Rapid monitoring of alkaline phosphatase in raw milk using 1,1′-oxalyldiimidazole chemiluminescence detection†
Lucienne
Park
ab,
Hojae
Bae
cd,
Young-Teck
Kim
e and
Ji Hoon
Lee
*b
aPoolesville High School, Poolesville, MD 20837, USA
bLuminescent MD, LLC, Hagerstown, MD 21742, USA. E-mail: jhlee@luminescentmd.com; Fax: +1-301-193-9092; Tel: +1-301-393-9091
cCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, 65 Landsdowne Street, Cambridge, MA 02139, USA
dHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA 02139, USA
eDepartment of Packaging Science, Clemson University, Clemson, SC 29634, USA
First published on 22nd November 2010
Abstract
A simple biosensor with 1,1′-oxalyldiimidazole chemiluminescence (ODI-CL) detection capable of rapidly quantifying and screening alkaline phosphatase (ALP) in raw and pasteurized milk was developed as an indicator for confirming whether commercial milk is properly pasteurized. Fluorescein was formed when standards containing 1.0% milk with different activities of ALP and samples containing 1.0% raw milk were incubated with fluorescein diphosphate (FDP) for 15 min at room temperature. The relative CL intensity of fluorescein measured with the addition of 80 mM H2O2 and ODI formed from the reaction of 2.0 μM bis(2,4,6-trichlorophenyl) oxalate and 10.0 μM 4-methyl imidazole in ethyl acetate was proportional to the concentration of ALP in milk. The range (39∼2500 mU/L) of linear calibration curve (R2 = 0.998) for the quantification of ALP in milk using ODI-CL detection was wider than those using currently applied fluorescence and 1,2-dioxetane CL detections. Also, the limit of detection (3.7 mU/L) determined using the former detection, which has good precision, was lower than those reported using the latter detections. In conclusion, the cost-effective and highly sensitive biosensor with ODI-CL detection can be applied to monitor whether milk is pasteurized according to acceptable ALP activities threshold level (350 mU/L) for public safety newly adopted by US and EU and the internal investigation level (100 mU/L) proposed by EU.
Introduction
Alkaline Phosphatase (ALP) naturally existing in all raw milks is applied as an indicator to confirm whether milk has been pasteurized. This is because the temperature required to inactivate ALP is higher than those (62.8 °C for 30 min and 71.7 °C for 15 s) required to kill pathogens such as mycobacdvanceterium tuberculosis.1,2 In 2003, US Pasteurized Milk Ordinance (US PMO) adopted 350 mU/L ALP, as a new public health level, instead of 500 mU/L.3 Also, EU has revised the acceptable health level of ALP to 350 mU/L and adopted 100 mU/L ALP as an internal investigation level in 2006.3 Thus, recently, highly sensitive biosensors with fluorescence2,4,5 and 1,2-dioxetane chemiluminescence3 detection have been developed because it is impossible to sense ALP of 350 mU/L and lower using the conventional colorimetric method.2 Also, an immunoassay1,6,7 using antibody of ALP was developed specifically for quantitation of bovine milk alkaline phosphatase (ALP).Cost-effective 1,1′-oxalyldiimidazole chemiluminescence (ODI CL)8–11 capable of detecting fluorescent analytes is more sensitive than fluorescence detection with xenon or laser as a light source, powered by with high-voltage power supply. It is well-known that fluorescein diphosphate tetrammonium salt (FDP) is applied as a substrate for the quantification of ALP.12–14 When FDP reacts with ALP for a certain time fluorescein having high quantum yield is formed. This result indicates that a new biosensor with ODI CL detection, capable of quantifying and monitoring trace levels of ALP existing in raw and pasteurized milks, can be developed.
We developed the advanced biosensor with ODI CL detection for the quantification and monitoring of ALP in milk based on the reaction mechanism shown in Scheme 1. In this paper, we report in detail about the protocol of new analytical method as well as the differences between ODI CL detection and commercially available sensitive detections such as fluorescence and 1,2-dioxetane CL.
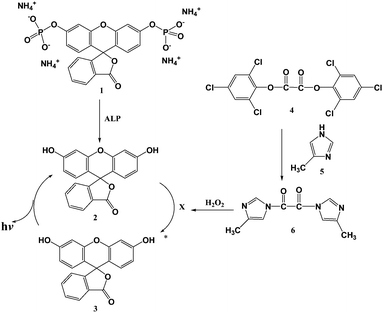 | ||
| Scheme 1 Quantification of ALP using ODI CL detection. 1. FDP, 2. Fluorescein under the ground state, 3. Fluorescein under the excited state, 4. Bis(2.4.6-trichlorophenyl) oxalate (TCPO), 5. 4-methylimidazole (4MImH), X: high energy intermediate capable of transferring energy to fluorescein. | ||
Experimental
Chemical and material
Alkaline phosphatase (ALP) from bovine intestinal mucosa was purchased from sigma (Saint Louis, MO, USA). 3.0% hydrogen peroxide and 0.05 M EDTA solutions were from VWR (Bridgeport, NJ, USA). Fluorescein Diphosphate tetrammonium salt (FDP) and Lysis buffer were purchased from Anaspec (Fremont, CA, USA). Magnesium Chloride (MgCl2) and Triton X-100 were from EMD (Gibbstown, NJ, USA). 4-Methylimidazole (4MImH, 98%) and Zinc Chloride (ZnCl2) were purchased from Alfa-Aesar (Ward Hill, MA, USA). Four different 1.0 M Tris-HCl buffers (pH 7.0, 7.5, 8.0, and 8.5) were purchased from Teknova (Hollister, CA, USA). Bis (2,4,6-trichlorophenyl) oxalate (TCPO) was purchased from TCI-America (Portland, OR, USA). Dimethyl sulfoxide (DMSO) was purchased from GI biochem (Boston, MA, USA). PhosphaGLO™AP Substrate containing 1,2-dioxetane was purchased from KPL (Gaithersburg, MD, USA). Glycerol was purchased from Mallinckrod Baker (Phillipsburg, NJ, USA). Pasteurized milk was purchased from a local food market. Raw milk was received from a local herd located in Hagerstown, Maryland, USA.Standard and sample preparation
ALP stock solution (25 U/L) was prepared daily in lysis buffer solution containing 50 mM Tris-HCl (pH 8.0) NaCl, 0.1% Triton X-100, and 1.0% pasteurized milk. Using the stock solution, 8 different standard solutions ranging between 0∼2500 mU/L were prepared daily by diluting in lysis buffer solution. In order to prepare sample solution, 0.1 ml of raw milk was diluted daily with 9.9 ml lysis buffer solution not containing 1.0% pasteurized milk. The rest of the raw milk was transferred into twenty 1.5 ml-centrifuge tubes and stored in a freezer (−20.0 °C).Preparation of FDP solution as a substrate
FDP (4.5 × 10−3 M) dissolved in DMSO was used as a stock solution. Using ten 1.0 ml amber vials, the stock solution was divided equally and stored in a freezer (−20.0 °C). Using aqueous solution containing 0.05M Tris-HCl pH 8.5, 0.1mM EDTA, 0.01 M MgCl2, 0.1 M ZnCl2, and 0.5% glycerol, four different concentrations (2.3, 4.5, 18.0, and 36.0 μM) of FDP working solutions were prepared. 4.5 μM FDP solution was mainly used in this research.Preparation of ODI CL reagents
1.0 mM TCPO was daily prepared in ethyl acetate as stock solutions. 5.0 mM 4MImH was prepared in ethyl acetate. It was stored under ambient condition for 2 weeks. 1,1′-oxalydi-4-methylimidazole (OD4MI), which is one of the ODI derivatives, was rapidly formed with the reaction of TCPO (100 μl) and 4MImH (100 μl) in 19.8 ml ethyl acetate.9–11 0.05 M H2O2 solution was prepared daily in iso-propyl alcohol.ODI CL detection
As shown in Fig. 1, ALP solution was mixed with FDP working solution and incubated for a certain amount of time (e.g., 2.5, 5.0, 15.0, 30.0, 55.0, 100 min) for producing fluorescein at room temperature (21.0 ± 2.0 °C). After the incubation, 10 μl of fluorescein added in a 12 × 75 mm borosilicate test tube was inserted into a sample holder of Lumat 9507 Luminometer with two dispensers (Berthold, Inc.). When the start button was pressed, the test tube containing fluorescein moved into the detection area. 25.0 μl of H2O2 was injected into the test tube through the first dispenser. After injecting 25.0 μl of ODI to the test tube through the second dispenser, relative ODI CL intensity was measured immediately for 0.5 s with an interval of 0.1 s.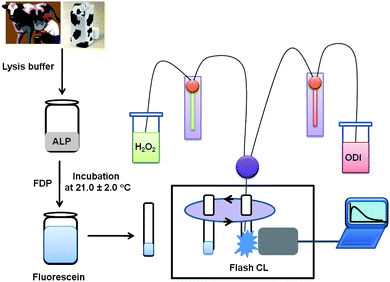 | ||
| Fig. 1 Quantification of ALP in milk using ODI CL detection. | ||
Fluorescence and 1,2-dioxetane CL detections
The mixture of 100 μl of ALP and 100 μl of FDP (4.5 μM) in a strip-well was inserted into a microplate reader (Infinite M 1000 of Tecan, Inc.) and incubated for 30 min at room temperature. After the incubation, the relative fluorescence intensity of fluorescein formed in each strip-well was measured at 518 nm emission wavelength (excitation wavelength: 494 nm).The mixture of 100 μl of ALP and 100 μl of 1,2-dioxetane, a CL reagent optimized by KPL in a strip-well, was inserted into a luminometer (Luminoskan Ascent of ThermoScientific, Inc.) and incubated for 15 min at room temperature. After the incubation, the relative CL intensity, integrated for 1.0 s, was read.
Results and discussion
Effect of temperature
As shown in Fig. 2, the results observed when standards containing lower than or similar to 500 mU/L ALP were mixed with 1.63 μM FDP and incubated at room temperature for 20 min were similar to those obtained at 36 °C. These results indicate that the reaction between FDP and lower ALP than 500 mU/L to produce fluorescein is not dependent on the temperature (between room temperature and 36 °C) within the acceptable error range. Relative CL intensity observed in the presence of 750 mU/L ALP at 36 °C was higher than that obtained at room temperature. However, relative CL intensity observed in the presence of 1000 mU/L ALP at 36 °C was lower than that obtained at room temperature. This results indicates that the concentration of fluorescein formed from the reaction between 16.3 μM FDP and 1000 mU/L ALP at 36 °C was so high that relative CL intensity of fluorecein observed in this condition was self-quenched.![Effect of temperature for incubating the mixture of ALP and FDP. Condition: [FDP] = 16.3 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, Incubation time: 20 min.](/image/article/2011/AY/c0ay00383b/c0ay00383b-f2.gif) | ||
| Fig. 2 Effect of temperature for incubating the mixture of ALP and FDP. Condition: [FDP] = 16.3 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, Incubation time: 20 min. | ||
Based on the results shown in Fig. 2, we conducted all the experiments at room temperature to determine an optimum condition capable of rapidly quantifying lower ALP than 350 mU/L with a wide calibration curve.
Effect of FDP concentration
As shown in Fig. 3, the sensitivity of ODI CL detection for the quantification ALP in raw milk is dependent on the concentration of FDP. The sensitivity of ODI CL in the presence of 4.5 μM FDP was better than those in the presence of lower or higher FDP than 4.5 μM. Relative CL intensities observed in the presence of 9.0 μM FDP after incubation of 20 min or longer were slightly lower than that obtained in the presence of 2.25 μM and higher than that measured in the presence of 18.0 μM. The results obtained with 9.0 μM aren't included in Fig. 3 in order to show more clearly the results measured with 2.25 and 18.0 μM. Relative CL intensities measured when 18.0 or 36.0 μM FDP was mixed with 350 mU/L ALP and incubated for 20 min or longer were self-quenched. Based on the results shown in Fig. 2, 4.5 μM FDP was selected for the quantification of trace levels of ALP in raw milk.![Effect of FDP concentration for the quantification of ALP using ODI CL detection at room temperature. Condition: [ALP] = 350 mU/L, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, Background measured in the absence of FDP was 2789 ± 350.](/image/article/2011/AY/c0ay00383b/c0ay00383b-f3.gif) | ||
| Fig. 3 Effect of FDP concentration for the quantification of ALP using ODI CL detection at room temperature. Condition: [ALP] = 350 mU/L, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, Background measured in the absence of FDP was 2789 ± 350. | ||
Effect of incubation time
As shown in Fig. 4, the sensitivity of ODI CL detection depends on the incubation time between FDP and ALP for producing fluorescein. With the increase of incubation time, relative CL intensity was enhanced. However, relative CL intensity measured when the mixture of higher ALP than 1500 mU/L and 4.50 μM FDP for 30 min was self-quenched.![Effect of incubation time for the quantification of ALP at room temperature. Condition: [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM.](/image/article/2011/AY/c0ay00383b/c0ay00383b-f4.gif) | ||
| Fig. 4 Effect of incubation time for the quantification of ALP at room temperature. Condition: [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM. | ||
Table 1 shows that the ratio of between signal and background, which was measured in the presence of 4.50 μM FDP and 350 mU/L of ALP (Cut-off value of US PMO), depends on the incubation time to form fluorescein from the reaction of FDP and ALP. Using the ratio (4.5) of signal to noise obtained when the mixture of FDP and ALP was incubated for only 2 min 30 s, it is possible to rapidly monitor whether a milk sample contains higher ALP than 350 mU/L. However, we expect that 15 min-incubation is more accurate than 2 min 30 s-incubation to quantify and monitor raw and pasteurized milks. This is because the ratio of signal to noise determined after 15 min-incubation is about 5 times higher than that obtained after 2 min 30 s-incubation. The signal to background ratio obtained with the 55 min-incubation was about 5 times higher than that with the 15 min-incubation. These results indicate that the sensitivity of ODI CL detection for the quantification of ALP after 55 min of incubation is better than that after 15 min of incubation. However, the sensitivity enhancement of ODI CL detection determined with longer incubation time than 15 min is not as important as rapid monitoring of ALP existing in raw and pasteurized milks. Table 1 shows that it is not necessary to incubate for longer than 55 min because relative CL intensity measured with the longer incubation than 55 min is self-quenched.
Effect of pH
In general, the pH for measuring the highest CL intensity using ODI CL detection is 6.5.9,11 With the increase of basicity, ODI CL intensity is decreased.9,11 However, Fig. 5 indicates that pH of Tris-HCl buffer for enhancing the sensitivity of ODI CL detection for the quantitation of ALP is 8.5. This result indicates that the concentration of fluorescein formed from the reaction of ALP and FDP at pH 8.5 is much higher than that at pH 7.0 under the experimental condition shown in Fig. 5. Based on the results, we selected pH 8.5 Tris HCl buffer to determine the activity of ALP in raw milk using a linear calibration curve.![Effect of pH for the quantification of ALP at room temperature. Condition: [ALP] = 350 mU/L, [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, incubation time: 15 min.](/image/article/2011/AY/c0ay00383b/c0ay00383b-f5.gif) | ||
| Fig. 5 Effect of pH for the quantification of ALP at room temperature. Condition: [ALP] = 350 mU/L, [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, incubation time: 15 min. | ||
Comparison of ODI CL detection with fluorescence and 1,2-dioxetane CL detections
As shown in Fig. 6, a linear calibration curve for quantifying ALP using ODI CL detection was obtained under the optimum condition determined using the experimental results described above. The calibration curve of ODI CL detection (39∼2500 mU/L) was much wider than those of fluorescence (156∼1250 mU/L) and 1,2-dioxetane CL (156∼625 mU/L) detections. The inset shown in Fig. 6 indicates that ODI CL detection can sense ALP as low as 39 mU/L. The linearity (y = 0.0051x + 0.1836, R2 = 0.9975) of calibration curve of ODI CL detection is also better than those of fluorescence (y = 0.0041x + 0.3376, R2 = 0.9948) and 1,2-dioxetane CL (y = 0.0019x + 0.7379, R2 = 0.9904) detections. In addition, the limit of detection (LOD = background + 3σ, 3.7 mU/L) of ODI CL detection was much lower than those of fluorescence (48.2 mU/L) and 1,2-dioxetane CL (21.1 mU/L) detections. σ is the standard deviation determined with backgrounds (n = 20) measured in the absence of FDP. The limit of quantification (LOQ = background + 10σ, 20 mU/L) of ODI CL detection determined for sensing of ALP using highly sensitive ODI CL detection is 17.5 times lower than 350 mU/L.![Linear calibration curve for the quantification of ALP Condition at room temperature: [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, [1,2-dioxetane] = concentration optimized by KPL.](/image/article/2011/AY/c0ay00383b/c0ay00383b-f6.gif) | ||
| Fig. 6 Linear calibration curve for the quantification of ALP Condition at room temperature: [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, [1,2-dioxetane] = concentration optimized by KPL. | ||
In addition, the total analytical time of ODI CL detection for the quantification of ALP in raw milk is much faster than that of immunoassays with absorbance detection.6,7
Table 2 shows that milk samples containing a wide range of ALP can be quantified and monitored using ODI CL detection. Also, the precision of ODI CL detection was better than those of fluorescence and 1,2-dioxetane CL detections.
| SAMPLE NUMBER | ODI CL | Fluorescence | 1,2-Dioxetane CL | |||
|---|---|---|---|---|---|---|
| Activity (mU/L) | CV (%) | Activity (mU/L) | CV (%) | Activity (mU/L) | CV (%) | |
| a N = 7, ND: non-decision, NA: not available, ODI CL: [FDP] = 4.50 μM, [TCPO] = 10 μM, [H2O2] = 50 mM, [4MImH] = 50 μM, Incubation time: 15 min, Fluorescence: [FDP] = 4.50 μM, Incubation time: 30 min, 1,2-dioxetane CL: [FDP] = 4.50 μM, optimized 1,2-dioxetane purchased from KPL, Incubation time: 15 min. | ||||||
| A | 510.3 | 3.1 | 459.8 | 6.6 | 477.4 | 5.6 |
| B | 1085.9 | 3.5 | 892.2 | 8.3 | ND | NA |
| C | 2051.5 | 4.0 | ND | NA | ND | NA |
Based on the results shown in Fig. 6 and Table 2, we expect that ODI CL detection can be applied as an advanced analytical method capable of quantifying and monitoring ALP existing in raw and pasteurized milks under the new regulations adopted by US as well as EU for public safety.
Conclusions
Using ODI CL detection and FDP, we developed an analytical method capable of rapidly quantifying and monitoring trace levels of ALP existing in milk according to the ALP activities threshold level (350 mU/L) recently revised by US and EU. The sensitivity and precision of ODI CL detection with a wide linear calibration curve were better than those of the widely used fluorescence and 1,2-dioxetane detections. In addition, ALP, which is an enzyme, is widely used as an analytical marker of highly sensitive enzyme immunoassay (EIA). Based on the results obtain in this research, we expect that ODI CL detection can be applied as a new biosensor of EIA using ALP.Notes and references
- C. Payne and R. A. Wilbey, Int. J. Dairy Technol., 2009, 62, 308 CrossRef CAS.
- C. C. Chen, Y. C. Tai, S. C. Shen, Y. Y. Tu, M. C. Wu and H. M. Chang, Food Chem., 2006, 95, 213 CrossRef CAS.
- R. S. Salter and J. Fitchen, J. AOAC Int., 2006, 89, 1061 CAS.
- J. Montalibet, K. I. Skorey and B. P. Kennedy, Methods, 2005, 35, 2–8 CrossRef CAS.
- K. Yoshitomi, Int. J. Food Sci. Technol., 2004, 39, 349 CrossRef CAS.
- N. Geneix, E. Dufour, A. Venien and D. Levieux, J. Dairy Res., 2007, 74, 290 CrossRef CAS.
- D. Levieux, N. Geneix and A. Levieux, J. Dairy Res., 2007, 74, 296 CrossRef CAS.
- J. H. Lee, J. C. Rock, S. B. Park, M. A. Schlautman and E. R. Carraway, J. Chem. Soc., Perkin Trans. 2, 2002, 802 RSC.
- J. H. Lee, J. T. Je, M. A. Schlautman and E. R. Carraway, Chem. Commun., 2003, 270 RSC.
- J. H. Lee, J. Je, J. Hur, M. A. Schlautman and E. R. Carraway, Analyst, 2003, 128, 1257–61 RSC.
- J. H. Lee, J. Je, A. Tartaglia, J. Hur, M. A. Schlautman and E. R. Carraway, J. Photochem. Photobiol., A, 2006, 182, 28 CrossRef CAS.
- Q. G. Wang, Y. M. Wang, G. Luo and W. S. B. Yeung, J. Liq. Chromatogr. Relat. Technol., 2001, 24, 1953 CrossRef CAS.
- Y. Murakami, T. Morita, T. Kanekiyo and E. Tamiya, Biosens. Bioelectron., 2001, 16, 1009 CrossRef CAS.
- H. Nakajima, S. Ishino, H. Masuda, T. Shimosaka, T. Nakagama, T. Hobo and K. Uchiyama, Chem. Lett., 2005, 34, 358 CrossRef CAS.
Footnote |
| † This project was performed based on the intern program (LMD 09-03) of Luminescent MD, LLC. |
| This journal is © The Royal Society of Chemistry 2011 |
