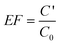Sensitive determination of cadmium in water, beverage and cereal samples by a novel liquid-phase microextraction coupled with flame atomic absorption spectrometry
Qiuhua
Wu
,
Chunxia
Wu
,
Chun
Wang
,
Xuena
Lu
,
Xiaomeng
Li
and
Zhi
Wang
*
Key Laboratory of Bioinorganic Chemistry, College of Science, Agricultural University of Hebei, Baoding, 071001, China. E-mail: zhiwang1963@yahoo.com.cn; Fax: +86-312-7521513; Tel: +86-312-7521513
First published on 24th November 2010
Abstract
A new microextraction technique termed dispersive liquid–liquid microextraction based on solidification of floating organic droplet (DLLME-SFO) coupled with flame atomic absorption spectrometry (FAAS) has been developed for the determination of trace cadmium in water, beverage and cereal samples. In the DLLME-SFO, cadmium was first complexed with 8-hydroxyquinoline, and then extracted into a small volume of the extraction solvent (1-dodecanol) with methanol as a dispersive solvent. Then, the extractant was analyzed by FAAS. The main factors affecting the DLLME-SFO, such as the type and volume of the extraction solvent and dispersive solvent, extraction time, sample volume, the amount of chelating agent, and salt addition were optimized. Under the optimum conditions, the established method showed a good linearity within a range of 1–50 ng mL−1, high enhancement factor (133), low limit of detection (0.3 ng mL−1), satisfactory repeatabilities (the relative standard deviation (RSD) = 3.7%, n = 6), and high recoveries (from 91.8 to 104.4%). The method was applied to determine the cadmium in three different samples (water, beverage and cereal samples) and two certified reference materials. The results indicated that the method can be applied for the determination of trace cadmium in real samples with complex matrices.
Introduction
Cadmium (Cd) is well known to be one of the most hazardous elements to human health with a biological half-life in the range of 10–30 years, mainly accumulated in the kidney, liver and lungs.1 This element was indicated as a human carcinogen and a causative factor in renal toxicity, pancreatic cancer, enhanced tumor growth, cardiovascular diseases, and in particular, hypertension.2 It can also inhibit the action of the zinc enzymes by displacing the zinc.3 Moreover, it can exist in the environment owing to emissions from industrial plants, manure and atmospheric deposition, and improper waste disposal.4,5 For humans, the main exposure to cadmium comes from daily intake of water, food and cigarette smoke. Therefore, sensitive, selective, and accurate monitoring of trace cadmium in environmental, biological and food samples is of significant importance from the human health and environmental point of view.Many modern instrumental methods including neutron activation analysis (NAA),6electrothermal atomic absorption spectrometry (ETAAS),7graphite furnace atomic absorption spectrometry (GFAAS),8,9 inductively coupled plasma emission spectrometry (ICP-EAS),10inductively coupled plasma mass spectrometry (ICP-MS),11 and flame atomic absorption spectrometry (FAAS)12,13 have been extensively used for the determination of trace cadmium in various matrix samples. Particularly, flame atomic absorption spectrometry (FAAS) is an attractive alternative of choice due to its simplicity, short analysis time, low cost, good precision and selectivity, and high sample throughput.14 However, the determination of trace cadmium ions in biological and food samples by FAAS is still difficult due to various factors, particularly their low abundance levels in the samples, insufficient sensitivity of the FAAS technique as well as the high complexity of the sample matrices.15 Therefore, for a sensitive determination of the trace amount of cadmium, an instrumental technique often has to be complemented by an effective sample preconcentration step.
For the determination of trace levels of cadmium, several sample preparation methods have been reported, including liquid–liquid extraction (LLE),16solid-phase extraction (SPE),17coprecipitation,18 ion-exchange,19cloud point extraction20,21 and flow injection on-line preconcentration technique.22 Nevertheless, these methods are somewhat time-consuming and tedious, and often require large amount of samples and toxic organic solvents. Recently, much attention has been paid to the development of miniaturized, more efficient and environment-friendly extraction techniques that could greatly reduce organic solvent consumption. For this purpose, several different types of liquid-phase microextraction (LPME) techniques have emerged as new attractive alternatives for the determination of trace levels of cadmium, such as single-drop micro-extraction (SDME),23 dispersive liquid–liquid microextraction (DLLME),24–26 and solidified floating organic drop microextraction (SFODME).27,28 However, they also suffer from some shortcomings. For example, in SDME, the instability of liquid drop is often encountered. In SFODME, the extraction time is usually prolonged and often needs about 30 min. In DLLME, high-density extraction solvents, which are toxic and environment-unfriendly, are often required.
In order to overcome these disadvantages, more recently, Leong et al. developed a novel microextraction technique termed dispersive liquid–liquid microextraction based on solidification of floating organic droplet (DLLME-SFO).29 It is based on the combination of DLLME with the solidification of floating organic drop. In this method, an appropriate mixture of dispersive solvent and extraction solvent with low-density and proper melting point is rapidly injected into an aqueous sample by syringe. A cloudy solution containing the fine droplets of the extraction solvent dispersed entirely in the aqueous phase is formed. The analytes in the sample are extracted into the fine droplets, which are further separated by centrifugation. The floated extractant droplet can be collected easily by solidifying it at low temperature because its melting point is near room temperature. Then it melted immediately and the melted organic solvent is used for analytes determination. The large contact surface area between the sample and the droplets of the extraction solvent speeds up the mass transfer of the analytes. Accordingly, the analysis time for DLLME-SFO can be as fast as DLLME and is much shorter than SFODME. DLLME-SFO not only can enhance the extraction efficiency but also can reduce the consumption of high-toxicity organic solvent. The advantages of DLLME-SFO method are simplicity of operation, rapidity, low cost, high recovery, and the use of lower toxicity extraction solvents compared with those used in DLLME.30,31 Furthermore, there is no need to use conical bottom glass tubes, which are easily damaged and hard to clean.
The main objective of this paper is to explore the applicability of the DLLME-SFO in combination with FAAS for the determination of trace cadmium in water, beverage and cereal samples. Various parameters that could affect the extraction efficiency were investigated and optimized. The result reveals that the developed method is simple, rapid, efficient, practical and environment-friendly. To the best of our knowledge, this may be the first report about the application of the DLLME-SFO method for the determination of trace cadmium.
Experimental
Reagents and materials
The stock standard solution of Cd2+ at a concentration of 1000 μg mL−1 was purchased from the National Institute of Standards (Beijing, China). Working standard solutions were prepared daily from the stock solutions by serial dilution. The chelating agent, 0.1 mol L−18-hydroxyquinoline (Beijing Chemistry Reagent Company, Beijing, China), was prepared in methanol. 1-Dodecanol, 1-undecanol, n-hexadecane, and bromohexadecane were obtained from Beijing Chemical Reagents Company (Beijing, China). Sodium chloride (NaCl), acetone, acetonitrile, tetrahydrofuran (THF), ethanol, and methanol were from Tianjin Fuchen Chemical Reagent Factory (Tianjin, China). The pH of solutions was adjusted by Britton-Robinson wide-ranging buffer solution, i.e., by mixing different ratios of M/25 mixture acids (H3PO4, CH3COOH and H3BO3) and sodium hydroxide (0.2 mol L−1). The water used throughout the work was double-distilled on a SZ-93 automatic double-distiller purchased from Shanghai Yarong Biochemistry Instrumental Factory (Shanghai, China). All chemicals used were of high-purity grade reagents.For water samples, the tap water was obtained from Yixian (Baoding, China), rain water from Wumazhuang (Baoding, China), Spring water, Smart, and Black tea from Jiaxing supermarket (Baoding, China). The cereal samples (rice and millet) were obtained from Damaiyuan supermarket (Baoding, China).
Cadmium standard solutions (1–10 μg mL−1) in nitric acid solution were prepared daily and their absorbance was read along with samples.
Instruments
A Hitachi Z-5000 atomic absorption spectrometer (Japan) equipped with Zeeman background correction and a cadmium hollow-cathode lamp was used for absorbance measurements at a wavelength of 228.8 nm according to the instrument instructions. The instrumental parameters were adjusted according to the manufacturer's recommendations. All pH measurements were carried out using a pHS-3C digital pH meter equipped with a combined glass-calomel electrode (Hangzhou Dongxing Instrument Factory, Hangzhou, China). A Model LD5-2A centrifuge (Beijing Jingli Instrument Factory, Beijing, China) was used for centrifugation.Sample preparation
Beverage and water samples were directly subjected to DLLME-SFO and subsequent measurements by FAAS.Cereal samples were pretreated by dry ashing digestion. The samples were pulverized and passed through a 250-μm sieve. 1.0 g of the sample was accurately weighed and put into a quartz crucible. The crucible was heated on an electric hot plate to make the sample undergo carbonation and then was transferred to muffle furnace at 500 °C for 3 h until the ash was close to white. After that, the ash was dissolved with 5 mL 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 nitric acid solution and was transferred to a 100 mL volumetric flask, to which distilled water was added to complete the volume. Then the solution was filtrated through a 0.45 μm filter under reduced pressure, and the resultant filtrate was further subjected to DLLME-SFO.
1 nitric acid solution and was transferred to a 100 mL volumetric flask, to which distilled water was added to complete the volume. Then the solution was filtrated through a 0.45 μm filter under reduced pressure, and the resultant filtrate was further subjected to DLLME-SFO.
DLLME-SFO procedure
For DLLME-SFO, an aliquot of 20.0 mL sample solution were placed in a 25 mL screw cap glass test tube. 2.0 mL Britton-Robinson wide-ranging buffer (pH = 7) and 0.75 mL 0.1 mol L−18-hydroxyquinoline were added into it. A mixed solution of 100 μL 1-dodecanol (extraction solvent) and 1 mL methanol (dispersive solvent) was rapidly injected into the above solution, and then the mixture was vortexed for 1 min. A cloudy solution that consists of very fine droplets of 1-dodecanol dispersed into aqueous sample was formed, and the analytes were extracted into the fine droplets. After centrifugation at 3500 rpm for 5 min, a liquid organic droplet was floated on the top of the test tube. The glass tube was then immersed in an ice bath for 5 min, and the floated organic droplet was solidified. Then the solidified extractant was transferred into a 1.5 mL conical vial where it melted rapidly at room temperature. 400 μL methanol was added to the extractant and the cadmium concentration was determined by FAAS.Calculation of enrichment factor
The enrichment factor (EF) was defined as the ratio between the cadmium concentration in the extractant (C′) and the initial concentration of the analyte (C0) in the aqueous sample.29 In order to evaluate the effect of some relevant experimental conditions on the extraction, EF was calculated according to the following equation:where EF is the enrichment factor, C0 is the initial analyte concentration in 20 mL aqueous samples, and C′ is the analyte concentration in the final mixture solution of the floating solvent and 400 μL methanol. C′ was calculated according to the linear regression equation of A = 0.09053 + 0.04743C (where A is absorbance and C is the concentration of cadmium in μg mL−1), which was established by a series of cadmium standard solutions at five concentration levels of 2.0, 4.0, 6.0, 8.0 and 10.0 μg mL−1.
Results and discussion
In the experiment, 20.0 mL of double-distilled water spiked with 20 ng mL−1Cd2+ ions was used to study the extraction performance under different experimental conditions. All experiments were performed in triplicate and the means of the results were used in plotting of the optimization curves.Selection of extraction and dispersive solvent
In DLLME-SFO, the selection of an appropriate extraction solvent is critical for obtaining an efficient extraction. The extraction solvent used for DLLME-SFO should have suitable physicochemical properties such as low volatility, low toxicity, lower density than water and a low melting point around room temperature (in the range from 10 to 30 °C). In addition, it must be immiscible with water and can dissolve the analytes better than water to promote the extraction of the analytes. Considering these requirements, 1-dodecanol, 1-undecanol, n-hexadecane and bromohexadecane were selected as the potential extraction solvents. On the other hand, the dispersive solvent can also play an important role for the extraction performance. The most important point for the selection of the dispersive solvent is its miscibility with both water and the extraction solvents. Moreover it should form a dispersive solution when injected together with the extraction solvent into aqueous samples. Thereby, acetone, methanol, THF, acetonitrile and ethanol were investigated. In the study, all combinations of using 1-dodecanol, 1-undecanol, n-hexadecane, and bromohexadecane as extraction solvent with acetone, methanol, ethanol, acetonitrile and THF as dispersive solvent were investigated. It was found that n-hexadecane and bromohexadecane can not be dissolved in any of the five dispersive solvents possibly due to their strong hydrophobicity. The combination of 1-dodecanol and methanol was found to provide the best extraction efficiency for cadmium. Consequently, further experiments were performed with 1-dodecanol as extraction solvent and methanol as dispersive solvent.Effect of extraction solvent volume
Optimization of the extraction solvent volume is necessary to achieve an efficient extraction. Experiments were performed with different volumes of 1-dodecanol ranging from 50 to 400 μL as the extraction solvent (Fig. 1). It was observed that the extraction efficiency was increased by increasing the volume of 1-undecanol up to 100 μL and then decreased when the volume of 1-undecanol was changed from 100 to 400 μL. The reason for this could be that with increased volume of 1-dodecanol, the volume of the resultant floating phase was increased, and subsequently the concentration of the analytes in the floating phase could be decreased due to the dilution effect when the volume of 1-dodecanol was higher than 100 μL. Therefore, 100 μL 1-dodecanol was chosen.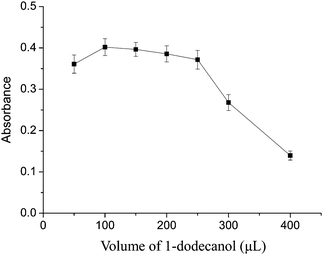 | ||
| Fig. 1 Effect of the volume of extraction solvent (1-dodecanol). Extraction conditions: sample volume, 20.0 mL; extraction solvent, 1-dodecanol; dispersive solvent, 1.5 mL methanol; pH, 6; 8-hydroxyquinoline amount, 1 mL. | ||
Effect of the dispersive solvent volume
The effect of the dispersive solvent volume on the extraction efficiency was also investigated since the variation of the volume of the dispersive solvent could affect the dispersion of the extraction solvent in water solution and thus affect the extraction efficiency. For the optimization of the methanol volume, a series of experiments were conducted using different volumes of methanol from 0.5 to 2.5 mL. The result is shown in Fig. 2. According to Fig. 2, the extraction efficiency increased when the volume of methanol was increased from 0.5 to 1.0 mL, and then slightly decreased. The reason could be that at a lower volume of methanol than 1.0 mL, the extraction solvent could not be dispersed well in the sample solution and a dispersive cloudy state could not be formed stably, therefore, the extraction efficiency was low. At higher volume of methanol, the solubility of the analytes in the aqueous sample solution could be increased, leading to a decrease of the extraction efficiency. Consequently, 1.0 mL methanol was chosen as the optimal dispersive solvent volume.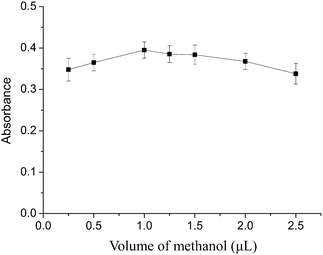 | ||
| Fig. 2 Effect of the volume of dispersive solvent (methanol). Extraction conditions: sample volume, 20.0 mL; extraction solvent, 100 μL 1-dodecanol; dispersive solvent, methanol; pH, 6; 8-hydroxyquinoline amount, 1 mL. | ||
Effect of sample solution pH
The pH of the sample solution is one of the essential factors for both the formation of metal-chelate and the subsequent DLLME-SFO. The dependence of the extraction efficiency on the solution pH was studied over the pH range from 2 to 12 while keeping the other parameters constant. The results illustrated in Fig. 3 revealed that the best extraction efficiency for cadmium was obtained at a pH about 7. Under this pH condition, the ionic and hydrophilic Cd2+ could be complexed well with 8-hydroxyquinoline, and produce a neutral oxinate chelate which is extractable into 1-dodecanol. In acidic medium, only a weak complexation of Cd2+ with 8-hydroxyquinoline occurred. On the other hand, at a higher pH than 7, metal hydroxide species such as soluble M(OH)+ and/or insoluble precipitate of M(OH)n were present. Hence, a pH of 7.0 was selected.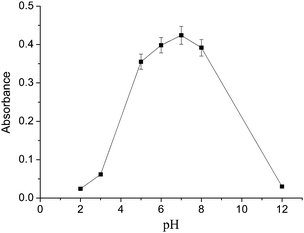 | ||
| Fig. 3 Effect of the sample solution pH on the extraction efficiency of the cadmium. Extraction conditions: sample volume, 20.0 mL; extraction solvent, 100 μL 1-dodecanol; dispersive solvent, 1 mL methanol; 8-hydroxyquinoline amount, 1 mL. | ||
Effect of the amount of 8-hydroxyquinoline
The chelating agent 8-hydroxyquinoline is one of the most widely used chelating reagents for cadmium ions in analytical chemistry.32,33 The amount of chelating agent is also a key variable to be optimized. The influence of the amount of 8-hydroxyquinoline (0.1 mol L−1) was investigated by changing its volume from 0.05 to 1.5 mL. As is shown in Fig. 4, the extraction efficiency was increased by the addition of the 8-hydroxyquinoline up to 0.75 mL, and then reached a plateau. Further addition of 8-hydroxyquinoline had no remarkable influence on the extraction efficiency, but the background absorbance was increased when the amount of 8-hydroxyquinoline was higher than 0.75 mL. Therefore, 0.75 mL of 0.1 mol L−18-hydroxyquinoline was added.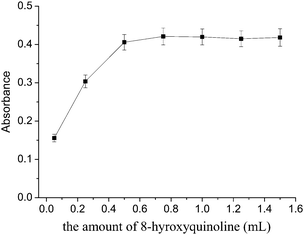 | ||
| Fig. 4 Effect of the amount of 8-hydroxyquinoline on the extraction efficiency of the cadmium. Extraction conditions: sample volume, 20.0 mL; extraction solvent, 100 μL 1-dodecanol; dispersive solvent, 1 mL methanol; pH, 8. | ||
Effect of reaction time and extraction time
Reaction time was defined as the time interval elapsed between the addition of 8-hydroxyquinoline solution and the addition of extraction solvent dissolved in dispersive solvent. Reaction time may affect the extent of the formation of metal-chelate, and therefore affect the extraction efficiency. In this study, the effect of reaction time on the extraction efficiency was examined in the range from 0 to 10 min. The selection of extraction time is also important in DLLME-SFO as in most extraction procedures. The extraction time was defined as the time interval elapsed between the start of the addition of the mixture of the extraction solvent and dispersive solvent to the sample and the time before centrifugation. The influence of extraction time on the extraction efficiency was studied over the range from 1 to 9 min. It was revealed that both the reaction and the extraction processes are rapid and independent of the time, which could be attributed to the fact that the equilibrium of the metal-chelate reaction can be achieved quickly, and the transfer of metal-chelate from the aqueous phase to the extraction solvent is also fast because of the large surface area between extraction solvent and the aqueous sample phase after the formation of a dispersive cloudy solution.Effect of salt addition
In order to evaluate the effect of salt addition, NaCl in concentrations ranging from 0 to 20% (w/v) was added to the aqueous solution while the other conditions were kept constant. Generally, the addition of salt can decrease the solubility of the analytes in the aqueous phase and promote the transfer of the analytes towards the organic phase due to the salting-out effect, and thus, improve the extraction efficiency. On the other hand, with increased concentration of NaCl, the resultant increased volume of the organic phase could reduce the enrichment factor. The experimental results indicated that the extraction efficiency was nearly constant by increasing the concentration of NaCl. Based on the experimental results, further extractions were carried out without the addition of NaCl.Effect of co-existing ions
The effects of common coexisting ions in real samples on the absorbance of cadmium were further evaluated to demonstrate the selectivity of the established method in the presence of potential interference ions. In this experiment, 20.0 mL solutions containing 250 ng mL−1 of cadmium and various amounts of foreign ions (Pb2+, Hg2+, Cu2+, Ni2+, Co2+, Zn2+, Mg2+, Fe3+, Mn2+, Ca2+, SO42−, Al3+, Cr3+, Na+, Cl−, K+, NO3−) were treated according to the above-established procedures for the interfering studies. The concentration ratio of cadmium to foreign ions was investigated starting at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000 and then was gradually decreased. An ion was considered as interfering when it caused 10% deviation for the determination of cadmium. The results (see Table 1) indicated that at the ratio of 1
1000 and then was gradually decreased. An ion was considered as interfering when it caused 10% deviation for the determination of cadmium. The results (see Table 1) indicated that at the ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000, among the foreign ions investigated, Na+, K+, Cl−, SO42−, NO3−, Ca2+ and Mg2+ had no significant interferences while the other remaining ions had interferences. The number of interfering ions deceased when the concentration ratio was decreased, and none of the foreign ions investigated had significant interferences when the concentration ratio was down to 1
1000, among the foreign ions investigated, Na+, K+, Cl−, SO42−, NO3−, Ca2+ and Mg2+ had no significant interferences while the other remaining ions had interferences. The number of interfering ions deceased when the concentration ratio was decreased, and none of the foreign ions investigated had significant interferences when the concentration ratio was down to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. For real-world samples, the contents of the above mentioned co-existing ions are usually lower than these interfering concentration levels.34,35 Therefore, the developed method is applicable for the determination of cadmium in real samples, and no further sample treatment or masking reagents are needed.
100. For real-world samples, the contents of the above mentioned co-existing ions are usually lower than these interfering concentration levels.34,35 Therefore, the developed method is applicable for the determination of cadmium in real samples, and no further sample treatment or masking reagents are needed.
Under the above optimized experimental conditions, the enrichment factor of the DLLME-SFO for cadmium was obtained as 133 (n = 3).
Linearity, repeatability and limits of detection (LODs)
Analytical characteristics of the DLLME-SFO method such as linear range, correlation coefficients, LODs and repeatability were all investigated for the determination of cadmium. Under the optimum conditions, the calibration graph was linear in the range of 1–50 ng mL−1. The corresponding regression equation was A = 0.01445 + 0.0038C (where A is absorbance and C is the concentration of cadmium in ng mL−1) with the correlation coefficient (r) of 0.9981. The LODs, defined as 3SB/m (where SB and m are the standard deviation of the blank and the slope of the calibration graph, respectively), was found to be 0.3 ng mL−1. Repeatability was carried out by spiking blank samples at the concentration of 10 ng mL−1, and the relative standard deviations (RSDs) for six replicate experiments were 3.7%.Real samples analysis
The performance of the presented method was verified by the analysis of cadmium in different samples i.e., water, beverage and cereal samples under the optimum conditions established above. As a result (see Table 2), no residues of cadmium were found in rain water, Smart, and millet. The concentration of cadmium in the tap water, spring water, black tea, and rice samples were determined to be 2.17, 6.44, 9.06, and 9.96 ng g−1, respectively. The recoveries of the method, expressed as the mean percentage between the amounts found and the ones spiked, were in the range from 91.8 to 104.4%, indicating a good performance of the DLLME-SFO method for the determination of cadmium in water, beverage and cereal samples and its independence of the sample matrix.| Sample | Certified/ng mL−1 | Added/ng mL−1 | Found/ng mL−1 | Recovery (%) |
|---|---|---|---|---|
| a nd: not detected. b R: recovery of the method. | ||||
| GSBZ50009-88 | 5 ± 0.6 | — | 4.83 ± 0.49 | 96.6 |
| 15 ± 1.8 | — | 14.09 ± 1.56 | 93.9 | |
| GSB07-1185-2000 | 10 ± 0.9 | — | 10.20 ± 0.69 | 102.0 |
| 30 ± 2.7 | — | 29.57 ± 2.55 | 98.6 | |
| Tap water | 0.0 | 2.17 ± 0.37 | — | |
| 5.0 | 6.78 ± 0.64 | 92.2 | ||
| 10.0 | 12.01 ± 0.43 | 98.4 | ||
| Rain water | 0.0 | nda | — | |
| 5.0 | 4.98 ± 0.31 | 99.6 | ||
| 10.0 | 9.44 ± 0.46 | 94.4 | ||
| Spring water | 0.0 | 6.44 ± 0.29 | — | |
| 5.0 | 11.03 ± 0.61 | 91.8 | ||
| 10.0 | 16.88 ± 0.57 | 104.4 | ||
| Smart | 0.0 | nda | — | |
| 5.0 | 4.63 ± 0.31 | 92.6 | ||
| 10.0 | 9.04 ± 0.57 | 90.4 | ||
| Black tea | 0.0 | 9.06 ± 0.46 | — | |
| 5.0 | 13.91 ± 0.44 | 97.0 | ||
| 10.0 | 18.55 ± 0.38 | 94.9 | ||
| Rice/ng g−1 | 0.0 | 9.96 ± 0.63 | — | |
| 5.0 | 14.69 ± 0.45 | 94.6 | ||
| 10.0 | 19.35 ± 0.37 | 93.9 | ||
| Millet/ng g−1 | 0.0 | nda | — | |
| 5.0 | 4.87 ± 0.35 | 97.4 | ||
| 10.0 | 9.53 ± 0.28 | 95.3 |
The accuracy of the proposed method was further evaluated by determining Cd2+ in National Standard Reference Material for Environment Water (GSBZ50009-88 and GSB07-1185-2000) after the appropriate dilution. The results presented in Table 2 are in good agreement with the certified values. Thus, the method is reliable for the determination of cadmium in real samples.
Comparison of DLLME-SFO with other sample preparation techniques
The presented DLLME-SFO method with other sample preparation techniques in combination with FAAS36–41 for the extraction and determination of cadmium was compared (Table 3). The comparison of the results indicated that the linearity, LOD, RSD and the enrichment factor of the DLLME-SFO method were comparable with, and in some cases better than those of the other methods except for the on-line LLE with APDC13 and the SPE with NaDDTC.37 However, it should be mentioned that this technique requires much shorter extraction time than SPE and ultrasound-assisted emulsification microextraction (USAEME). Furthermore, DLLME-SFO is easy to operate without the need of any additional special instrumentation. In comparison with DLLME, the extraction solvent of lower toxicity was used in DLLME-SFO. All these results demonstrate that DLLME-SFO is indeed simple, rapid, efficient, easy to use and environment-friendly.| Methods | Chelating agent | Linearity/ng mL−1 | LOD/ng mL−1 | RSD (%) | EF | Extraction time/min | Samples | Ref. |
|---|---|---|---|---|---|---|---|---|
| a APDC, ammonium pyrrolidine dithiocarbamate. b NaDDTC, sodium diethyldithiocarbamate. c CPE, cloud point extraction. d TAN, 1-(2-thiazolylazo)-2-naphthol. e USAEME, ultrasound-assisted emulsification-microextraction. f SME, solvent microextraction. g 8-QH, 8-hydroxyquinoline. | ||||||||
| On-line LLE | APDC a | 0.06–6.0 | 0.02 | 3.2 | 155 | 33 | water | 13 |
| IL-silica column | Dithizone | 1–800 | 0.60 | 3.7 | 75 | — | water | 36 |
| SPE | NaDDTC b | 30–110 | 0.08 | 1.9 | — | >60 | water, rock | 37 |
| On-line SPE | 1,10-Phenanthroline | 0.5–60 | 0.14 | 2.2 | 116 | >60 | human hair, water | 38 |
| CPE c | TAN d | 5–25 | 0.75 | 3.2 | 15.1 | 10 | water | 39 |
| USAEME e | NaDDTC | 10–600 | 0.91 | 2.6 | 95 | 20 | water | 40 |
| SME f | APDC | 50–7000 | 0.8 | 2.2 | 93 | 30 | meat, fish | 41 |
| DLLME-SFO | 8-QH g | 1–50 | 0.3 | 3.7 | 133 | 5 | water, beverage, cereal | this method |
Conclusions
A novel DLLME-SFO method coupled with FAAS has been developed for the preconcentration and determination of low levels of cadmium in real samples. The method was proved to be simple, rapid and environment-friendly. Compared with other conventional sample preparation methods, the method offers advantages such as cheapness, ease of operation, speed of analysis, minimum use of toxic organic solvent and high enrichment factor. The method was successfully applied to determine the low concentrations of cadmium in aqueous samples such as tap water and beverage, as well as in complex matrix samples such as rice and millet with a relatively high sensitivity and good repeatability.Acknowledgements
This research was supported by the Natural Science Foundations of Hebei (B2010000657) and the Scientific Research Foundation of Agricultural University of Hebei (Fsz200905).References
- T. D. A. Maranhão, D. L. G. Borges, M. A. M. S. da Veiga and A. J. Curtius, Spectrochim. Acta, Part B, 2005, 60, 667–672 CrossRef.
- A. C. Davis, P. Wu, X. Zhang, X. Hou and B. T. Jones, Appl. Spectrosc. Rev., 2006, 41, 35–75 CrossRef CAS.
- K. Pyrzynska and K. Kilian, Water Res., 2007, 41, 2839–2851 CrossRef CAS.
- E. L. D. Silva, E. M. Ganzarolli and R. R. U. de Queiróz, Anal. Lett., 2005, 38, 2089–2101 CrossRef.
- V. A. Lemos, C. G. Novaes, A. D. S. Lima and D. R. Vieira, J. Hazard. Mater., 2008, 155, 128–134 CrossRef CAS.
- N. Lavi and Z. B. Afassi, Analyst, 1990, 115, 817–822 RSC.
- E. I. V. Aonso, L. P. Gil, T. S. Cordero, A. G. Torres and J. M. C. Pavon, J. Anal. At. Spectrom., 2001, 16, 293–295 RSC.
- B. Rezaei, S. Meghdadi and R. F. Zarandi, J. Hazard. Mater., 2008, 153, 179–186 CrossRef CAS.
- T. Oymak, S. TokalIoglu, V. Yilmaz, S. Kartal and D. Aydin, Food Chem., 2009, 113, 1314–1317 CrossRef CAS.
- R. F. Lara, R. G. Wuilloud, J. A. Salonia, R. A. Olsina and L. D. Martinez, Fresenius J. Anal. Chem., 2001, 371, 989–993 CrossRef CAS.
- L. Xia, B. Hu, Z. C. Jiang, Y. L. Wu, L. Li and R. Chen, J. Anal. At. Spectrom., 2005, 20, 441–446 RSC.
- M. H. A. Melo, S. L. C. Ferreira and R. E. Santelli, Microchem. J., 2000, 65, 59–65 CrossRef CAS.
- A. N. Anthemidis, G. A. Zachariadis, C. G. Farastelis and J. A. Stratis, Talanta, 2004, 62, 437–443 CrossRef CAS.
- B. W. Yang and Z. F. Fan, At. Spectrosc., 2008, 29, 193–197 CAS.
- M. Ghaedi, F. Ahmadi and M. Soylak, J. Hazard. Mater., 2007, 147, 226–231 CrossRef CAS.
- J. Wang and E. H. Hansen, Anal. Chim. Acta, 2002, 456, 283–292 CrossRef CAS.
- S. Mustafa and N. Ibrahim, Chem. Anal., 2005, 50, 705–715.
- K. Atsumi, T. Minami and J. Ueda, Anal. Sci., 2005, 21, 647–649 CrossRef CAS.
- E. Pehlivan and T. Altun, J. Hazard. Mater., 2007, 140, 299–307 CrossRef CAS.
- C. G. Yuan, G. B. Jiang, Y. Q. Cai, B. He and J. F. Liu, At. Spectrosc., 2004, 25, 170–176 CAS.
- X. S. Zhu, X. H. Zhu and B. S. Wang, Microchim. Acta, 2006, 154, 95–100 CrossRef CAS.
- J. Nakajima, Y. Hirano and K. Oguma, Anal. Sci., 2003, 19, 585–588 CAS.
- A. N. Anthemidis and I. S. I. Adam, Anal. Chim. Acta, 2009, 632, 216–220 CrossRef CAS.
- A. N. Anthemidis and K. I. G. Ioannou, Anal. Chim. Acta, 2010, 668, 35–40 CrossRef CAS.
- E. Z. Jahromi, A. Bidari, Y. Assadi, M. R. M. Hosseini and M. R. Jamali, Anal. Chim. Acta, 2007, 585, 305–311 CrossRef.
- M. Rezaee, Y. Assadi, M. R. M. Hosseini, E. Aghaee, F. Ahmadi and S. Berijani, J. Chromatogr., A, 2006, 1116, 1–9 CrossRef CAS.
- S. Dadfarnia, A. M. H. Shabani and E. Kamranzadeh, Talanta, 2009, 79, 1061–1065 CrossRef CAS.
- M. R. K. Zanjani, Y. Yamini, S. Shariati and J.Å. Jönsson, Anal. Chim. Acta, 2007, 585, 286–293 CrossRef.
- M. I. Leong and S. D. Huang, J. Chromatogr., A, 2008, 1211, 8–12 CrossRef CAS.
- Y. Yamini, M. Rezaee, A. Khanchi, M. Faraji and A. Saleh, J. Chromatogr., A, 2010, 1217, 2358–2364 CrossRef CAS.
- H. Xu, Z. Ding, L. Lv, D. Song and Y. Q. Feng, Anal. Chim. Acta, 2009, 636, 28–33 CrossRef CAS.
- T. P. Rao and J. M. Gladis, Anal. Sci., 2002, 18, 517–524 CAS.
- S. Cerutti, M. F. Silva, J. A. Gásquez, R. A. Olsina and L. D. Martinez, Spectrochim. Acta, Part B, 2003, 58, 43–50 CrossRef.
- J. S. Carletto, R. M. Luciano, G. C. Bedendo and E. Carasek, Anal. Chim. Acta, 2009, 638, 45–50 CrossRef CAS.
- X. W. Chen, L. L. Huang and R. H. He, Talanta, 2009, 78, 71–75 CrossRef CAS.
- P. Liang and L. Peng, Talanta, 2010, 81, 673–677 CrossRef CAS.
- C. Duran, A. Gundogdu, V. N. Bulut, M. Soylak, L. Elci, H. B. Sentürk and M. Tüfekci, J. Hazard. Mater., 2007, 146, 347–355 CrossRef CAS.
- A. M. H. Shabani, S. Dadfarnia and Z. Dehghani, Talanta, 2009, 79, 1066–1070 CrossRef CAS.
- E. L. Silva and P. D. S. Roldan, J. Hazard. Mater., 2009, 161, 142–147 CrossRef CAS.
- J. J. Ma, X. Du, J. W. Zhang, J. C. Li and L. Z. Wang, Talanta, 2009, 80, 980–984 CrossRef.
- N. Gordarzi, J. Agric. Food Chem., 2009, 57, 1099–1104 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |