 2008, 62, 406 CrossRef CAS.
- F. S. vom Saal, B. T. Akingbemi, S. M. Belcher, L. S. Birnbaum, D. A. Crain, M. Eriksen, F. Farabollini, L. J. Guillette, R. Hauser, J. J. Heindel, S. M. Ho, P. A. Hunt, T. Iguchi, S. Jobling, J. Kanno, R. A. Keri, K. E. Knudsen, H. Laufer, G. A. LeBlanc, M. Marcus, J. A. McLachlan, J. P. Myers, A. Nadal, R. R. Newbold, N. Olea, G. S. Prins, C. A. Richter, B. S. Rubin, C. Sonnenschein, A. M. Soto, C. E. Talsness, J. G. Vandenbergh, L. N. Vandenberg, D. R. Walser-Kuntz, C. S. Watson, W. V. Welshons, Y. Wetherill and R. T. Zoeller, Reprod. Toxicol., 2007, 24, 131 CrossRef CAS.
- O. Ballesteros, A. Zafra, A. Navalón and J. L. Vílchez, J. Chromatogr., A, 2006, 1121, 154 CrossRef CAS.
- H. Gallart-Ayala, E. Moyano and M. T. Galceran, J. Chromatogr., A, 2010, 1217, 3511 CrossRef CAS.
- E. Turiel and A. Martín-Esteban, Anal. Chim. Acta, 2010, 668, 87 CrossRef CAS.
- E. Herrero-Hernández, R. Carabias-Martínez and E. Rodríguez-Gonzalo, Anal. Chim. Acta, 2009, 650, 195 CrossRef CAS.
- M. Jiang, Y. Shi, R. L. Zhang, C. H. Shi, Y. Peng, Z. Huang and B. Lu, J. Sep. Sci., 2009, 32, 3265 CrossRef CAS.
- Y. Ji, J. Yin, Z. Xu, C. Zhao, H. Huang, H. Zhang and C. Wang, Anal. Bioanal. Chem., 2009, 395, 1125 CrossRef CAS.
- Q. Z. Feng, L. X. Zhao, W. Yan, J. M. Lin and Z. X. Zheng, J. Hazard. Mater., 2009, 167, 282 CrossRef CAS.
- J. Ou, L. Hu, L. Hu, X. Li and H. Zou, Talanta, 2006, 69, 1001 CrossRef CAS.
- Y. Watabe, K. Hosoya, N. Tanaka, T. Kondo, M. Morita and T. Kubo, Anal. Bioanal. Chem., 2005, 381, 1193 CrossRef CAS.
- F. G. Tamayo, E. Turiel and A. Martín-Esteban, J. Chromatogr., A, 2007, 1152, 32 CrossRef CAS.
- A. Ellwanger, C. Berggren, S. Bayoudh, C. Crecenzi, L. Karlsson, P. K. Owens, K. Ensing, P. Cormack, D. Sherrington and B. Sellergren, Analyst, 2001, 126, 784 RSC.
- H. Sambe, K. Hoshina, K. Hosoya and J. Haginaka, J. Chromatogr., A, 2006, 1134, 16 CrossRef CAS.
- H. Sambe, K. Hoshina, K. Hosoya and J. Haginaka, Analyst, 2005, 130, 38 RSC.
- J. Yin, Z. Meng, M. Du, C. Liu, M. Song and H. Wang, J. Chromatogr., A, 2010, 1217, 5420–5426 CrossRef CAS.
- J. L. Urraca, A. J. Hall, M. C. Moreno-Bondi and B. Sellergren, Angew. Chem., Int. Ed., 2006, 45, 5158 CrossRef CAS.
- Y. Liu, K. Hoshina and J. Haginaka, Talanta, 2010, 80, 1713 CrossRef CAS.
- E. Turiel, C. Perez-Conde and A. Martin-Esteban, Analyst, 2003, 128, 137 RSC.
- O. P. Heemken, H. Reincke, B. Stachel and N. Theobald, Chemosphere, 2001, 45, 245 CrossRef CAS.
- T. Furuichi, K. Kannan, J. P. Giesy and S. Masunaga, Water Res., 2004, 38, 4991.
- H. Fromme, T. Kuchler, T. Otto, K. Pilz, J. Muller and A. Wenzel, Water Res., 2002, 36, 1429 CrossRef CAS.
- H. Hakk and R. J. Letcher, Environ. Int., 2003, 29, 801 CrossRef CAS.
- M. Ike, M.-Y. Chen, E. Danzl, K. Sei and M. Fujita, Water Sci. Technol., 2006, 31, 203 Search PubMed.
- J.-H. Kang and F. Kondo, Chemosphere, 2002, 49, 493 CrossRef CAS.
- J. W. Voordeckers, D. E. Fennell, K. Jones and M. M. Häggblom, Environ. Sci. Technol., 2002, 36, 696 CrossRef CAS.
- J. Yin, S. Wang, G. Yang, G. Yang and Y. Chen, J. Chromatogr., B, 2006, 844, 142 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
