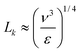Diatoms: Self assembled silica nanostructures, and templates for bio/chemical sensors and biomimetic membranes
Wenrong
Yang
*ab,
Pascal J.
Lopez
c and
Gary
Rosengarten
*a
aSchool of Mechanical Engineering, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: wenrong.yang@sydney.edu.au; g.rosengarten@unsw.edu.au
bAustralia centre for microscopy & microanalysis, The University of Sydney, Sydney, NSW 2006, Australia
cCNRS UMR-8197, Ecole Normale Supérieure, 46 rue d'Ulm, 75005, Paris, France
First published on 7th October 2010
Abstract
In this review we highlight recent advances in the understanding of biosilica production, biomodification of diatom frustules and their subsequent applications in bio/chemical sensors, and as a model membrane for filtration and separation.
 Wenrong Yang | Wenrong Yang received his PhD degree in chemistry in 2002 from the University of New South Wales under the mentorship of Prof. Justin Gooding and Prof. Bryn Hibbert. He worked then at Australian Commonwealth Scientific and Industrial Research Organisation (CSIRO) as a CSIRO post-doctoral fellow before returning to UNSW in 2005. He was awarded a University of Sydney Research Fellowship and joined the University of Sydney in 2007. His research interest involves nanostructuring surfaces to provide them with unique functionality, nanomaterial-based biosensors and molecular electronics. |
 Pascal J. Lopez | Pascal Jean Lopez heads the Diatom Biomineralization and Morphogenesis group at the Ecole Normale Supérieure, Paris (France). He obtained is Ph.D. in Microbiology from the University of Paris IX-Orsay (France) and completed a post-doctoral position in the Gene Expression Unit at the European Molecular Biology Laboratory, Heidelberg (Germany). His research interests include genomic and cell biology studies of the impact of environmental factors on silica morphogenesis, and the development of biophysical approaches to characterize biomaterials. |
 Gary Rosengarten | Gary Rosengarten heads the micro and nanotransport phenomena group in the School of Mechanical and Manufacturing Engineering at the University of New South Wales, Australia. He completed honours degrees in Physics and Mechanical Engineering at Monash University, and a Ph.D. at the University of NSW in Mechanical Engineering. His research interests include fluid flow and heat transfer for micro- and nano-systems specifically related to energy systems and surface effects in micro-fluidics. |
Introduction
Diatoms are unicellular marine organisms that have an amazing self-assembled micro- and nanoporous silica outer thecae (called a frustule). They use photosynthesis as an energy source and convert dissolved carbon dioxide into sugars. They are essential to all life on earth, producing in the order of 20% of the oxygen we breathe by capturing atmospheric carbon.1 When diatoms die, because their silica shell ensures they are denser than water, they sink to the bottom of the ocean, thus, in effect, producing a massive carbon dioxide sink. As diatoms are photosynthetic they can live within the top ∼200 m of water where sunlight can penetrate (known as the Euphotic zone), but generally reside much closer to the surface. They are the predominant photosynthetic organism in both fresh and sea water and have even been attributed to cause the largest global cooling event in the last 100 million years.2Diatoms also have a rich research history due to their unique silica shell which acts as an indicator for water quality,3–5 and because of their abundance and critical roles in the earth's carbon cycle,2,6 because they are a model for self assembled nanotechnology and biometics7–10 and because very recently they have been proposed as a sustainable source of biofuel.11–14 The genome of three simple diatoms has even been sequenced indicating, amongst other things, the genes that help in biosilica production and those that allow diatoms to thrive in aquatic environments.15,31
In the last ten years or so, since the explosion of nanotechnology as a separate science, there have been various reviews focusing on the unique diatom biosilica frustule (for example references 11 and 16–22) and the possible insights we can gain from their nano-structure. In this review we focus specifically on recent advances in the understanding of the biosilica production, biomodification of diatom frustules and their subsequent applications in biosensors, and on the interaction of fluids with the frustule and its role as a membrane.
Diatom structure
There are, purportedly, over 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 species of diatoms23 but their classification and taxonomy is still under debate.24,25 Their shapes and sizes vary from circular to triangular and from approximately five micrometres to a few hundred micrometres. They are broadly categorised into those having radial symmetry of the nanostructured pore pattern (centric) or bilateral symmetry (pennate). Examples of some nanostructures found in centric diatoms are shown in Fig. 1
000 species of diatoms23 but their classification and taxonomy is still under debate.24,25 Their shapes and sizes vary from circular to triangular and from approximately five micrometres to a few hundred micrometres. They are broadly categorised into those having radial symmetry of the nanostructured pore pattern (centric) or bilateral symmetry (pennate). Examples of some nanostructures found in centric diatoms are shown in Fig. 1
 | ||
| Fig. 1 Example of diatom frustule a) full Thalassiosira frustule,26 b) Thalassiosira valve and c) close up of Thalassiosira valve pores,27 d) pore structure of girdle band from Coscinodiscus.28,29. | ||
Diatoms possess the typical characteristics of standard plant cells: a nucleus that contains the DNA, mitochondria, chloroplasts for photosynthesis and a cell membrane. What they have that is unique is the extracellular porous rigid silica outer-shell or frustule. A schematic representation of the three dimensional structure of a centric diatom, such as a Coscinodiscus, is shown in Fig. 2. The frustule is made up of two caps called valves joined by a cylindrical section called the girdle bands. Diatoms reproduce primarily by mitotic divisions which are constrained by the rigid cell wall. The mother cell divides with one valve contributing to each of the two daughter cells. Thus a new valve is produced for each reproductive cycle and the new generation gets smaller until a critical size is reached and sexual reproduction (generally oogamy) is used to increase their size.
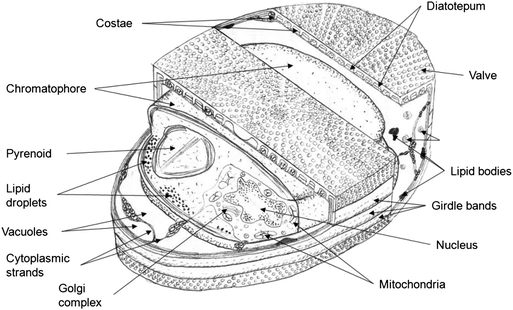 | ||
| Fig. 2 Schematic representation of a typical centric diatom, indicating the major components. | ||
Surrounding the frustule there is an organic layer coating all the silica which is most often too thin even to be seen in material prepared for TEM sectioning, but it does sometimes obscure detail in SEM images of uncleaned diatoms.26 The organic layer over the silica is thought to help in preventing silica dissolving.26 Most diatoms also have a distinct, continuous organic layer of acidic polysaccharides between the siliceous frustule and the plasma membrane called the diatotepum (see Fig. 3). It is postulated that the diatotepum helps keep the silica frustule together and possibly reduce the pore size to be more selective during filtration.30 Some diatoms, particularly those that attach to surfaces, have a considerable extracellular membrane material that forms the adhesive for attachment.31 In most centric diatoms the cell wall does not extend into the silica frustule leaving pores open for transport of nutrients.
 | ||
| Fig. 3 TEM image of a cryopreserved adhesive diatom (Bacillariophyceae) showing the diatotepum, plasma membrane P, chromatophore C, frustule F, and extra cellular material (EX) that acts as a surface adhesive.31 The organic casing is not visible. | ||
The intricate nanostructure of a diatom's silica frustule has been linked to a variety of functions including acting as a photonic crystal that guides light to help photosynthesis,32 being exceedingly strong in order to stave off predators18 and to counterbalance turgor pressure.33 The structure is species dependent and is thus genetically controlled. At this stage, other than knowing that the silica structure depends on the chemical environment they are grown in (e.g. the salinity), and that energy minimization through self assembly is involved, there is little consensus to the true function of the intricate nano-patterned silica cell wall. Some diatoms have larger pores on the outside such as Thalassiosira and some on the inside27 such as Coscinodiscus. While it is not clear why this is the case it may be linked to the environment they reside in, with those living in more turbulent water requiring quicker uptake into a larger ‘storage’ space where the nutrients can then diffuse into the plasma membrane (bigger pores outside). Further keys in understanding the role of the silica frustules are linked to understanding the silica formation process.
Diatom biomineralization
Over the last decade several complementary approaches have allowed important progress in our understanding of diatom biomineralization. The development of molecular tools,34 (for a review see35), and then the sequencing of the complete genome of currently three diatoms: Phaeodactylum tricornutum,36Thalassiosira pseudonana37 and Fragilariopsis cylindrus (available at http://genome.jgi-psf.org/Fracy1/Fracy1.home.html), have been essential steps. Among the other important approaches was the development of fluorescent probes used to label the silica shells. The first reported dye was the rhodamine-123 which is permeant to cell membranes, and typically concentrates in acidic organelles. Hence, because the compartment within which frustule morphogenesis occurs, namely the Silica Deposition Vesicle (i.e., SDV), is acidic the rhodamine-123 accumulates inside the SDV and becomes entrapped within the newly synthesized silica structures.38,39 Other weakly basic amines that selectively accumulate in cellular compartments with low internal, pH were also tested. Among, them, two particular dyes named DND-16040–42 and HCK-12343 (see Fig. 4) were shown to be particularly useful, giving for example, new information on the process of silica morphogenesis.44,45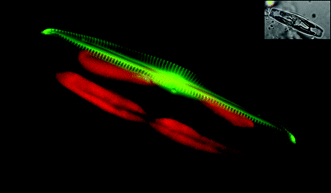 | ||
| Fig. 4 Fluorescent labelling of newly synthesized silica structure in a pennate diatom. Seminavis robustacells were grown in the presence of the fluorescent dye (HCK-123). After ca. 12 h, one valve of a dividing cell is labelled. Some details of the frustule (the central nodule, the raphe structure and the striae) can be clearly observed. In green the silica reporter, red the chloroplast auto-fluorescence, and the insert corresponds to the Nomarsky image. | ||
One of the most successful approaches relied on the purification of the organic components associated to the frustule. This procedure involves the development of protocols to first dissociate the cell-wall components and then to dissolve the silica phase. Experiments of characterization of the organic components entrapped inside the biomaterials have identified proteins, polysaccharides and long chain polyamines (LCPAs).46,47
It was shown that each diatom contains a specific mixture of LCPAs that have been characterized as poly(propyleneimine) chains with up to 20 repeat units, attached to putrescine, 1,3-diaminopropane or spermine.48 Interestingly, depending on the LCPAs fraction used it was possible to control the formation of nanostructured silicain vitro.49,50 In fact, both the methylation pattern and the polyamine chain length are likely to influence their ability to promote silica condensation rate, and therefore to play a role in creating species-specific silica nanostructures.51 The first cloned silaffins (silica affinity) gene came from the pennate diatom Cylindrotheca fusiformis.52 It was shown that peptides derived from Cf-Sil1 can enhance the precipitation of silica particles in vitro.52–56 A second Silaffins (Cf-Sil2) was also purified from this species but the gene has not yet been cloned. Three different silaffins have also been characterized from the model specie T. pseudonana.57 Even if silaffins do not show sequence similarity, they present domains enriched in lysines and serines residues. Another common feature, likely to be essential in silica polycondensation and assembly, is the presence of different kind of post-transcriptional modification including phosphorylation, methylation, hydroxylation, glycosylation and sulfation.58 A highly acidic phosphoprotein, named silacidins, was isolated from T. pseudonana.59 From in vitro experiments obtained with different combinations of polyamine/silcacidin to precipitate silica from a silicic acid solution, it was proposed that silacidins may serve as the polyanion required for silica formation directed by polyamines (and/or silaffins) in vivo.
Recently, Brunner and colleagues60 were able to purify chitin fibers after dissolution of T. pseudonanasilica frustules. Remarkably they show that the network-like chitin-based scaffold resembles the size and shape of the T. pseudonana biosilica. Knowledge on the organic material present in the silica biomaterial of diatoms has been used to develop a number of applications. In particular one specific peptide inspired from Cf-Sil1 and named R5, was used to control of silica nano-patterning61 or to perform enzymes immobilization.62–67Silica encapsulation can often stabilize an enzyme’s activity,68 making it an interesting and cost-effective route to develop new biosensors. Another attractive possibility to directly incorporate proteins within the silica matrix was to use the capability of diatom cells to target specific proteins to the frustule. One study has been published on the genetic manipulation of diatom silica wall biogenesis per se. Fusion between the C-terminal region of a silaffin (Tp-Sil3) and either the GFP reporter or an enzyme involved in Hydroxylamino aromatic compounds degradation (HabB) were constructed and used to transform the diatom T. pseudonana. It was shown that in some transformants the fusion protein was targeted to the frustule and that such incorporation into the silica biomaterial decreases the GFP photobleaching or stabilizes the hydroxylaminobenzene mutase activity.57 Genetic engineering combined with further information on the network of genes involved in the silicon metabolism and frustule formation should find some potential uses for biotechnology and materials science.
Molecular-level bio-modification of diatom frustules
Recent developments in chemical modification and bio-functionalization of silica have made it possible to generate a new class of bioactive silica nanostructures that can be conjugated with biomolecules such as proteins. The availability of these biomodified nano-constructs has opened up entirely new and exciting research directions in the field of biosensors. The rich chemistry of the silica structure of the diatom frustule provides great potential for bio-interfacing with biomolecules. Free hydroxyl groups on the surface can be used for chemical modification of the surface and subsequent tethering of biological or chemical moieties. Popular methods use long-chain alkane thiols to create self-assembled monolayers on the metal surface such as gold. The attractiveness of thiol chemistry is that well ordered monolayers can be formed relatively easily, with a reasonably strong bond formed between the organic molecule and the surface, and that a diverse range of molecules can be synthesized with which to modify a surface. However, these have compromised long-term stability of surface coatings. The advantages of thiol chemistry are somewhat offset by a number of disadvantages, including alkanethiols being oxidatively or reductively desorbed. Other disadvantages include: alkanethiols being desorbed at temperatures over 100 °C. In contrast, silanes are tightly bound through covalent bonds with the surface, and silane molecules can link to each other as well as the surface to form a polymer network within the coating. This means that functionalized silanes provide a more robust layer, which is essential for maintaining adhesion and strength for the grafting of relatively large biological moieties. In addition, silanes are good chelating ligands because of their reactivity with hydroxyls, and they can couple organic groups to virtually any oxide surface. The frustule is hydrated glass: SiO2·nH2O, which has free hydroxyl groups on the surface. These reactive groups allow the chemical modification of the surface and subsequent functionalization.The immobilisation of a biological sensing element (such as an enzyme, DNA, antibody or cell) with a transducer, either electrochemical, optical or piezoelectric, is the basis of a biosensor. There are two basic approaches to immobilising biomolecules onto a surface using the characteristic of silane to self-assemble on silica surfaces: (1). The most common non-covalent approach to immobilise biomolecules to the surface has been via electrostatic binding. This simple and gentle method provides the potential for control over the orientation of the immobilised biomolecules depending on the charge distribution of the biomolecules. The major drawback of electrostatic binding is that the strength of the bond is dependent on the solution conditions. Changes in ionic strength and pH can cause the protein to be lost from the surface. (2). Direct covalent attachment has the greatest potential for the development of biosensors due to the stability of the resultant covalent bond. A popular and highly versatile method for covalently attaching biomolecules to the surfaces is the use of carbodiimide coupling chemistry which couples amines to carboxylic acids. In the reaction N-ethyl-N-[dimethylaminopropyl] carbodiimide (EDC) converts the carboxylic acid into a reactive intermediate which is susceptible to attack by amines. In some cases EDC and N-hydroxysuccinimide (NHS) or N-hydroxysulfosuccinimide (NHS) are used as they produce a more stable reactive intermediate which has been shown to give a greater reaction yield69,70 (see Fig. 5). Silanes were, for example, shown to be useful to label diatom frustules either starting with purified biomaterials or live cells.39 The surface control provided over the molecular architecture of the sensing interface fabricated by using self-assembled monolayers of silane has been used for fabrication of elegant complex sensors. More exciting developments with nanostructured materials such as diatoms are expected in the near future with regard to biosensors.
 | ||
| Fig. 5 Schematic diagram showing the covalent attachment of a biomolecule to a SAM using EDC and NHS. | ||
Townley et al. used 3-aminopropyl trimethoxy silane (APS) to silanize diatom surfaces followed by reaction with the heterobifunctional crosslinker N-5-azido-2-nitrobenzoyloxysuccinimide (ANB-NOS).71 The crosslinker provides an amine-reactive N-hydroxysuccinimide (NHS) ester with a photoactivatable nitrophenyl azide, enabling antibodies to be tethered via their amine groups when exposed to UV light. They demonstrated surface modification using anti-IgY. The direct coupling of anti-IgY to the diatom surface is also of particular interest since IgY does not bind to protein A or protein G. This coupling can be performed through primary amine groups. Whilst very effective, binding can also occur near the antigen-binding domain of the antibody, which reduces the number of antibodies that retain biological activity and are able to bind antigen. A similar direct-coupling method enables orientation-dependent coupling by binding via the carbohydrate side chains of the antibody.
The silica surface of a diatom is amenable to simple chemical functionalization. An interesting example of this uses a DNA-modified diatom template for the control of nanoparticle assembly.72 The amino-functionalized diatoms were coupled to fluorophore-labelled thiolated DNA by using a hetero-bifunctional crosslinking agent. Then gold particles were coated with DNA complementary to that bound to the surface of the diatom. Subsequently, the gold particles were bound to the diatom surface via the sequence specific DNA interaction (see Fig. 6). Using this method, up to seven layers were added showing how a hierarchical structure could be built onto the template. So one can easily modify the diatom surfaces with many different functional groups designed specifically to interact with nanostructures of interest or to perform desired chemistry. Unlike the surfaces of some biological templates, the surface of diatoms exhibit remarkable species-specific nanoscopic details that cannot be rendered or synthesized by using conventional material fabrication techniques. These nanostructures may prove useful for various applications in sensors, catalysis and optics by using the unique properties of surface modification of diatoms.73
 | ||
| Fig. 6 (a). Fluorescence microscopy images of diatoms modified by fluorophore-labeled thiolated DNA. (b). The reaction of DNA-functionalized diatoms with complementary DNA-functionalized nanoparticles can be monitored by the naked-eye or by UV/Vis spectroscopy. | ||
Diatoms frustules possess intricate nanoscale features imbedded within naturally micro/nano-fabricated periodic two-dimensional pore arrays, displaying unparalleled diversity in structure and morphology. The deposition of gold and silver on a diatom frustule surface has been achieved by thermal evaporation.74,75 Furthermore, Losic et al.74 developed a procedure for pore size modifications of centric diatom species, using the atomic layer deposition of ultrathin films of TiO2. TiO2 was deposited by sequential exposures to TiCl4 and water. They also showed the controlled reduction of pore sizes while preserving the shape of the diatom membrane pores. Pore diameters of diatom membranes can be further tailored for specific applications by varying the number of cycles and by changing their surface functionality. Toster et al. demonstrated that the ordered pores of diatom frustule can be effectively used as templates to mediate gold nanoparticle growth by a facile method.76 Therefore, diatom frustules offer a advantage for synthesis of nanomaterials with well-defined and precisely controlled three dimensional morphologies and micro-to-nano scale features. Recently Losic et al. demonstrated the surface modification of diatom frustules with dopamine terminated Fe3O4 nanoparticles.77 This approach is based on a simple one-step electrostatically driven self-assembly of dopamine modified Fe3O4 nanoparticles onto the diatom surface. The labelling of modified diatoms using fluoro probes showed that amino groups of dopamine on diatom surface are functional and available for the further attachment of targeting molecules. It was suggested this type of three dimensional hybrid nanomaterials could be potentially used as magnetically guided micro-carriers for drug delivery applications. Further, Rorrer's research group demonstrated the biological fabrication of Ge-doped biosilica frustules by two-stage cell culture of the diatom Pinnularia, and they fabricated an electroluminescent device by the incorporation of these diatom frustules.78 This study represented a first step towards the realization of optoelectronic devices that utilize components fabricated through cell culture. Furthermore, the same team used the living diatom itself to metabolically insert nanostructured TiO2 into the periodic structure of its frustule biosilica,79 and they found addition of titanium to the diatom cells had no detrimental effects on the growth of the organism and preserved the nano- and microstructure of the frustule biosilica. The fabrication of these unique nano- and microstructured semiconductor materials would offer possible applications including dye-sensitized solar cells for enhanced light trapping efficiency and photocatalysts for enhanced breakdown of toxic chemicals.
Biosensors
The changes in photoluminescent (PL) properties of porous silicon and nanoscale semiconductor materials upon their interaction with biomolecules have been well studied. The research groups of Chan,80,81 Sailor82,83 and Gooding84,85 have investigated the application of porous silicon as optical biosensors where biomolecules including enzymes, DNA fragments and antibodies have been immobilized. Diatoms as photonic crystals consist of a periodically arranged set of dielectric materials that affect the propagation of light in a manner analogous to the way crystalline solids influence the flow of electrons. Using diatoms both as a large surface area matrix as well as an optical transducer of biomolecular interactions is relatively new. A number of research groups have explored diatom based photonic nanostructures as large surface area matrices for the immobilization of particular biomolecular agents and through spectral measurements study biomolecular interactions with high sensitivity. Apart from the sensitivity, there are other advantages of diatoms: first of all, they can monitor the binding reactions without the need to label one of the molecules involved in the binding reaction. Label-free monitoring of binding reactions not only simplifies any analysis by reducing errors in quantification, but also allows the biomolecules to be investigated directly in their natural environment. Secondly, the porosity of diatoms enables an intimate mix between the analytical sample and the detector, allowing monitoring of the biomolecular interactions in an effective way.Rorrer's research group86 investigated how antibody-functionalized diatom biosilica frustules serve as a microscale biosensor platform for selective and label-free PL-based detection of immunocomplex formation. They attached antibody rabbit immunoglobulin G (IgG) covalently to the frustule biosilica of the disk-shaped, 10 μm diameter diatom Cyclotella sp. by using silanol amination and a crosslinking step to a surface site density. It was demonstrated that functionalization of the diatom biosilica with the nucleophilic IgG antibody amplifies the intrinsinc blue PL of diatom biosilica by a factor of six. When the rabbit-IgG-functionalized diatom biosilica was bound to its complimentary antigen (goat anti-rabbit IgG), which is also nucleophilic, the peak PL intensity increased again by at least a factor of three. In fact, the increase of PL emission with nanoscale topology has been investigated with nucleophilic moieties or biomolecules that are attached to nanoscale semiconductors or other photoluminescent surfaces.87,88 These studies suggested that nucleophilic chemical groups increase the PL intensity by donating electrons to non-radiative defect sites on the photoluminescent surface, thereby decreasing non-radiative electron decay and increasing the radiative emission, resulting in a higher quantum efficiency of the functionalized photoluminescent surface.
Parker et al.89 demonstrate how the photonic properties of a diatom can be altered by growth with a metal pollutant. Both the optical and physical properties of the silica frustule of the diatom Coscinodiscus wailesii were affected by the presence of nickel sulfate in sea water. It was found that a sublethal concentration of the metal both significantly modified the size of the pores of the valves and quenched the intrinsic PL of the amorphous silica. De Stafano et al.90,91 recently chemically modified the frustules of the marine diatom Coscinodiscus to properly bind a highly selective bioprobe such as an antibody. By measuring the changes in the photoluminescence emission of diatoms frustules, they monitored the molecular recognition event between the antibody and its binding ligands.
Gas sensors
The photoluminescence (PL) emission from the silica frustule of diatoms has been explored by De Stafano92 for an optical gas sensor. They showed that the PL of Thalassiosira rotula is strongly dependent on the surrounding environment. Both the optical intensity and peaks are affected by gases and organic vapours. In the presence of NO2, acetone and ethanol, the photoluminescence was quenched because these substances attract electrons from the silica frustule of the diatoms and hence quench the PL. On the other hand, substances that donate electrons, such as xylene and pyridine, had the opposite effect, and increased PL intensity almost ten times. Both quenching and enhancements were reversible as soon as the atmosphere was replaced by air. Subsequently, the same research group investigated the modification of the PL properties of different light-emitting diatom samples induced by the presence of NO2. They demonstrated high-sensitivity gas detection at a low concentration, with a detection limit as low as 50 ppb obtained in the case of diatom frustules of highest specific surface. This study strongly implied that the luminescence activity of diatom frustules is related to surface-oxygen stoichiometric defects.93Further, Sandhage et al.94 conducted an inorganic molecular conversion reaction that preserves the size, shape and morphology of the diatom whilst changing its composition. They used a displacement reaction to convert biologically derived silica structures such as frustules into new compositions. Magnesium was shown to convert SiO2 diatoms by a vapour phase reaction at 900 °C to MgO of identical shape and structure, with a liquid Mg2Si by-product. Similarly when diatoms were exposed to titanium fluoride gas, the titanium displaced the silicon, yielding a diatom structure made up entirely of titanium dioxide; a material used in some commercial solar cells. Recently, this group extended its work on silica diatoms to work at lower temperatures.95 Two-dimensional microporous silicon was used as an attractive sensing interface for rapid gas detection with the high specific surface areas and structures of the 3D silicon frustule replicas allowing rapid gas detection. To test such gas detection, a simple device based on a silicon frustule replica was fabricated by using platinum electrodes connected to the ends of this replica on a silicon nitride substrate, which exhibited rapid changes in impedance upon exposure to gaseous nitric oxide (see Fig. 7). This type of sensor could be used in in microscale gas sensing. Subsequently the same group reported how the intricate nanostructured silica valves of diatom frustules may be coated with a thin (50 nm), conformal, and continuous layer of a functional oxide (SnO2) through dendritic amplification of hydroxy groups on the silica surfaces and then use of an automated surface sol–gel process.96 They fabricated a device from such SnO2-coated diatom frustule valves acts as a sensitive detector for NO gas by using a general process for depositing compact, continuous, and conformal coatings of synthetic inorganic oxides onto 3D nanostructured biosilica templates. To maximise the sensitivity and response of sensors an understanding of how fluids interacts with the diatom surfaces is imperative. In the next section we discuss recent progress in this field.
 | ||
| Fig. 7 (a) Secondary electron image of an electroded microporous silicon frustule replica. (b) Electrical response of this single silicon frustule sensor to NO(g). ΔZ is the impedance change upon exposure to NO(g), and Zo is the sensor impedance in pure flowing argon. | ||
How diatoms interact with fluids and their use as biomimetic membranes
In their natural aquatic environment diatoms are exposed to a large variety of molecules/particles that range in size from less than one nanometre (nitrates for example) to viruses and bacteria that are in the micrometre range. Their silica membrane surface is exposed to not only a large size range of particles but also particles in varying concentrations- anything from concentrations of 107 ml−1 for large bacteria (∼1 μm), to concentrations of 1016 ml−1 for nitrates.97,98 Diatoms manage with minimal energy consumption to dominate by somehow filtering deleterious particles from useful ones, without the use of complex high-energy-using moving components to control their microenvironment. Their surface does not foul (but they manage to foul water purification membranes99 themselves), they sort useful particles from harmful ones, and dominate environments with little energy consumption which leads the inevitable question: how and why? And what role does their silica frustule have in their success? Can we utilize the diatom's structure to design more efficient membranes?Diatoms have a long history of being used by mankind for filtration purposes. Diatomaceous earth, or diatomite, which is made from the rock consisting of sedimented dead diatoms has long been known as an excellent filter due to its relatively high porosity but very fine pores, and is used as a filter in a range of applications, such as in swimming pool filters and for water purification. Recently chemically modified diatomite has been shown to be effective in removing dissolved uranium ions from water (see Fig. 8).100 The authors used pure diatomite and hexadecyltrimethylammonium (HDTMA) modified diatomite and found the adsorption of uranium(VI) on the pure and the HDTMA diatomite varied with initial uranium concentration, sorbent/solution contact time and pH values of solution. The maximum adsorption capacity of the HDTMA-diatomite was 25.63 mol g−1 (158.8 mg g−1 or 15.9 wt/%), and for a pH of 8 and above 100% purification was achieved illustrating the usefulness of their high surface area to volume ratio.
 | ||
| Fig. 8 SEM of diatomite100 showing a range of different diatom frustules used in removing dissolved uranium irons. | ||
In order to help understand how diatoms filter we must examine their natural habitat. Free living diatoms are at the mercy of the ocean or lake flow, currents and turbulence. They are subject to the bulk water motion and, due to their size, are sensitive to very small scale motion. Here we look at what flow they are subject to (depicted in Fig. 9) and how it may affect their transport and thus filtration properties.
 | ||
| Fig. 9 Flow regimes for diatoms ranging from macroscale motion of the ocean to nanoscale transport through the frustules pores. | ||
Macroscale flow
We define macroscale as sizes greater than approximately 1 mm where the flow is affected by turbulence, currents and buoyancy changes. At this scale, and the microscale (∼1 μm to 1 mm), fluid properties can be considered as a continuum and the standard advection diffusion equation can be used to determine the transport phenomena. On the macroscale the diffusion coefficient, D, may vary in space due to local changes in temperature and salinity, but in the local microenvironment around a diatom D can be considered homogenous thus simplifying the problem. Numerical simulations to solve the advection/diffusion equation for specific geometries coupled with a nutrient uptake kinetic equations can be used determine impact of shape and external flow for diatoms nutrient acquisition.101–104 The information obtained using these methods may, for example, also be used for finding the best diatom for rapid drug absorption/release or biosensor response.Turbulence
Turbulence is a natural phenomenon that occurs in fluids when the inertial motion of the fluid overcomes the viscous forces tending to damp-out the motion. The ratio of these forces is called the Reynolds number. Turbulence thus occurs above a critical Reynolds number that depends on the nature and geometry of the flow. Turbulence is characterised by highly irregular, isotropic and fluctuating three dimensional flow with most of the energy contained in eddies (rotating packets of fluid). Diatoms are unique in water micro-organisms in that they have large vacuole that can store inorganic nutrients (e.g.nitrates and phosphates) meaning that they tend to thrive in areas with pulsating nutrients supplies such as highly turbulent areas of the ocean.6Sources of turbulent energy in the ocean, for example, are wave motion, winds, currents and moving marine creatures. At small scales, viscous forces dominate and turbulent energy is dissipated due to fluid friction and thus as heat. The Kolmogorov scale, Lk, defines the region where viscous forces start to dominate the dissipation and thus describes the size of the smallest eddies in the flow105 and is given by:
Microscale flow—the relationship between frustules shape and nutrient uptake
The typical Reynolds number of a 100 μm diameter diatom sinking at 350 μm s−1 is 0.35, which is well in the laminar regime. This value will vary depending on the relative speed and the size of the diatom but not enough to induce turbulent flow. In addition, as the ocean's turbulence manifests itself as linear laminar shear at the scale of a diatom, only laminar flow needs to be considered. The impact of cell shape on diffusion and advective nutrient transport to individual diatoms and diatom chains was investigated by Pahlow et al.102 For individual diatoms the nutrient uptake was shown to be higher than for elongated diatom shapes due to their higher surface area to volume ratio, particularly relative to that for spheres. This indicated that elongated diatoms may form a better sensor substrate.It has been shown111 using the analysis of101 that simply due to the shear forces associated with diatoms such as Coscinodiscus wailesii sinking and floating they will rotate with a period ranging from approximately 0.5 to 2 s, which is similar to the frequency of maximum turbulent energy dissipation in the ocean. This rotation will produce periodic pressure fluctuations in the pores that are in the order of the stagnation pressure, 0.5 ρv2, associated with the sinking velocity. That gives pressure fluctuations ranging from approximately 3.2 μPa to 60 μPa. These fluctuations may be superimposed onto the changing hydrostatic pressure associated with sinking and floating and help the diatom with filtration.
For an excellent review on the microscale flow around the surface of diatoms see Karp-Boss et al.103 who assume throughout their analysis that diatoms are perfect sinks so that nutrient concentration at the surface is zero. While admitting this is not physically correct it makes the problem a lot easier to solve.
When considering the uptake of nutrients into diatoms Paskiak and Gravis (1974) defined a new parameter being the ratio of diffusive transport to nutrient uptake kinetics using the Michaelis–Menten equation. This ratio defines the rate limiting process as either being diffusion limited or uptake limited. It was shown by them, and since by other authors, that various processes are diffusion limited (e.g.CO2 uptake112). However in all these analyses a single diffusion coefficient has been used based on the free solution value.
Nanoscale effects and methods of filtering
Currently all the studies of nutrient uptake in diatoms have only considered microscale flow around diatoms, or in other words have considered only a bulk free diffusion coefficient, and a diatom plasma membrane that has a nutrient uptake limit. In reality, however, the nanostructured frustule between the bulk solution and the cell plasma membrane will have a significant effect on the transport properties. This is depicted in Fig. 9 for a girdle band pore and in Fig. 10 for a valve pore showing the pore structure and the associated diffusion coefficients and concentration gradient taking into account the nanoscale confining effects. | ||
| Fig. 10 Schematic of a diatom valve pore structure (not to scale) and associated diffusion coefficients and concentration though the pore assuming pure diffusion and no advection. | ||
While allowing nutrients through the frustule, diatoms reject suspended bacteria and viruses. The method they use to do this is not understood, especially with regard to the role of the frustule. It is clear that the organic components play a role similar to regular cells without a hard outer silica layer. This is illustrated in Fig. 11 that shows small molecules like fluorescein (equivalent diameter ∼1 nm) and propidium iodide (<1 nm) cannot penetrate a live diatom, but when the organic membrane was ruptured with methanol, the dyes readily entered the cell.
 | ||
| Fig. 11 Confocal images in the midplane of a Coscinodiscus wailesii in fluorescein (left) and propidium iodide (right). a) In a live diatom, b) Same diatom after organic membrane ruptured with methanol. | ||
There have been a handful of studies investigating the role of the nano-structure on the transport through and along diatoms. The first in the area were by Hale and Mitchell113,114 who looked at the lateral motion of Brownian fluorescent particles over cleaned diatom frustules and over mimics etched in silicon. They showed how the surface topography deflected the path of Brownian particles (∼0.3–0.5 μm) away from the mean flow direction even when the flow was not dominated by diffusion. They proposed that the diatom surface topography may help diatoms sort and filter.
There are a few roles that the nanostructures of the frustule can play in terms of facilitating filtration and sorting based on the type of flow the diatoms experience. The methods that the solvent (water) and the solutes (molecules and nanoparticles, virus, bacteria etc.) interact with the diatom are dictated by advection and diffusion. In order to determine the dominate transport mechanism around diatoms, the Peclet number, is often used which is defined as the ratio of advective to diffusive transport and is given by
The first attempt at measuring the diffusion coefficient through clean diatom valves was by Losic et al.115 Their basic set-up used a valve glued to the end of a capillary tube and they measured the fluorescent emission spectra, showing selectivity of the frustule to particle size. Bhatta et al.116 then did more detailed experiments showing the diffusion coefficient of small molecules like fluoresceine was reduced through a Coscinodiscus valve. In order to get a more accurate value of the diffusion coefficient Bhatta et al.117 then used fluorescent correlation spectroscopy FCS, which has a measurement volume very close to that of the large pores in a Coscinodiscus valve. The diffusion coefficient of a fluorescent dye in a pore was shown to be reduced significantly relative to the free solution value.117 The decrease of the diffusion coefficient is well documented for confined diffusion. Experiments show that even with molecules that are only 5% of the pore diameter the diffusion coefficient is reduced by about 20% relative to free solution.118 This decrease due to confinement is shown schematically in Fig. 10. For a good review on all aspects of hindered diffusion see Deen.119 Additionally as diatoms live in a coilloidal mixture, the rapid changes in pore area in the valve could help in entropic trapping.120
As well as pore shape effecting diffusive transport, it is also known to effect advection of both solvent and solute, the frictional pressure drop and fouling.121 For example Bowen and Sharif122 showed a rounded pore shape is the best for maximising flux when electrostatic interactions are involved. The unique diatom pore may be optimised as an ideal antifouling membrane.
Depending on the macroscale conditions diatoms may be subject to a constant shear field, fluctuating shear, or stagnant water. The fluctuating pressure field mentioned previously associated with turbulence and rotation may provide the periodic force required to drive Brownian ratchet filtering mechanism.123,124 The girdle band pores shown in Fig. 1d offer the change in shear forces required for operation of a ratchet, with the diatom pore shape being similar to that made artificially in silicon for a Brownian ratchet.28
Additionally filtering may also utilize electrostatic interactions via the electric double layer or even Van de Waals forces. If the negatively charged silica frustule was exposed to diatoms in sea water with an ionic strength of 0.7 M the Debye length would be very small relative to the pore size so that it would have little effect on the transport properties. However, when a very thin layer of polysaccharides are attached to the silica this may change with interaction lengths possibly approaching around 30 nm125 which is close the size of the smallest diatom pores without the organic layer. This is area ripe for further investigation. Thus there is still much to learn about transport through diatoms frustules and we expect soon that they may form a model for efficient and selective nanofluidic transport for both solvents and solutes.126
While we believe the main application for the study of diatom frustules will be to inspire new architectures for synthetic membranes, diatoms may also be used directly, not only as thick random porous membrane filters such as diatomite as shown in Fig. 8, but more interestingly, as a single layer array of diatom valves to form a macroscale membrane. Cleaned valves could be self-assembled on a porous silicon support substrate and then permanently attached by forming an oxide layer on the silicon. As the biosilica is very thin for the smallest pore layer (∼50–100 nm), such a membrane could offer excellent selectivity with very low hydrodynamic resistance.
Conclusion and future perspectives
Diatoms possess intricate nanoscale features imbedded within naturally micro/nano-fabricated periodic two-dimensional pore arrays, displaying unparalleled diversity in structure and morphology. Undoubtedly, diatoms will play an important role in biosensors and separation science because of their many unique properties. Diatoms generate silica structures without the need for complex chemical processes, providing an exceptionally cheap material for analytical science. Recent developments in chemical modification and biofunctionalization of these materials have made it possible to generate a new class of bioactive silica nanostructures that are conjugated with biomolecules such as proteins. The nanoporus structures of the frustules may shed light on how to best structure a membrane for low energy separation and to avoid fouling as they contain many features know for low energy separation, and even form the active structure of a manmade membrane themselves. The availability of these biomodified nano-constructs has opened up entirely new and exciting research directions in the field of biosensors and filtration.Acknowledgements
We acknowledge CSIRO Advanced Membrane Technologies For Water Treatment Research Cluster. PJL’s research was in part supported by the CNRS-program “Interface physique, biologie et chimie ”.References
- C. B. Field, M. J. Behrenfeld, J. T. Randerson and P. Falkowski, Science, 1998, 281, 237–240 CrossRef CAS.
- D. L. Rabosky and U. Sorhannus, Nature, 2009, 457, 183–186 CrossRef CAS.
- J. P. Descy and M. Coste, in International Association of Theoretical and Applied Limnology - Proceedings, Vol 24, Pt 4, ed. V. Sladecek and A. Sladeckova, E Schweizerbart'sche Verlagsbuchhandlung, Stuttgart, 1991, vol. 24, pp. 2112–2116 Search PubMed.
- B. C. Chessman and S. A. Townsend, Ecological Indicators, 10, pp. 620–626 Search PubMed.
- M. G. Kelly and B. A. Whitton, J. Appl. Phycol., 1995, 7, 433–444 Search PubMed.
- P. G. Falkowski, M. E. Katz, A. H. Knoll, A. Quigg, J. A. Raven, O. Schofield and F. J. R. Taylor, Science, 2004, 305, 354–360 CrossRef CAS.
- B. Fei, Z. G. Hu, H. F. Lu and J. H. Xin, Small, 2007, 3, 1921–1926 CrossRef CAS.
- G. Begum, R. K. Rana, S. Singh and L. Satyanarayana, Chem. Mater., 2010, 22, 551–556 CrossRef CAS.
- S. M. Allan, M. R. Weatherspoon, P. D. Graham, Y. Cai, M. S. Haluska, R. L. Snyder and K. H. Sandhage, in Advances in Ceramic Coatings and Ceramic-Metal Systems, ed. D. Zhu and K. Plucknett, 2005, vol. 26, pp. 289–296 Search PubMed.
- I. Gebeshuber, M. Drack and M. Scherge, Tribology - Materials Surfaces & Interfaces, 2008, 2, 200–212 Search PubMed.
- A. Bozarth, U. G. Maier and S. Zauner, Appl. Microbiol. Biotechnol., 2009, 82, 195–201 CrossRef CAS.
- R. Radakovits, R. E. Jinkerson, A. Darzins and M. C. Posewitz, Eukaryotic Cell, 2010, 9, 486–501 CrossRef CAS.
- Q. Hu, M. Sommerfeld, E. Jarvis, M. Ghirardi, M. Posewitz, M. Seibert and A. Darzins, Plant J., 2008, 54, 621–639 CrossRef CAS.
- P. T. Pienkos and A. Darzins, Biofuels, Bioprod. Biorefin., 2009, 3, 431–440 Search PubMed.
- E. V. Armbrust, J. A. Berges, C. Bowler, B. R. Green, D. Martinez, N. H. Putnam, S. G. Zhou, A. E. Allen, K. E. Apt, M. Bechner, M. A. Brzezinski, B. K. Chaal, A. Chiovitti, A. K. Davis, M. S. Demarest, J. C. Detter, T. Glavina, D. Goodstein, M. Z. Hadi, U. Hellsten, M. Hildebrand, B. D. Jenkins, J. Jurka, V. V. Kapitonov, N. Kroger, W. W. Y. Lau, T. W. Lane, F. W. Larimer, J. C. Lippmeier, S. Lucas, M. Medina, A. Montsant, M. Obornik, M. S. Parker, B. Palenik, G. J. Pazour, P. M. Richardson, T. A. Rynearson, M. A. Saito, D. C. Schwartz, K. Thamatrakoln, K. Valentin, A. Vardi, F. P. Wilkerson and D. S. Rokhsar, Science, 2004, 306, 79–86 CrossRef CAS.
- J. Parkinson and R. Gordon, Trends Biotechnol., 1999, 17, 190–196 CrossRef CAS.
- R. Gordon, D. Losic, M. A. Tiffany, S. S. Nagy and F. A. S. Sterrenburg, Trends Biotechnol., 2009, 27, 116–127 CrossRef CAS.
- C. E. Hamm, R. Merkel, O. Springer, P. Jurkojc, C. Maier, K. Prechtel and V. Smetacek, Nature, 2003, 421, 841–843 CrossRef CAS.
- C. E. Hamm, J. Nanosci. Nanotechnol., 2005, 5, 108–119 CrossRef CAS.
- R. Gordon and J. Parkinson, J. Nanosci. Nanotechnol., 2005, 5, 35–40 CrossRef CAS.
- R. W. Drum and R. Gordon, Trends Biotechnol., 2003, 21, 325–328 CrossRef CAS.
- S. Neethirajan, R. Gordon and L. J. Wang, Trends Biotechnol., 2009, 27, 461–467 CrossRef CAS.
- D. G. Mann and S. J. M. Droop, Hydrobiologia, 1996, 336, 19–32.
- L. K. Medlin and I. Kaczmarska, Phycologia, 2004, 43, 245–270 CrossRef.
- E. C. Theriot, J. J. Cannone, R. R. Gutell and A. J. Alverson, European journal of phycology, 2009, 99999, 1–14 Search PubMed.
- F. Round, R. Crawford and D. Mann, The diatoms: biology & morphology of the genera, Cambridge Univ Pr, 1990 Search PubMed.
- D. Losic, G. Rosengarten, J. G. Mitchell and N. H. Voelcker, J. Nanosci. Nanotechnol., 2006, 6, 982–989 CrossRef CAS.
- S. Matthias and F. Muller, Nature, 2003, 424, 53–57 CrossRef CAS.
- L. Dusan, G. M. James and H. V. Nicolas, Adv. Mater., 2009, 21, 2947–2958 CrossRef.
- H. A. von Stosch, Structural and histochemical observations on the organic layers of the diatom cell wall, 1981 Search PubMed.
- Y. Wang, Y. Chen, C. Lavin and M. Gretz, J. Phycol., 2000, 36, 367–378.
- T. Fuhrmann, S. Landwehr, M. El Rharbi-Kucki and M. Sumper, Appl. Phys. B: Lasers Opt., 2004, 78, 257–260 CrossRef CAS.
- A. Schmid, Protoplasma, 1994, 181, 43–60 CrossRef.
- N. Poulsen, P. M. Chesley and N. Kroger, J. Phycol., 2006, 42, 1059–1065 CrossRef.
- P. J. Lopez, J. Descles, A. E. Allen and C. Bowler, Curr. Opin. Biotechnol., 2005, 16, 180–186 CrossRef CAS.
- C. Bowler, A. E. Allen, J. H. Badger, J. Grimwood, K. Jabbari, A. Kuo, U. Maheswari, C. Martens, F. Maumus, R. P. Otillar, E. Rayko, A. Salamov, K. Vandepoele, B. Beszteri, A. Gruber, M. Heijde, M. Katinka, T. Mock, K. Valentin, F. Verret, J. A. Berges, C. Brownlee, J. P. Cadoret, A. Chiovitti, C. J. Choi, S. Coesel, A. De Martino, J. C. Detter, C. Durkin, A. Falciatore, J. Fournet, M. Haruta, M. J. Huysman, B. D. Jenkins, K. Jiroutova, R. E. Jorgensen, Y. Joubert, A. Kaplan, N. Kroger, P. G. Kroth, J. La Roche, E. Lindquist, M. Lommer, V. Martin-Jezequel, P. J. Lopez, S. Lucas, M. Mangogna, K. McGinnis, L. K. Medlin, A. Montsant, M. P. Oudot-Le Secq, C. Napoli, M. Obornik, M. S. Parker, J. L. Petit, B. M. Porcel, N. Poulsen, M. Robison, L. Rychlewski, T. A. Rynearson, J. Schmutz, H. Shapiro, M. Siaut, M. Stanley, M. R. Sussman, A. R. Taylor, A. Vardi, P. von Dassow, W. Vyverman, A. Willis, L. S. Wyrwicz, D. S. Rokhsar, J. Weissenbach, E. V. Armbrust, B. R. Green, Y. Van de Peer and I. V. Grigoriev, Nature, 2008, 456, 239–244 CrossRef CAS.
- E. V. Armbrust, J. A. Berges, C. Bowler, B. R. Green, D. Martinez, N. H. Putnam, S. Zhou, A. E. Allen, K. E. Apt, M. Bechner, M. A. Brzezinski, B. K. Chaal, A. Chiovitti, A. K. Davis, M. S. Demarest, J. C. Detter, T. Glavina, D. Goodstein, M. Z. Hadi, U. Hellsten, M. Hildebrand, B. D. Jenkins, J. Jurka, V. V. Kapitonov, N. Kroger, W. W. Lau, T. W. Lane, F. W. Larimer, J. C. Lippmeier, S. Lucas, M. Medina, A. Montsant, M. Obornik, M. S. Parker, B. Palenik, G. J. Pazour, P. M. Richardson, T. A. Rynearson, M. A. Saito, D. C. Schwartz, K. Thamatrakoln, K. Valentin, A. Vardi, F. P. Wilkerson and D. S. Rokhsar, Science, 2004, 306, 79–86 CrossRef CAS.
- M. A. Brzezinski and D. J. Conley, J. Phycol., 1994, 30, 45–55 CrossRef CAS.
- W. Wang, T. Gutu, D. K. Gale, J. Jiao, G. L. Rorrer and C. H. Chang, J. Am. Chem. Soc., 2009, 131, 4178–4179 CrossRef CAS.
- Z. Diwu, C. S. Chen, C. Zhang, D. H. Klaubert and R. P. Haugland, Chem. Biol., 1999, 6, 411–418 CrossRef CAS.
- K. Leblanc and D. A. Hutchins, Limnology and Oceanography Methods, 2005, 3, 462–476 Search PubMed.
- K. Shimizu, Y. Del Amo, M. A. Brzezinski, G. D. Stucky and D. E. Morse, Chem. Biol., 2001, 8, 1051–1060 CrossRef CAS.
- J. Descles, M. Vartanian, A. El Harrak, M. Quinet, N. Bremond, G. Sapriel, J. Bibette and P. J. Lopez, New Phytol., 2008, 177, 822–829 Search PubMed.
- B. Tesson and M. Hildebrand, J. Struct. Biol., 2010, 169, 62–74 CrossRef CAS.
- M. Vartanian, J. Descles, M. Quinet, S. Douady and P. J. Lopez, New Phytol., 2009, 182, 429–442 Search PubMed.
- N. Kroger, R. Deutzmann, C. Bergsdorf and M. Sumper, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 14133–14138 CrossRef CAS.
- M. Sumper and N. Kroger, J. Mater. Chem., 2004, 14, 2059–2065 RSC.
- M. Sumper and E. Brunner, ChemBioChem, 2008, 9, 1187–1194 CrossRef CAS.
- M. Sumper, S. Lorenz and E. Brunner, Angew. Chem., Int. Ed., 2003, 42, 5192–5195 CrossRef CAS.
- M. Sumper and G. Lehmann, ChemBioChem, 2006, 7, 1419–1427 CrossRef CAS.
- A. Bernecker, R. Wieneke, R. Riedel, M. Seibt, A. Geyer and C. Steinem, J. Am. Chem. Soc., 2010, 132, 1023–1031 CrossRef CAS.
- N. Kroger, R. Deutzmann and M. Sumper, Science, 1999, 286, 1129–1132 CrossRef CAS.
- N. Kroger, R. Deutzmann and M. Sumper, J. Biol. Chem., 2001, 276, 26066–26070 CrossRef CAS.
- N. Kroger, S. Lorenz, E. Brunner and M. Sumper, Science, 2002, 298, 584–586 CrossRef.
- M. R. Knecht and D. W. Wright, Chem. Commun., 2003, 3038–3039 RSC.
- R. R. Naik, P. W. Whitlock, F. Rodriguez, L. L. Brott, D. D. Glawe, S. J. Clarson and M. O. Stone, Chem. Commun., 2003, 238–239 RSC.
- N. Poulsen, C. Berne, J. Spain and N. Kroger, Angew. Chem., Int. Ed., 2007, 46, 1843–1846 CrossRef.
- N. Kroger and N. Poulsen, Annu. Rev. Genet., 2008, 42, 83–107 CrossRef CAS.
- S. Wenzl, R. Hett, P. Richthammer and M. Sumper, Angew. Chem., Int. Ed., 2008, 47, 1729–1732 CrossRef CAS.
- E. Brunner, P. Richthammer, H. Ehrlich, S. Paasch, P. Simon, S. Ueberlein and K. H. van Pee, Angew Chem Int Ed Engl, 2009, 48, 9724–9727 CAS.
- L. L. Brott, R. R. Naik, D. J. Pikas, S. M. Kirkpatrick, D. W. Tomlin, P. W. Whitlock, S. J. Clarson and M. O. Stone, Nature, 2001, 413, 291–293 CrossRef CAS.
- L. J. Chien and C. K. Lee, Biotechnol. Bioeng., 2007, 100, 223–230.
- H. R. Luckarift, J. C. Spain, R. R. Naik and M. O. Stone, Nat. Biotechnol., 2004, 22, 211–213 CrossRef CAS.
- W. D. Marner 2nd, A. S. Shaikh, S. J. Muller and J. D. Keasling, Biotechnol. Prog., 2009, 25, 417–423 CrossRef.
- R. R. Naik, M. M. Tomczak, H. R. Luckarift, J. C. Spain and M. O. Stone, Chem. Commun., 2004, 1684–1685 RSC.
- D. H. Nam, K. Won, Y. H. Kim and B. I. Sang, Biotechnol Prog, 2009, 25, 1643–1649 CAS.
- P. W. Whitlock, S. V. Patwardhan, M. O. Stone and S. J. Clarson, in Symposium on Polymer Biocatalysis and Biomaterials held at the 2006 ACS National Meeting, ed. H. N. Cheng and R. A. Gross, Boston, MA, ACS SYMPOSIUM SERIES edn, 2006, p. 412 Search PubMed.
- I. Gill and A. Ballesteros, Trends Biotechnol., 2000, 18, 282–296 CrossRef CAS.
- W. R. Yang, D. B. Hibbert, R. Zhang, G. D. Willett and J. J. Gooding, Langmuir, 2005, 21, 260–265 CrossRef CAS.
- J. J. Gooding, F. Mearns, W. Yang and J. Liu, Electroanalysis, 2003, 15, 81–96 CrossRef CAS.
- H. E. Townley, A. R. Parker and H. White-Cooper, Adv. Funct. Mater., 2008, 18, 369–374 CrossRef CAS.
- N. L. Rosi, C. S. Thaxton and C. A. Mirkin, Angew. Chem., Int. Ed., 2004, 43, 5500–5503 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103–1169 CrossRef CAS.
- D. Losic, G. Triani, P. J. Evans, A. Atanacio, J. G. Mitchell and N. H. Voelcker, J. Mater. Chem., 2006, 16, 4029–4034 RSC.
- D. Losic, J. G. Mitchell and N. H. Voelcker, New J. Chem., 2006, 30, 908–914 RSC.
- J. Toster, K. S. Iyer, R. Burtovyy, S. S. O. Burgess, I. A. Luzinov and C. L. Raston, J. Am. Chem. Soc., 2009, 131, 8356 CrossRef CAS.
- D. Losic, Y. Yu, M. S. Aw, S. Simovic, B. Thierry and J. Addai-Mensah, Chem. Commun., 2010, 46, 6323–6325 RSC.
- C. Jeffryes, R. Solanki, Y. Rangineni, W. Wang, C. H. Chang and G. L. Rorrer, Adv. Mater., 2008, 20, 2633 CrossRef CAS.
- C. Jeffryes, T. Gutu, J. Jiao and G. L. Rorrer, ACS Nano, 2008, 2, 2103–2112 CrossRef CAS.
- S. Chan, Y. Li, L. J. Rothberg, B. L. Miller and P. M. Fauchet, Materials Science & Engineering C-Biomimetic and Supramolecular Systems, 2001, 15, 277–282 Search PubMed.
- S. Chan, P. M. Fauchet, Y. Li, L. J. Rothberg and B. L. Miller, Phys. Status Solidi A, 2000, 182, 541–546 CrossRef CAS.
- V. S. Y. Lin, K. Motesharei, K. P. S. Dancil, M. J. Sailor and M. R. Ghadiri, Science, 1997, 278, 840–843 CrossRef CAS.
- K. P. S. Dancil, D. P. Greiner and M. J. Sailor, J. Am. Chem. Soc., 1999, 121, 7925–7930 CrossRef CAS.
- K. A. Kilian, T. Bocking, S. Ilyas, K. Gaus, M. Gal and J. J. Gooding, 2006 International Conference on Nanoscience and Nanotechnology, 2006, Vols. 1 and 2, 671–674 Search PubMed.
- K. A. Kilian, T. Boecking and J. J. Gooding, Chem. Commun., 2009, 630–640 RSC.
- D. K. Gale, T. Gutu, J. Jiao, C. H. Chang and G. L. Rorrer, Adv. Funct. Mater., 2009, 19, 926–933 CrossRef CAS.
- A. Dorfman, N. Kumar and J. Hahm, Adv. Mater., 2006, 18, 2685 CrossRef CAS.
- N. Kumar, A. Dorfman and J. Hahm, Nanotechnology, 2006, 17, 2875–2881 CrossRef CAS.
- H. E. Townley, K. L. Woon, F. P. Payne, H. White-Cooper and A. R. Parker, Nanotechnology, 2007, 18, 295101 CrossRef.
- L. De Stefano, A. Larnberti, L. Rotiroti and M. De Stefano, Acta Biomater., 2008, 4, 126–130 CrossRef CAS.
- L. De Stefano, L. Rotiroti, M. De Stefano, A. Lamberti, S. Lettieri, A. Setaro and P. Maddalena, Biosens. Bioelectron., 2009, 24, 1580–1584 CrossRef CAS.
- L. De Stefano, I. Rendina, M. De Stefano, A. Bismuto and P. Maddalena, Appl. Phys. Lett., 2005, 87, 233902 CrossRef.
- S. Lettieri, A. Setaro, L. De Stefano, M. De Stefano and P. Maddalena, Adv. Funct. Mater., 2008, 18, 1257–1264 CrossRef CAS.
- K. H. Sandhage, M. B. Dickerson, P. M. Huseman, M. A. Caranna, J. D. Clifton, T. A. Bull, T. J. Heibel, W. R. Overton and M. E. A. Schoenwaelder, Adv. Mater., 2002, 14, 429 CrossRef CAS.
- Z. H. Bao, M. R. Weatherspoon, S. Shian, Y. Cai, P. D. Graham, S. M. Allan, G. Ahmad, M. B. Dickerson, B. C. Church, Z. T. Kang, H. W. Abernathy, C. J. Summers, M. L. Liu and K. H. Sandhage, Nature, 2007, 446, 172–175 CrossRef CAS.
- M. R. Weatherspoon, M. B. Dickerson, G. Wang, Y. Cai, S. Shian, S. C. Jones, S. R. Marder and K. H. Sandhage, Angew. Chem., Int. Ed., 2007, 46, 5724–5727 CrossRef.
- H. W. Ducklow, D. A. Purdie, P. J. L. Williams and J. M. Davies, Science, 1986, 232, 865–867 CrossRef CAS.
- P. G. Falkowski, R. T. Barber and V. Smetacek, Science, 1998, 281, 200–206 CrossRef CAS.
- A. S. Al-Amoudi, Desalination, 2010, 259, 1–10 CrossRef CAS.
- M. Sprynskyy, I. Kovalchuk and B. Buszewski, J. Hazard. Mater., 2010, 181, 700–707 CrossRef CAS.
- M. A. Koehl, P. Jumars and L. Karp-Boss, in Out of the Past, ed. T. Norton, British Phycological Associatioin, Belfast, 2003 Search PubMed.
- M. Pahlow, U. Riebesell and D. Wolf-Gladrow, Limnol. Oceanogr., 1997, 42, 1660–1672 CrossRef.
- L. Karp-Boss, E. Boss and P. Jumars, Oceanogr. Mar. Biol. Annu. Rev, 1996, 34, 71–107 Search PubMed.
- M. M. Musielak, L. KARP-BOSS, P. A. JUMARS and L. J. FAUCI, J. Fluid Mech., 2009, 638, 401–421 Search PubMed.
- M. C. Potter and D. C. Wiggert, Mechanics of Fluids. Third Ed., Brooks/Cole, Pacific Grove, CA, 2002 Search PubMed.
- F. Peters and C. Marrasé, Mar. Ecol.: Prog. Ser., 2000, 205, 291–306 CrossRef.
- A. V. Soloviev, N. V. Vershinsky and V. A. Bezverchnii, Deep-Sea Res., Part A, 1988, 35, 1859–1874 CrossRef.
- S. Clarson, M. Steinitz-Kannan, S. Patwardhan, R. Kannan, R. Hartig, L. Schloesser, D. Hamilton, J. Fusaro and R. Beltz, Silicon, 2009, 1, 79–90 Search PubMed.
- F. Peters, L. Arin, C. Marrasé, E. Berdalet and M. Sala, Journal of Marine Systems, 2006, 61, 134–148 Search PubMed.
- T. Kiørboe, Sci. Mar., 2001, 65 Search PubMed.
- G. Rosengarten, Can we learn from nature to design membranes? The intricate pore sturcture of the diatom., Pohang, South Korea, 2009 Search PubMed.
- U. Riebesell, D. Wolf-Gladrow and V. Smetacek, 1993.
- M. S. Hale and J. G. Mitchell, Nano Lett., 2001, 1, 617–623 CrossRef CAS.
- M. S. Hale and J. G. Mitchell, Nano Lett., 2002, 2, 657–663 CrossRef CAS.
- D. Losic, G. Rosengarten, J. G. Mitchell and N. H. Voelcker, J. Nanosci. Nanotechnol., 2006, 6, 982–989 CrossRef CAS.
- H. Bhatta and G. Rosengarten, Diffusion in Solids and Liquid, 2008 Search PubMed Accepted for publication.
- H. Bhatta, J. Enderlein and G. Rosengarten, J. Nanosci. Nanotechnol., 2009, 9, 6760–6766 CrossRef CAS.
- R. Beck and J. Schultz, Science, 1970, 170, 1302 CrossRef CAS.
- W. Deen, AIChE J., 1987, 33, 1409–1425 CrossRef.
- A. D. Dinsmore, A. G. Yodh and D. J. Pine, Nature, 1996, 383, 239–242 CrossRef CAS.
- K. L. Tung and C. J. Chuang, Desalination, 2002, 146, 129–134 CrossRef CAS.
- W. R. Bowen and A. O. Sharif, Colloids Surf., A, 2002, 201, 207–217 CrossRef CAS.
- S. M.a. F. Muller, Nature, 2003, 424, 53–57 CrossRef CAS.
- B.-q. Ai and L.-g. Liu, J. Chem. Phys., 2008, 128, 024706–024705 CrossRef.
- R. Yongsunthon, S. K. Lower, J. W. B. G. M. G. AllenI. Laskin and S. Sima, in Advances in Applied Microbiology, Academic Press, 2005, vol. Volume 58, pp. 97–124 Search PubMed.
- W. Sparreboom, A. Van Den Berg and J. Eijkel, Nat. Nanotechnol., 2009, 4, 713–720 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2011 |