DOI:
10.1039/C0AY00531B
(Paper)
Anal. Methods, 2011,
3, 192-197
Received
1st September 2010
, Accepted 14th October 2010
First published on 15th November 2010
Abstract
The possibility to utilize nanocomposite films as easy-to-handle surfaces for surface assisted laser desorption ionization-mass spectrometry (SALDI-MS) of small molecules, such as pharmaceutical compounds, was evaluated. The signal-to-noise values of acebutolol, propranolol and carbamazepine obtained on the nanocomposite surfaces were higher than the values obtained on plain PLA surface showing that the nanoparticles participate in the ionization/desorption process even when they are immobilized in the polymer matrix. The advantages of nanocomposite films compared to the free nanoparticles used in earlier studies are the ease of handling and reduction of instrument contamination since the particles are immobilized into the polymer matrix. Eight inorganic nanoparticles, titanium dioxide, silicon dioxide, magnesium oxide, hydroxyapatite, montmorillonite nanoclay, halloysite nanoclay, silicon nitride and graphitized carbon black at different concentrations were solution casted to films with polylactide (PLA). There were large differences in signal intensities depending on the type of drug, type of nanoparticle and the concentration of nanoparticles. Polylactide with 10% titanium oxide or 10% silicon nitride functioned best as SALDI-MS surfaces. The limit of detection (LOD) for the study was ranging from 1.7 ppm up to 56.3 ppm and the signal to noise relative standard deviations for the surface containing 10% silicon nitride was approximately 20–30%. Scanning electron microscopy demonstrated in most cases a good distribution of the nanoparticles in the polymer matrix and contact angle measurements showed increasing hydrophobicity when the nanoparticle concentration was increased, which could influence the desorption and ionization. Overall, the results show that nanocomposite films have potential as surfaces for SALDI-MS analysis of small molecules.
Introduction
Matrix assisted laser desorption ionization-mass spectrometry (MALDI-MS) has become a routine tool for the analysis of high molecular weight synthetic polymers and biopolymers. However, usually MALDI analysis is limited to compounds having molecular weight above 500 g mol−1 due to interfering cluster ions which mask the signals of interest at low molecular weight range.1,2 Several approaches have been applied for analysis of small molecules (50 m/z to 500 m/z) to overcome the matrix problems including surface assisted laser desorption ionization-mass spectrometry (SALDI-MS). The traditional organic matrices were replaced with a surface that is able to absorb the light and thereby transfer energy to the analytes without generating cluster ions and therefore is applicable for analysis of low molecular weight compounds.3
Metal4–6 and metal oxide7,8 micro- and nanoparticles, and etched surfaces of silicon9 have been applied for SALDI-MS analysis of drugs, proteins and peptides. Zinc oxide nanoparticles also functioned as surfaces for analysis of low molecular weight (400 g mol−1) polypropylene glycol, polystyrene and polymethylmethacrylate.8 The sensitivity and molecular weight distributions were comparable to the MALDI-MS spectra obtained with 2,5-hydroxybenzoic acid (DHB) as matrix. Other suitable SALDI surfaces include graphite,10carbon nanotubes,11 activated carbon12 and graphitized carbon black13 (GCB). Moreover, oxidized graphitized carbon black particles were shown to be great desorption/ionization surfaces for analysis of pharmaceutical compounds such as propranolol that was extracted from Baltic Sea blue mussels.14 The oxidized graphitized carbon black contains carboxylic acid groups, which enhances the efficiency of the ionization/desorption process compared to the non-oxidized GCB. More recently, GCB disks were used for screening of pesticides in aqueous environment.15 Good sensitivity and reproducibility were in many cases shown offering high-speed analysis of low molecular weight compounds.
Recently silicon nitride nanoparticles were shown to be excellent SALDI surfaces for detection of drugs.16 Furthermore, porous polymer monoliths,17 poly(glycidyl methacrylate/divinylbenzene),18 inorganic materials,19surfactant suppressed matrices20 and high molecular weight matrices such as meso-tetrakis(pentafluorophenyl)porphyrin21 have been used for analysis of small molecules or proteins. A modification of pyrolytic highly oriented graphite polymer film with cationic polymer polyethyleneimine increased the signal intensity for perfluorooctanoic acid tenfold.22 Different nanomaterials have been shown to be suitable as surfaces for SALDI analysis of small molecules. However, nanoparticles are difficult to handle and lead to instrument contamination. This could be avoided if nanoparticles were immobilized into polymer matrix. The aim of our study was, thus, to investigate if immobilized nanoparticles still can facilitate the ionization/desorption process. In other words, if nanocomposite films would function as easy-to-handle surfaces for rapid SALDI-MS analysis of small molecules such as pharmaceutical compounds. Eight different nanoparticles, titanium dioxide, magnesium oxide, silicon nitride, graphitized carbon black, silicon dioxide, halloysite nanoclay, montmorillonite nanoclay and hydroxyapatite were, thus, incorporated into polylactide matrix and evaluated as SALDI surfaces for the analysis of human drugs.
Experimental
Materials
Polylactide (PLA) was PLA 5200 D from NatureWorks LLC. Eight different nanoparticles were evaluated for the development of new surfaces for SALDI-MS studies; hydroxyapatite (particle size < 200 nm, Aldrich Chemistry), silicon dioxide (SiO2) (particle size 5–15 nm (BET), 99.5% metal basis, Aldrich Chemistry), halloysite nanoclay (Aldrich Chemistry), montmorillonite nanoclay (surface modified with 35–45 wt% dimethyl dialkyl (C14–C18) amine, Aldrich Chemistry), titanium dioxide (TiO2) (particle size < 100 nm (BET), 99.9% metal basis, Aldrich Chemistry), magnesium oxide (MgO) (98%, Aldrich Chemistry), silicon nitride (Si3N4) (Sigma Aldrich), graphitized carbon black (LARA laboratori analitici di ricerca associati). Chloroform (HPLC grade, Fisher Scientific) was used in order to dissolve the polylactide and mix it with nanoparticles. The drugs carbamazepine, propranolol and acebutolol were all obtained from Sigma Aldrich in powder form. The methanol, acetonitrile and triflouroacetic acid (TFA) used to prepare the drug standard solutions were also from Sigma Aldrich. TFA is a toxic chemical that should be handled carefully. The container of TFA should be kept in a well ventilated place since inhalation or skin contact of TFA is very toxic. Additionally, it can cause burns, toxic gases and is highly flammable. Wear suitable gloves, eye and face protection.
Surface preparation
Polylactide pellets were dissolved in chloroform and mixed with 0, 5, 10 or 30 weight% of nanoparticles. The total mass of polylactide and nanoparticles was 5 g. The chloroform was heated up to 40–50 °C and stirred to dissolve the PLA. After two hours the solution was spread out on a glass mould. The chloroform was then allowed to evaporate in the fume hood after which the films were separated from the glass mould and put into a vacuum oven in order to remove the residual chloroform. Chloroform is a harmful solvent, irritating to skin and harmful by inhalation. Chloroform is considered inflammable, but hazardous products such as hydrogen chloride can be formed upon heating. In addition contact of chloroform with some pure metals such as aluminium and magnesium should be avoided. All work with chloroform should be done in fume hood using protective clothing and gloves. Fourteen different PLA nanocomposites and one pure PLA film were prepared to examine their potential as surface assisting materials for SALDI-MS analysis.
SALDI-MS analysis
The SALDI-MS spectra were obtained using a Voyager DE-STR time-of-flight mass spectrometer (Applied Biosystems) in reflector mode and a 337 nm nitrogen laser source. The acceleration voltage was set to 20 kV, grid voltage to 65% and the delay time to 150 ns. Three different standard solutions of acebutolol, carbamazepine and propranolol with a final concentration of 150 ppm were studied. The standard solutions were prepared first with a solution of methanol at a concentration of 2000 ppm and further diluted with 50% acetonitrile and 0.1% TFA. The nanocomposite surface was cut into 1.5 × 1.5 cm2 pieces. 3 µL of different drug solutions were spotted on top of each surface. The surfaces were then placed on a modified stainless steel MALDI plate. The solutions were allowed to dry and were then analyzed. The spectrum obtained for each drug on each surface is an accumulation of 50 shots from 5–10 different spots (on each solution spot) with 5–10 shots per spot. The maximum tolerable laser intensity (MTLI) was determined for each surface by gradually increasing the laser intensity and shooting within the sample spot, until a clean background was no longer observed. The limit of detection (LOD) was defined as signal-to-noise ratio = 3. The observed signal-to-noise ratio was averaged from the resulting spectra. The mass range was from 50 m/z to 500 m/z.
The surface morphology of all samples was studied by ultra-High Resolution FE-SEM (Hitachi S-4800). Surface samples were placed on a metal stub and sputter coated with a 2 nm layer of palladium.
Contact angle (CA)
All surfaces were tested for their hydrophilic properties with a CAM200 contact angle instrument. 4 µL of Milli-Q water were dropped on all surfaces in order to calculate the contact angle (KSV Instruments Ltd., Helsinki, Finland). The experiment was recorded with a video camera that took pictures of the droplet. The pictures were utilized to calculate the contact angel with the software that uses the Young–Laplace method.
Results and discussion
The possibility to utilize nanocomposite films as easy-to-handle surfaces for SALDI-MS analysis of pharmaceutical samples instead of the previously used free nanoparticles was evaluated. The nanocomposite films consisted of a polylactide matrix blended with different nanoparticles at different concentrations. The nanoparticles were silicon dioxide, titanium dioxide, halloysite nanoclay, montmorillonite nanoclay, silicon nitride, magnesium oxide, graphitized carbon black and hydroxyapatite. Standard solutions of propranolol, acebutolol and carbamazepine were used as test drugs to be analyzed on the surface of the different nanocomposite films. The surfaces were also characterized by scanning electron microscopy and contact angle measurements.
Nanocomposites as surfaces for SALDI-MS
As a first step the maximum tolerable laser intensity was determined for each surface to evaluate if a clean background can be obtained in the low m/z region without interference from surface cluster ions. MTLI value is important especially when high laser intensity is used for MS/MS or post-source decay fragmentation. At higher intensities than MTLI, the spectrum may be noisy and clusters from the surface could appear. A high MTLI value is a necessary property for the SALDI surfaces to be able to tolerate high irradiation intensities. PLA fulfilled this requirement. In addition use of PLA is favorable since it is a non-toxic polymer made from renewable resources. Fig. 1 shows a background spectrum acquired from the surface containing PLA mixed with 10% titanium dioxide. The spectrum is clean except for the occasionally detected ion at m/z = 64.1 corresponding to TiO fragment from TiO2 nanoparticles. There were some variations on the MTLI values depending on the nanoparticles, however, all surfaces tolerated high irradiation. The MTLI reproducibility was good, for example the relative standard deviation of MTLIs determined for PLA with 10% TiO2 from five spectra was ±3%. Pure PLA and the nanocomposites all showed to be potent surfaces for SALDI-MS from the point of view that no significant background ions were detected.
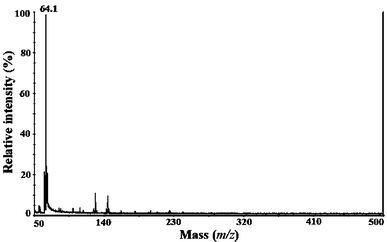 |
| | Fig. 1 The SALDI-MS background spectrum of PLA/10% TiO2 surface. | |
Averages of the signal-to-noise (S/N) values for the drugs on different surfaces were calculated and summarized in Table 1. The calculations were made on the proton adducts [M + H]+ since these were consistently present in most of the spectra. Fig. 2 shows the spectrum of acebutolol on the surface of PLA with 10% TiO2 showing the proton [M + H]+, sodium [M + Na]+ and potassium [M + K]+ adducts. Fig. 3 shows the carbamazepine spectrum desorbed and ionized from the same surface showing also the sodium [M + Na]+ and potassium [M + K]+ adducts in addition to the proton adduct. Carbamazepine spectra also had a fragmentation product at m/z 193.4 corresponding to the loss of carboxamide group. Fig. 4 demonstrates the mass spectrum of propranolol on the surface of PLA with 10% TiO2 showing again the hydrogen adduct [M + H]+. The ion at m/z = 64.1 seen in Fig. 1–4 corresponds probably to TiO fragment from TiO2. It was seen occasionally in the spectra taken on TiO2 nanocomposite surfaces. In the same way m/z 69.9 corresponding to radical fragment of [Si2N]˙ was seen occasionally in the spectra taken on silicon nitride nanocomposite surface. The appearance of this fragment was also reported in a previous study where free silicon nitride nanoparticles were used as SALDI-MS surfaces.16 The values presented in Table 1 show that nanocomposite films can be used as SALDI surfaces for detection and analysis of small molecules such as drugs. Moreover, Table 1 also indicates that pure PLA itself was not a good surface. The incorporation of nanoparticles in the polymer matrix gave higher S/N values compared to pure PLA. The nanoparticles, thus, facilitated the ionization and desorption of analytes even when they were immobilised in the polymer matrix. The acebutolol was detected on all the surfaces, except on pure PLA surface, while the other two drugs were not detected in all cases. There were large differences in the S/N ratios depending on the type and concentration of nanoparticles and type of drug. The surface containing 10% silicon nitride was by far the best surface for the analysis of all three drugs. It gave up to 9 times higher S/N values compared to pure steel plate and the limits of detection for all samples (LOD) were ranging from 1.7 ppm to 56.3 ppm. The standard deviations for S/N values on 10% silicon nitride as a surface were 20–30% depending on the analyte. This means the reproducibility was comparable to that obtained in an earlier study where the same compounds were determined on the surface of free silicon nitride nanoparticles.16 The films containing 10% TiO2, 10% montmorillonite nanoclay and 30% montmorillonite nanoclay were also good SALDI surfaces. In most cases the surfaces containing 10% nanoparticles gave higher S/N values than the surfaces containing 30% nanoparticles, an exception was the GCB surface where 30% GCB gave much better results than 10% GCB. All results were obtained by using intensities lower than the MTLI of the surface and the spectra in the low mass range were in most cases totally clear from cluster ions even when intensities close to MTLI were used. The MTLI values of the surfaces were high enough as the drugs were desorbed at intensities lower than the MTLI values.
Table 1 Average signal-to-noise (S/N) ratios for the drugs on the different surfaces
| Surface |
Acebutolol
|
Carbamazepine
|
Propranolol
|
Contact angle |
|
S/N [M + H]+ |
S/N [M + H]+ |
S/N [M + H]+ |
|
n.d. = not detected, * = also detected with sodium and/or potassium adduct.
|
| Pure PLA |
n.d.a |
20.1 |
n.d. |
65.5° |
| 5% TiO2 |
35* |
n.d. |
29* |
|
| 10% TiO2 |
158* |
26* |
18 |
66.2° |
| 30% TiO2 |
63* |
47* |
26 |
79.1° |
| 10% GCB |
8* |
n.d. |
n.d. |
67.8° |
| 30% GCB |
210* |
50* |
n.d. |
69.8° |
| 10% Silicon nitride |
175* |
211* |
260 |
70.3° |
| 30% Silicon nitride |
23 |
n.d. |
n.d. |
77.9° |
| 10% Montmorillonite |
25* |
80* |
226 |
77.7° |
| 30% Montmorillonite |
13 |
224 |
18 |
80.7° |
| 10% MgO |
25 |
n.d. |
8 |
77.3° |
| 10% Hallosite nanoclay |
47* |
35* |
12 |
71.3° |
| 10% Hydroxyapatite |
37 |
25 |
n.d. |
82.7° |
| 10% SiO2 |
23* |
84* |
11 |
73.6° |
| Steel plate |
21* |
121* |
39 |
|
 |
| | Fig. 2 SALDI-MS spectrum of acebutolol showing a proton adduct (m/z 337.3), sodium adduct (m/z 359.3) and potassium adduct (m/z 375.3) on the PLA/10% TiO2 surface. | |
 |
| | Fig. 3 SALDI-MS spectrum of carbamazepine showing a proton adduct (m/z 237.5), sodium adduct (m/z 259.4) and potassium adduct (m/z 275.5) on the PLA/10% TiO2 surface. The fragment ion at m/z 193.4 probably corresponds to the loss of carboxamide group. | |
The effect of surface and analyte hydrophobicity
Table 1 shows the contact angles (average values) for the different surfaces. Larger angle indicates a more hydrophobic surface. The contact angle measurements indicated that all the surfaces were rather hydrophobic. As seen in Table 1 all the contact angles for the nanocomposites were higher compared to pure PLA showing that the addition of nanoparticles made the surfaces more hydrophobic. Looking at the S/N and contact angle values in Table 1 indicates that certain amount of nanoparticles enhanced the desorption/ionization of the drugs but a larger amount led to greater hydrophobicity, which could contribute to the lower signal-to-noise values obtained for most spectra taken on the surfaces containing 30% nanoparticles. Considering the analyte hydrophobicity, in most cases the highest S/N values were obtained for acebutolol, which is the least hydrophobic of the studied analytes. Propranolol, the most hydrophobic analyte, gave generally the lowest S/N values. However, there were some exceptions to this trend and in the case of 10% montmorillonite nanoclay and 10% silicon nitride reverse behavior was observed as the signal intensity increased with increasing analyte hydrophobicity. Based on the rather small number of analytes and surfaces it is difficult to draw any definite conclusions on the effect of hydrophilicity. However, some indication is given that the hydrophobicity of the surface and analytes could influence the signal intensities. In earlier study the hydrophilicity of oxidized graphitized carbon was shown to affect the desorption of analytes.23
Distribution of the nanoparticles on the nanocomposite surfaces
The distribution of the nanoparticles on the nanocomposite surfaces was studied by SEM. Fig. 5 shows the SEM micrographs of selected surfaces. The different nanoparticles distributed in the polymer matrix are in most cases clearly seen as white dots. As mentioned earlier, the nanocomposites with 10% nanoparticles gave in many cases better S/N values and the reason for this could be deduced from the SEM micrographs. Fig. 5a and b show a rather homogenous distribution of titanium dioxide nanoparticles with spherical shapes in the 10% and 30% films, respectively. In correlation the SALDI results showed that both compositions functioned well as SALDI surfaces. Silicon nitride was the material that showed largest differences for the 10 and 30% blends. The nanocomposite with 10% silicon nitride gave by far the best results of all the studied surfaces, while the 30% composite was surprisingly one of the poorest SALDI surfaces. In correlation with the SALDI-MS results the SEM micrographs shown in Fig. 5c and d illustrate large differences for these two surfaces. Fig. 5c shows the silicon nitride nanoparticles as white dots on the 10% composite surface. The 30% silicon nitride surface (Fig. 5d), however, shows a more homogeneous surface structure where the individual nanoparticles cannot be clearly distinguished. The montmorillonite nanoclay surfaces in Fig. 5e and f again show rather evenly distributed nanoparticles for both 10% and 30% films. The results in Table 1 confirm that montmorillonite nanoclay in general showed good or excellent results at both compositions. Fig. 5g shows that the size of GCB nanoparticles varied from small to rather large ones, which could also be aggregates. In contrast to the other nanocomposites the surface containing 30% GCB nanoparticles showed considerably higher S/N values.
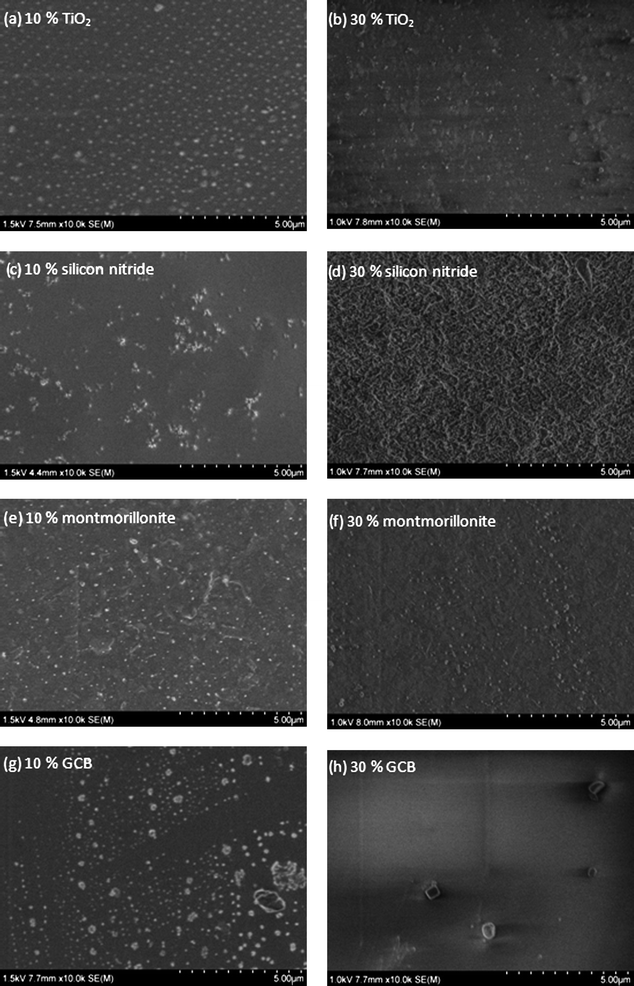 |
| | Fig. 5
SEM micrographs of the PLA nanocomposite films: (a) PLA/titanium dioxide 10%, (b) PLA/titanium dioxide 30%, (c) PLA/silicon nitride 10% (d) PLA/silicon nitride 30%, (e) PLA/montmorillonite nanoclay 10%, (f) PLA/montmorillonite nanoclay 30%, (g) PLA/GCB 10% and (h) PLA/GCB 30%. | |
Conclusion
It was shown that polymeric nanocomposite films function as surfaces for SALDI-MS analysis of small molecules such as drugs and could be used instead of free non-immobilized nanoparticles. Preventing source contamination, ease of operation and the potential for automation of sample spotting are the benefits of this new strategy. A clean background spectrum in the low m/z region was obtained for the pure nanocomposite surfaces. The signal-to-noise values obtained for acebutolol, carbamazepine and propranolol on the different nanocomposite surfaces were much higher compared to the values obtained on pure PLA surface showing that the nanoparticles contributed to the ionization/desorption process even when they were incorporated in the polymer matrix. However, there were large differences in the signal-to-noise ratios depending on the type of drug, type of nanoparticle and the concentration of nanoparticles in the polymer matrix. The 10% silicon nitride surface gave by far the highest signal-to-noise ratios of all the surfaces studied. The results show that nanocomposite films have potential as novel surfaces for SALDI-MS of low molecular weight compounds.
References
- G. Zhong and H. Lin, Anal. Bioanal. Chem., 2007, 387, 1939–1944 CrossRef CAS.
- L. H. Cohen and A. Gusev, Anal. Bioanal. Chem., 2002, 373, 571–586 CrossRef CAS.
- M. Tanaka, H. Waki, Y. Ido, S. Akita and T. Yoshida, Rapid Commun. Mass Spectrom., 1988, 2, 151–153 CAS.
- J. Wei, J. M. Buriak and G. Siuzdak, Nature, 1999, 399, 243–246 CrossRef CAS.
- M. Schurenberg, K. Dreisewerd and F. Hillenkamp, Anal. Chem., 1999, 71, 221–229 CrossRef.
- C.-L. Su and W.-L. Tseng, Anal. Chem., 2007, 79, 1626–1633 CrossRef CAS.
- L. Hua, J. Chen, L. Ge and S. N. Tan, J. Nanopart. Res., 2007, 9, 1133–1138 CrossRef CAS.
- T. Watanabe, H. Kawasaki, T. Yonezawa and R. Arakawa, J. Mass Spectrom., 2008, 43, 1063–1071 CrossRef CAS.
- T. Watanabe, K. Okumura, H. Kawasaki and R. Arakawa, J. Mass Spectrom., 2009, 44, 1443–1451 CrossRef CAS.
- J. Sunner, E. Dratz and Y.-C. Chen, Anal. Chem., 1995, 67, 4335–4342 CrossRef CAS.
- C. Pan, S. Xu, L. Hu, X. Su, J. Ou, H. Zou, Z. Guo, Y. Zhang and B. Guo, J. Am. Soc. Mass Spectrom., 2005, 16, 883–892 CrossRef CAS.
- Y.-C. Chen, J. Shiea and J. Sunner, Rapid Commun. Mass Spectrom., 2000, 14, 86–90 CrossRef CAS.
- M. Shariatgorji, N. Amini, G. Thorsen, C. Crescenzi and L. L. Ilag, Anal. Chem., 2008, 80, 5515–5523 CrossRef CAS.
- N. Amini, M. Shariatgorji and G. Thorsén, Anal. Chem., 2009, 20, 1207–1213 CAS.
- N. Amini, M. Shariatgorji, C. Crescenzi and G. Thorsén, Anal. Chem., 2010, 82, 290–296 CrossRef CAS.
- M. Shariatgorji, N. Amini and L. L. Ilag, J. Nanopart. Res., 2009, 11, 1509–1512 CrossRef CAS.
- D. S. Pepeterson, Q. Luo, E. F. Hilder, F. Svec and J. M. J. Frechet, Rapid Commun. Mass Spectrom., 2004, 18, 1504–1512 CrossRef CAS.
- I. Feuerstein, M. Najam-ul-Haq, M. Rainer, L. Trojer, R. Bakry, N. H. Aprilita, G. Stecher, C. W. Huck, G. K. Bonn, H. Klocker, G. Bartsch and A. Guttman, J. Am. Soc. Mass Spectrom., 2006, 17, 1203–1208 CrossRef CAS.
- T. Kinumi, T. Saisu, M. Takayama and H. Niwa, J. Mass Spectrom., 2000, 35, 417–422 CrossRef CAS.
- D. C. Grant and R. J. Helleur, Rapid Commun. Mass Spectrom., 2007, 21, 837–845 CrossRef CAS.
- F. O. Ayorinde, P. Hambright, T. N. Porter and Q. L. Keith, Jr, Rapid Commun. Mass Spectrom., 1999, 13, 2474–2479 CrossRef CAS.
- H. Kawasaki, N. Takahashi, H. Fujimori, K. Okumura, T. Watanabe, C. Matsumura, S. Takemine, T. Nakano and R. Arakawa, Rapid Commun. Mass Spectrom., 2009, 23, 3323–3332 CrossRef CAS.
- N. Amini, M. Shariatgorji and L. L. Ilag, J. Am. Soc. Mass Spectrom., 2009, 20, 1207–1213 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 




