HPLC method for the quantification of phenolic acids, phenolic aldehydes, coumarins and furanic derivatives in different kinds of toasted wood used for the ageing of brandies
Sara
Canas
*a,
António Pedro
Belchior
a,
Maria Isabel
Spranger
a and
Raúl
Bruno-de-Sousa
b
aINRB I.P., INIA/Dois Portos, 2565-191, Dois Portos, Portugal. E-mail: sara.canas@inrb.pt; Fax: +351 261712426; Tel: +351 261712106
bInstituto Superior de Agronomia, Departamento de Química Agrícola e Ambiental, Universidade Técnica de Lisboa, Tapada da Ajuda, Lisboa, Portugal
First published on 15th November 2010
Abstract
A simple, rapid and accurate HPLC method allowing the quantification of phenolic acids, phenolic aldehydes, coumarins and furanic derivatives in different kinds of toasted wood used in the ageing of wine brandies was developed and validated. The validated method presents good linearity, low limits of detection and quantification (LOD ranging between 0.03 µg L−1 for umbelliferone and 1.10 mg L−1 for ellagic acid, and LOQ ranging between 0.09 µg L−1 for umbelliferone and 3.66 mg L−1 for ellagic acid), high sensitivity, good repeatability (relative standard deviations ranging between 0.25% and 2.21%) and suitable recovery (mean values higher than 90% for all the concentrations added and compounds, except for vanillic acid). It can therefore be of a great interest for research studies and for quality control in routine analyses requested by the brandy producers, coopers and technicians, as a tool to know the low molecular weight composition of the toasted wood. The analysis of four different kinds of toasted wood (chestnut, Portuguese oak, Limousin oak and American oak) demonstrates the applicability of the method on the characterization and differentiation of the wood botanical species.
Introduction
The low molecular weight phenolic compounds and furanic derivatives are not present in the distillate, except furfural.1,2 They are released from the wood into the brandy during the ageing period, which corresponds to a minimum of six months for the brandies produced out of delimited regions, and two years for those produced within delimited regions in Europe (Reg. CE No. 110/2008).3 Their extraction rate depends on several factors, such as the botanical species and the geographical origin of the wood,4,5 the toasting intensity of the wood6,7 and the barrel size.8 Concerning the botanical species, oak is the most used and also the most studied wood, but other species, namely chestnut, was largely used for this purpose. Several studies show the great influence of the technological operations of the barrel-making process, mainly the toasting procedure, on the wood chemical composition, especially on low molecular weight compounds.9,10The quantification of low molecular weight phenolic compounds and furanic derivatives in different kinds of toasted wood used in the ageing of brandies is of considerable importance owing to their remarkable role on the chemical composition of the aged brandy and its evolution over the time,11–13 on its sensory properties,7,14,15 as well as on its nutraceutical quality.16,17 So, the knowledge on the wood chemical composition is crucial to brandy producers, technicians and coopers in order to choose the wood wisely and judiciously, as well as to researchers.
In the literature, the determination of these wood compounds by HPLC has been reported,4,9,18–20 but as far as we know was never published a validated analytical method for this purpose.
This paper reports the development and validation of an HPLC method for the quantification of phenolic acids, phenolic aldehydes, coumarins and furanic derivatives present in different kinds of wood used for barrel-making. The application of the method is demonstrated by the characterization and differentiation of four kinds of toasted wood—Portuguese oak, French oak from Limousin, American oak and Portuguese chestnut.
Experimental
Apparatus
The HPLC system Lachrom Merck Hitachi (Merck, Darmstadt, Germany) consisted of a quaternary pump L-7100, a column oven L-7350, a UV detector L-7400, a fluorescence detector L-7480 (connected in series to the UV detector) and an autosampler L-7250, and was coupled to a HSM D-7000 software for management, acquisition and treatment of data.The identification of chromatographic peaks was made by comparison of their relative retention times with those of external standards, as well as by their UV spectra. The chromatographic purity of the peaks and the UV spectra (200–400 nm) were performed using a Waters system (Milford, USA) equipped with a photodiode-array detector (Waters 996), with the same chromatographic conditions, managed by Millennium 2010 software.
Chemicals
The compounds listed in Table 1 were used as HPLC standard compounds during method development and validation. All of them were used without further purification. The standard calibration and internal standard solutions were prepared fresh prior to use with ethanol/water (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 v/v) adjusted to pH 4.2 with hydrochloric acid ACS 37% (Sigma-Aldrich, Steinheim, Germany).
45 v/v) adjusted to pH 4.2 with hydrochloric acid ACS 37% (Sigma-Aldrich, Steinheim, Germany).
| Compound | CAS number | Suppliera | Purity |
|---|---|---|---|
| a Fluka (Buchs, Switzerland) and Aldrich (Sigma-Aldrich, Steinheim, Germany). | |||
| 5-Hydroxymethylfurfural (HMF) | 67-47-0 | Fluka | >97 |
| Furfural (Furf) | 98-01-1 | Fluka | >99 |
| 5-Methyl furfural (5mfurf) | 620-02-0 | Fluka | 97 |
| Ellagic acid dehydrate (Ellag) | 476-66-4 | Fluka | >98 |
| Gallic acid monohydrate (Gall) | 149-91-7 | Fluka | >99 |
| Vanillic acid (Van) | 121-34-6 | Fluka | >97 |
| Syringic acid (Syrg) | 530-57-4 | Fluka | “Purum” |
| Ferulic acid (Ferul) | 537-98-4 | Fluka | >99 |
| Vanillin (Vanil) | 121-33-5 | Fluka | >99 |
| Syringaldehyde (Syrde) | 134-96-3 | Aldrich | 98 |
| Coniferaldehyde (Cofde) | 458-36-6 | Aldrich | 98 |
| Sinapaldehyde (Sipde) | 4206-58-0 | Aldrich | 98 |
| Umbelliferone (Umb) | 93-35-6 | Fluka | >98 |
| Scopoletin (Scop) | 92-61-5 | Fluka | >98 |
| 4-Hydroxybenzaldehyde (IS) | 123-08-0 | Aldrich | 98 |
All HPLC solvents used were gradient grade purchased from Merck. They were filtered through 0.45 µm membrane (Millipore, New Bedford, USA) and degasified in an ultrasonic bath.
Wood samples
The staves of four different kinds of wood were seasoned in the open air, at a cooperage industry (JM Gonçalves) in the Northern of Portugal. Their anatomical study allowed identifying the botanical species: Portuguese oak was Quercus pyrenaica Willd. (P), French oak from Limousin region was Quercus robur L. (L), American oak from the North America was a mixture of Quercus alba L./Quercus stellata Wangenh. and Quercus lyrata Walt./Quercus bicolor Willd. (A), and chestnut from the Northern of Portugal was Castanea sativa Mill. (C).20The staves of each kind of wood were used to make six 250 L barrels, which were divided in three groups. The barrels of each group were then submitted to heat treatment, with one of the following toasting levels—light (QL), medium (QM) and heavy (QF). The toasting process was controlled by the cooper, which is about 10 min for light toasting, 20 min for medium toasting and 25 min for heavy toasting, over a fire of corresponding wood offcuts. After the heat treatment, chips were cut from each barrel in order to get samples. So, a total of 24 samples of toasted wood were obtained from the two factorial design established: four wood botanical species × three toasting levels × two replications. The samples were identified by PQL, PQM, PQF, LQL, LQM, LQF, AQL, AQM, AQF, CQL, CQM, and CQF according to the corresponding barrel.
Wood extraction
In order to reproduce extraction conditions similar to those in brandy, the wood chips were grounded in a hammer-mill (Wiley, USA). The maceration of 50 g of wood powder (20 mesh) was carried out under rotary agitation for 180 min at 20 °C, with 1000 mL of an ethanol/water solution (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 v/v) adjusted to pH 4.2 with hydrochloric acid, as described by Caldeira et al.22 The wood extracts were filtered through a glass microfibre filter, Whatman GF/C 47 mm (Maidstone, UK), on a Büchner funnel (Sartorius, Goettingen, Germany). The effect of the filtration was studied in a previous assay performed in triplicate with standard solutions prepared with ethanol/water (55
45 v/v) adjusted to pH 4.2 with hydrochloric acid, as described by Caldeira et al.22 The wood extracts were filtered through a glass microfibre filter, Whatman GF/C 47 mm (Maidstone, UK), on a Büchner funnel (Sartorius, Goettingen, Germany). The effect of the filtration was studied in a previous assay performed in triplicate with standard solutions prepared with ethanol/water (55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 v/v) adjusted to pH 4.2 with hydrochloric acid. The filtration was optimized in order to achieve the maximum recovery of the studied compounds from the wood extracts. The range of variation (concentration before filtration − concentration after filtration) was: −2.2% for HMF, −2.4% for furfural, −2.5% for 5-methyl furfural, −11.3% for ellagic acid, −1.6% for gallic acid, −1.9% for vanillic acid, −5.7% for syringic acid, −1.6% for ferulic acid, −1.3% for vanillin, 0.1% for syringaldehyde, 0.1% for coniferaldehyde, 0.0% for sinapaldehyde, 0.4% for umbelliferone and −0.1% for scopoletin.
45 v/v) adjusted to pH 4.2 with hydrochloric acid. The filtration was optimized in order to achieve the maximum recovery of the studied compounds from the wood extracts. The range of variation (concentration before filtration − concentration after filtration) was: −2.2% for HMF, −2.4% for furfural, −2.5% for 5-methyl furfural, −11.3% for ellagic acid, −1.6% for gallic acid, −1.9% for vanillic acid, −5.7% for syringic acid, −1.6% for ferulic acid, −1.3% for vanillin, 0.1% for syringaldehyde, 0.1% for coniferaldehyde, 0.0% for sinapaldehyde, 0.4% for umbelliferone and −0.1% for scopoletin.
Statistical analysis
Regression analysis and analysis of variance were performed. In the application of the developed method, the comparison of the different averages was performed by calculation of least significant difference (LSD). All calculations were carried out using Statistica vs. '98 edition (Statsoft Inc., Tulsa, USA).Results and discussion
Method development
The HPLC method was developed and optimized in order to achieve the best resolution of the maximum number of peaks of low molecular weight phenolic compounds and furanic derivatives present in different kinds of toasted wood, in the shortest analysis time. Difficulty arose due to the chemical complexity of the toasted wood, as well as the high reactivity of phenolic compounds present. To solve this problem several factors were studied namely the instrumental operating conditions (column, temperature, flow rate, injection volume and wavelength), the solvents, the elution program, and the use of an internal standard (IS). The possibility of direct injection of the samples was also investigated.A 250 mm × 4 mm i.d. Lichrospher RP18 (5 µm) column (Merck) was chosen as the stationary phase with a 40 mm × 4 mm i.d. guard column of the same material, since it gave the best separation and resolution of chromatographic peaks of the wood extractable compounds analyzed and minimized the interferents. The other chromatographic conditions were progressively modified until their optimization: column temperature of 40 °C, flow rate of 1 mL min−1, and injection volume of 20 µL.
As regards the wavelength, at UV range the compounds were detected at their maximum absorption, i.e., 280 nm for phenolic acids and furanic derivatives, and 320 nm for phenolic aldehydes (Table 2). Since coumarins are present in wood at ppm levels, they were detected by fluorescence.18 Among the wavelengths tested, 325 nm (excitation) and 454 nm (emission) gave the best results.
| Peak no. | Retention timea/min | λ max /nm | Identification |
|---|---|---|---|
| a Mean ± standard deviation. b Máxima absorption wavelength. | |||
| 1 | 7.19 ± 0.15 | 271 | Gall |
| 2 | 12.50 ± 0.24 | 285 | HMF |
| 3 | 15.66 ± 0.38 | 233, 276 | Furf |
| 4 | 20.81 ± 0.37 | 221, 285 | IS |
| 5 | 22.09 ± 0.67 | 262, 295 | Van |
| 6 | 23.18 ± 0.71 | 295 | 5mfurf |
| 7 | 24.99 ± 1.05 | 276 | Syrg |
| 8 | 25.08 ± 0.57 | 238, 280–309 | Vanil |
| 9 | 27.73 ± 0.61 | 238, 309 | Syrde |
| 10 | 30.68 ± 1.04 | 236, 296–323 | Ferul |
| 11 | 32.50 ± 0.58 | 243, 306–342 | Cofde |
| 12 | 33.33 ± 0.85 | 246, 347 | Sipde |
| 13 | 39.06 ± 1.49 | 252, 365 | Ellag |
| 14 | 28.19 ± 0.50 | — | Umb |
| 15 | 30.19 ± 0.64 | — | Scop |
Concerning the elution program, a binary gradient was selected, using solvent A—water/formic acid (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v) and solvent B—methanol/water/formic acid (70
2 v/v) and solvent B—methanol/water/formic acid (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 28
28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 v/v/v) as follows: 0% B isocratic in 3 min, linear gradient from 0% to 40% B in 22 min, from 40% to 60% B in 18 min, 60% B isocratic in 12 min, linear gradient from 60% to 80% B in 5 min, 80% B isocratic in 5 min.
2 v/v/v) as follows: 0% B isocratic in 3 min, linear gradient from 0% to 40% B in 22 min, from 40% to 60% B in 18 min, 60% B isocratic in 12 min, linear gradient from 60% to 80% B in 5 min, 80% B isocratic in 5 min.
The separation of the compounds is very sensitive to the eluent composition probably due to their structural similarity. The slow increase of B (0–40%) in 22 minutes was necessary to achieve better sensitivity and separation of gallic acid, vanillic acid and syringic acid, and furanic derivatives at UV, while the less increase of B (40–60%) but with a high content of methanol in the next 18 minutes allows the separation of phenolic aldehydes, ferulic acid and ellagic acid at UV, and coumarins at fluorescence. Interferents of high polarity, namely hydrolysable tannins, are well separated in the beginning of the elution program (until 5 minutes) and others of less polarity are separated after 40 minutes.
Our study also demonstrates that there is no need for sample preparation prior to the HPLC analysis, which makes this method less expensive, less time-consuming, and more accurate, and therefore advantageous for the analysis of low molecular weight compounds of wood. So, the standard solutions and the wood extracts were added with the IS (4-hydroxybenzaldehyde, 20 mg L−1), filtered through 0.45 µm membrane (Titan, Scientific Resources Ltd, Gloucester, UK) and analyzed by direct injection.
Using the optimized conditions, well-resolved chromatograms of standards and toasted wood extracts were obtained (Fig. 1). Peak identification is shown in Table 2.
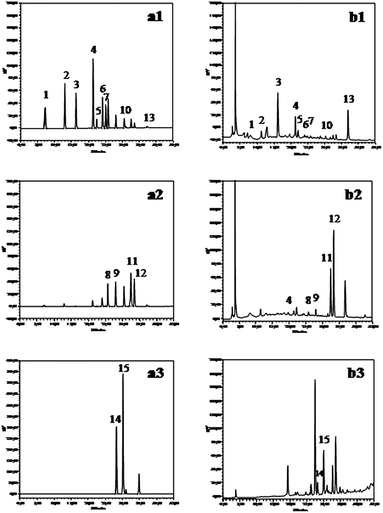 | ||
| Fig. 1 Chromatograms of standards (a) and extract of toasted wood (b), detected at 280 nm (1), 320 nm (2) and 325/454 nm (3). | ||
Method validation
The validation of the method was based on the following performance criteria: linearity,23 analytical limits,24 sensitivity,25 repeatability,26 and recovery.25| Rangea/mg L−1 | a | s a | b | s b | LODa/mg L−1 | LOQa/mg L−1 | S/V s mg−1 L−1 | |
|---|---|---|---|---|---|---|---|---|
| a µg L−1 for coumarins; a—intercept; b—slope; sa—standard deviation of the intercept; sb—standard deviation of the slope; LOD—limit of detection; LOQ—limit of quantification; and S—sensitivity. | ||||||||
| HMF | 0.32–20 | −1.24 × 102 | 2.40 × 103 | 9.14 × 104 | 2.67 × 102 | 0.10 | 0.32 | 0.09 |
| Furf | 0.05–20 | −3.07 × 103 | 4.95 × 103 | 2.06 × 105 | 4.74 × 102 | 0.02 | 0.05 | 0.21 |
| 5mfurf | 0.27–5 | 1.41 × 103 | 1.45 × 103 | 2.25 × 105 | 6.90 × 102 | 0.08 | 0.27 | 0.22 |
| Ellag | 3.66–200 | −7.36 × 104 | 2.80 × 104 | 2.75 × 104 | 2.64 × 102 | 1.10 | 3.66 | 0.028 |
| Gall | 3.21–300 | −2.36 × 104 | 2.10 × 104 | 3.30 × 104 | 1.55 × 102 | 0.96 | 3.21 | 0.03 |
| Van | 1.26–30 | −1.48 × 102 | 9.37 × 103 | 4.71 × 104 | 5.63 × 102 | 0.38 | 1.26 | 0.05 |
| Syrg | 2.96–100 | 1.64 × 103 | 1.14 × 104 | 1.82 × 104 | 2.07 × 102 | 0.89 | 2.96 | 0.02 |
| Ferul | 2.71–40 | −5.68 × 103 | 7.49 × 103 | 3.05 × 104 | 3.38 × 102 | 0.81 | 2.71 | 0.03 |
| Vanil | 0.70–50 | 1.69 × 102 | 7.50 × 103 | 3.84 × 104 | 2.73 × 102 | 0.21 | 0.70 | 0.04 |
| Syrde | 0.33–50 | −1.91 × 102 | 8.37 × 103 | 4.21 × 104 | 3.05 × 102 | 0.10 | 0.33 | 0.04 |
| Cofde | 0.15–50 | −1.48 × 103 | 1.37 × 104 | 6.76 × 104 | 4.98 × 102 | 0.05 | 0.15 | 0.07 |
| Sipde | 0.23–50 | −1.27 × 103 | 8.63 × 103 | 4.38 × 104 | 3.14 × 102 | 0.07 | 0.23 | 0.04 |
| Umb | 0.09–9 | −2.61 × 103 | 1.87 × 103 | 6.11 × 108 | 5.46 × 105 | 0.03 | 0.09 | 611.30 |
| Scop | 0.50–1500 | 4.33 × 104 | 5.51 × 104 | 2.08 × 107 | 8.01 × 104 | 0.15 | 0.50 | 20.79 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 45 v/v adjusted to pH 4.2 with hydrochloric acid in duplicate. Considering the most usual concentrations of the analyzed compounds in the different kinds of toasted wood used for the ageing of brandies, the limit of detection and the limit of quantification are low for all of them (Table 3).
45 v/v adjusted to pH 4.2 with hydrochloric acid in duplicate. Considering the most usual concentrations of the analyzed compounds in the different kinds of toasted wood used for the ageing of brandies, the limit of detection and the limit of quantification are low for all of them (Table 3).
| Repeatability | Recovery | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample A | Sample B | |||||||||
| x /mg L−1 | s /mg L−1 | r /mg L−1 | RSDr (%) | x /mg L−1 | s /mg L−1 | r /mg L−1 | RSDr (%) | Conc. addeda/mg L−1 | Recovery (%) | |
| a µg L−1 for coumarins; x—mean concentration of ten values; s—standard deviation; r—repeatability; and RSDr—relative standard deviation of repeatability. | ||||||||||
| HMF | 2.0 | 0.02 | 0.08 | 1.18 | 0.8 | 0.02 | 0.05 | 2.21 | 2 | 96.8 |
| Furf | 3.1 | 0.04 | 0.12 | 1.18 | 2.1 | 0.04 | 0.11 | 1.68 | 2 | 99.3 |
| 5mfurf | 2.7 | 0.03 | 0.09 | 1.01 | 0.3 | 0.00 | 0.01 | 1.42 | 0.3 | 93.8 |
| Ellag | 116.5 | 1.38 | 4.41 | 1.18 | 51.2 | 0.71 | 2.25 | 1.38 | 10 | 90.0 |
| Gall | 26.3 | 0.32 | 1.01 | 1.21 | 10.8 | 0.03 | 0.09 | 0.25 | 40 | 96.8 |
| Van | 3.3 | 0.05 | 0.17 | 1.60 | 1.4 | 0.03 | 0.10 | 2.19 | 4 | 82.8 |
| Syrg | 11.9 | 0.13 | 0.42 | 1.12 | 9.6 | 0.11 | 0.37 | 1.20 | 10 | 98.3 |
| Ferul | 6.0 | 0.02 | 0.07 | 0.36 | 7.5 | 0.06 | 0.20 | 0.82 | 5 | 98.9 |
| Vanil | 2.0 | 0.02 | 0.07 | 1.03 | 2.5 | 0.04 | 0.12 | 1.54 | 7.5 | 99.4 |
| Syrde | 2.9 | 0.01 | 0.03 | 0.31 | 3.9 | 0.03 | 0.09 | 0.76 | 7.5 | 100.4 |
| Cofde | 2.9 | 0.01 | 0.04 | 0.45 | 4.7 | 0.08 | 0.26 | 1.75 | 7.5 | 99.3 |
| Sipde | 8.4 | 0.03 | 0.10 | 0.36 | 8.9 | 0.16 | 0.53 | 1.85 | 5 | 99.3 |
| Umb | 0.1 | 2.03 × 10−3 | 6.48 × 10−3 | 1.48 | 0.03 | 4.03 × 10−3 | 1.29 × 10−2 | 1.42 | 1 | 96.3 |
| Scop | 54.1 | 1.15 | 3.67 | 2.13 | 1.10 × 103 | 11.2 | 35.9 | 1.02 | 200 | 99.1 |
Application of developed method
The analysis of four different kinds of toasted wood used in the ageing of brandies (Table 5) shows the application and the interest of the method, since the values are within the corresponding concentration ranges and are higher than the quantification limit of the method.| Effect | P | L | A | C | |
|---|---|---|---|---|---|
| a x ± s—mean ± standard deviation of six values; means followed by the same letter in a row are not significantly different at 0.05*, 0.01** or 0.001*** level of significance; and ns—without significant difference. b Indicates value <quantification limit; P—Portuguese oak; L—Limousin oak; A—American oak; and C—chestnut. | |||||
| HMF | * | 11.3 ± 5.2c | 5.6 ± 2.7a | 6.9 ± 5.5ab | 10.9 ± 5.6bc |
| Furf | ns | 2.1 ± 1.7 | 1.4 ± 0.5 | 1.1 ± 0.7 | 1.4 ± 1.1 |
| 5mfurf | * | 0.9 ± 0.8a | 1.3 ± 0.6a | 1.0 ± 0.6a | 1.9 ± 0.5b |
| Ellag | ns | (2.03 ± 1.54) × 102 | (1.67 ± 0.23) × 102 | 83.3 ± 9.9 | (1.71 ± 0.62) × 102 |
| Gall | *** | 68.0 ± 16.9b | 12.9 ± 3.5a | 21.4 ± 8.1a | (2.65 ± 0.29) × 102c |
| Van | *** | 3.9 ± 0.9a | 4.0 ± 1.5a | 8.1 ± 1.5a | 25.5 ± 10.8b |
| Syrg | *** | 10.6 ± 2.7b | 6.6 ± 1.8ab | 2.7b ± 2.0a | 53.1 ± 13.8c |
| Ferul | ** | 18.6 ± 1.5a | 18.5 ± 1.1a | 16.5 ± 1.2a | 25.8 ± 6.0b |
| Vanil | ns | 2.6 ± 1.6 | 2.7 ± 1.2 | 1.6 ± 1.2 | 19.8 ± 30.0 |
| Syrde | ns | 7.9 ± 5.9 | 9.6 ± 5.2 | 7.8 ± 7.7 | 12.1 ± 9.8 |
| Cofde | * | 9.1 ± 6.3ab | 12.0 ± 5.6b | 7.8 ± 6.0a | 6.3 ± 4.7a |
| Sipde | ns | 28.1 ± 23.6 | 32.8 ± 18.9 | 21.8 ± 19.5 | 20.2 ± 16.9 |
| Umb | *** | 0.8 ± 0.4a | 1.2 ± 0.3b | 1.3 ± 0.4b | 0.5 ± 0.4a |
| Scop | *** | 81.8 ± 8.1a | (4.38 ± 1.31) × 102b | (2.09 ± 0.39) × 103c | (1.14 ± 0.29) × 102a |
| Total | *** | (3.66 ± 1.87) × 102b | (2.74 ± 0.56) × 102ab | (1.82 ± 0.38) × 102a | (6.13 ± 0.86) × 102c |
The results of the variance analysis show significant differences in the contents of low molecular weight compounds between the different kinds of toasted wood analysed with this method, independently of variability within the wood botanical species and the variability induced by the toasting procedure.10 Independently of the toasting level, the majority of the analyzed compounds contribute to that differentiation. The total content of the analyzed compounds varies between 182.0 mg L−1 and 612.5 mg L−1, being higher in chestnut wood, and gradually lower from Portuguese oak to American oak.
The content of HMF is higher in Portuguese oak and is the lowest in Limousin oak. The chestnut and the American oak wood present intermediate contents of this furanic derivative.
The results emphasize the role of 5-methyl furfural, gallic acid, vanillic acid, syringic acid and ferulic acid as chemical markers, which contents are much higher in chestnut wood than in oak species studied. Gallic acid possibly results from the hydrolysis of wood gallotannins27 and digallic acid during the toasting of the wood.18 Vanillic acid is both produced by the oxidation of vanillin and guaiacylpropane units, while syringic acid is mainly originated by the oxidation of syringaldehyde during toasting.9
In addition, coniferaldehyde and scopoletin are chemical markers of Limousin oak and American oak wood, respectively, as observed in the corresponding untoasted woods.5 Regarding umbelliferone, there is a cluster formed by American oak and Limousin oak, which presents higher contents, and another cluster formed by Portuguese oak and chestnut, with lower contents.
Vanillin is a very important compound due to its contribution to vanilla sensory attribute in the aged brandies.28 In spite of its higher content in chestnut than in oak wood, as verified in untoasted wood,5 the high variability associated to the toasting procedure10 seems to justify the absence of significant differences between toasted woods.
The role of ellagic acid in wood differentiation was also modified by the toasting procedure, since this phenolic acid is a chemical marker of untoasted oak wood.5 In addition, ellagic and gallic acids are the most abundant compounds in toasted wood, as observed in the untoasted wood.5 Ellagic acid derives from ellagitannins degradation by the toasting procedure and its accumulation may also be a consequence of its high fusion point (>450 °C).29
The phenolic aldehydes are mainly formed during the toasting of the wood.9 Syringaldehyde and sinapaldehyde (with syringylpropane structure) are more abundant than vanillin and coniferaldehyde (with guaiacylpropane structure) in the toasted wood. This is probably due to the higher thermal stability of the syringylpropane configuration.
The HMF is the most important furanic derivative in the toasted woods. This result is unexpected, since HMF is a degradation product of cellulose during the toasting of the wood.30 In fact, the hemicelluloses are preferentially degraded during this process31 originating furfural, which is usually the most abundant furanic derivative in toasted wood used in cooperage.32 The great increase of furanic derivatives in toasted wood in relation to untoasted wood is of particular importance owing to their role as key-odorant compounds, positively correlated with dried fruits and caramel sensory attributes of the aged brandies.28
Conclusion
This study allowed the development of a method extremely sensitive, with good repeatability, recovery, and low limits of detection and quantification, which is appropriate for research and routine analysis of toasted wood used in the ageing of brandies. The analytical method could also be seen as a suitable method for the wood traceability in cooperage industry.A considerable advantage of this method is the good separation and quantification of the target compounds without sample preparation.
The application of the method in the analysis of the low molecular weight compounds of different kinds of toasted wood used in the ageing of brandies demonstrated its reliability.
Acknowledgements
The research was carried out with financial support of Project PAMAF 2052.References
- H. J. Jeuring and J. E. M. Kuppers, J. - Assoc. Off. Anal. Chem., 1980, 63, 1215–1218 CAS.
- I. Caldeira, O. Anjos, V. Portal, A. P. Belchior and S. Canas, Anal. Chim. Acta, 2009, 660, 43–52.
- Comission Regulation, Off. J. Eur. Communities: Legis., 2008, 16–54 Search PubMed.
- D. P. Miller, G. S. Howell, C. S. Michaelis and D. I. Dickmann, Am. J. Enol. Vitic., 1992, 43, 333–338 Search PubMed.
- S. Canas, M. C. Leandro, M. I. Spranger and A. P. Belchior, Holzforschung, 2000, 54, 255–261 CrossRef CAS.
- S. Canas, M. C. Leandro, M. I. Spranger and A. P. Belchior, J. Agric. Food Chem., 1999, 47, 5023–5030 CrossRef CAS.
- I. Caldeira, M. C. Clímaco, R. Bruno de Sousa and A. P. Belchior, J. Food Eng., 2006, 76, 202–211 CrossRef CAS.
- S. Canas, M. Vaz and A. P. Belchior, in Les eaux-de-vie traditionnelles d'origine viticole, ed. A. Bertrand, Lavoisier—TEC & DOC, Paris, 2008, pp. 143–146 Search PubMed.
- F. Sarni, M. Moutounet, J.-L. Puech and Ph. Rabier, Holzforschung, 1990, 44, 461–466 CrossRef CAS.
- S. Canas, A. P. Belchior, A. Falcão, J. A. Gonçalves, M. I. Spranger and R. Bruno de Sousa, Cienc. Tec. Vitivinic., 2007, 22, 5–14 Search PubMed.
- J. F. Guymon and E. A. Crowell, Qual. Plant. Mater. Veg., 1968, 16, 302–333 Search PubMed.
- J. Artajona, E. Barbero, M. Llobet, J. Marco and F. Parente, in Les eaux-de-vie traditionnelles d'origine viticole, ed. A. Bertrand, Lavoisier—TEC & DOC, Paris, 1991, pp. 197–205 Search PubMed.
- C. Viriot, A. Scalbert, C. Lapierre and M. Moutounet, J. Agric. Food Chem., 1993, 41, 1872–1879 CrossRef CAS.
- I. Caldeira, A. P. Belchior, M. C. Clímaco and R. Bruno de Sousa, Anal. Chim. Acta, 2002, 458, 55–62 CrossRef CAS.
- I. Caldeira, A. M. Mateus and A. P. Belchior, Anal. Chim. Acta, 2006, 563, 264–273 CrossRef CAS.
- C. Da Porto, S. Calligaris, E. Celotti and M. C. Nicoli, J. Agric. Food Chem., 2000, 48, 4241–4245 CrossRef CAS.
- S. Canas, V. Casanova and A. P. Belchior, J. Food Compos. Anal., 2008, 21, 626–633 CrossRef CAS.
- M. H. Salagoity-Auguste, C. Tricard, F. Marsal and P. Sudraud, Am. J. Enol. Vitic., 1987, 37, 301–303 Search PubMed.
- E. Cadahia, L. Munoz, B. F. Simón and M. C. J. Garcia-Vallejo, J. Agric. Food Chem., 2001, 49, 1790–1798 CrossRef CAS.
- B. F. Simón, M. Sanz, E. Cadahia, P. Poveda and M. Broto, J. Agric. Food Chem., 2006, 54, 8314–8321 CrossRef CAS.
- A. Carvalho, Cienc. Tec. Vitivinic., 1998, 13, 71–105 Search PubMed.
- I. Caldeira, R. Pereira, M. C. Clímaco, A. P. Belchior and R. Bruno de Sousa, Anal. Chim. Acta, 2004, 513, 125–134 CrossRef CAS.
- ISO 8466/1, International Organization for Standardization, Genève, 1990.
- J. Caporal-Gautier, J. M. Nivet, P. Algranti, M. Guilloteau, M. Histe, M. Lallier, J. J. N. Guyen-Huu and R. Russotto, STP Pharma Prat., 1992, 2, 205–239 Search PubMed.
- M. J. P. Monteiro and A. Bertrand, Feuillet Vert OIV, 1994, 970, 1–45 Search PubMed.
- ISO 5725/2, International Organization for Standardization, Genève, 1994.
- N. Vivas, L. Lagune and Y. Glories, Proceedings of the XVIIIth International Conference of the Groupe Polyphénols, Bordeaux, 1996, pp. 253–254 Search PubMed.
- I. Caldeira, R. Bruno de Sousa, A. P. Belchior and M. C. Clímaco, Cienc. Tec. Vitivinic., 2008, 23, 97–110 Search PubMed.
- Ph. Rabier and M. Moutounet, in Les eaux-de-vie traditionnelles d'origine viticole, ed. A. Bertrand, Lavoisier—TEC & DOC, Paris, 1990, pp. 220–230 Search PubMed.
- J. E. Hodge, in Chemistry and Physics of Flavour, ed. H. W. Schultz, E. A. Day and L. M. Libey, AVI Publis. Co, Westport, 1967, pp. 465–491 Search PubMed.
- D. Fengel and G. Wegner, in Wood: Chemistry, Ultrastructure, Reactions, Walter de Gruyter, Berlin, 1989, pp. 319–344 Search PubMed.
- C. S. Biermann, G. Mc Ginnis and T. P. Schultz, J. Agric. Food Chem., 1987, 35, 713–716 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |
