DOI:
10.1039/C0AN00362J
(Paper)
Analyst, 2011,
136, 201-204
Rational strategy of magnetic relaxation switches for glycoprotein sensing†
Received
31st May 2010
, Accepted 10th September 2010
First published on 14th October 2010
Abstract
A rational strategy of magnetic relaxation switches was proposed here to detect a1-acid glycoprotein (AGP), an acute phase a-globulin plasma glycoprotein. The assay was based on the relaxation time change between the aggregation of magnetic nanoparticles with concanavalin A and the redispersion with AGP, which can avoid the prozone effect and improve the detection accuracy. The assay was an easy and efficient method with two mixing steps and one measurement step, showing a detection limit of 0.66 nM in 0∼0.3 μg mL−1AGP, which was far lower than its normal level in human plasma.
Introduction
Glycosylation is the process of saccharides such as glucoses, linking to protein or lipid molecules on the control of enzyme. It is an important form of co-translational and post-translational modification. The oligosaccharide chains attached to the proteins are glycans, which serve as a variety of structural and functional roles in membrane and secreted proteins. And the proteins linked to glycans are glycoproteins, which are found inside, outside and within cell membranes.1Glycoproteins are important integral membrane proteins in cell–cell interactions. Furthermore, protein glycosylation is involved in many biological functions, such as the folding, transporting and targeting2 of protein, as well as signal transduction.3 Usually, the extent of glycosylation and its abnormal variation are the sign of cancer and other diseases.4 Some tumor markers and therapeutic targets, such as prostate specific antigen (PSA) of prostate cancer, Her2/neu of mammary cancer, CA125 of ovarian cancer are glycoproteins.5
The detection of glycoprotein is routinely accomplished by SDS-PAGE gels and Western blots with colorimetric or fluorescent detection, which need complicated and time-consuming procedures. Therefore, many methods such as HPLC,6mass spectrometry7 and NMR are currently utilized to realize rapid and efficient detection of glycoproteins. Meanwhile, nanomaterials have been developed to detect glycoprotein and study carbohydrate-protein interaction. Because of the unique optical property, gold and silver nanoparticles are chosen to study the carbohydrate-protein interaction sensitively with resonance light scattering.8 Similarly, core shell nanoparticles Fe3O4@Au can perform sensitive detection with the aid of external magnetic field.9 In addition, it can also be performed by the absorption or fluorescence change between dispersion and aggregation of Au nanoparticles.10,11 However, most of these assays are based on the optical properties of the sample, which may be seriously hampered in complex media.
In this study, we present the detection of glycoproteins with magnetic relaxation switches (MRS).12MRS is a novel detection method based on the transverse relaxation time (T2) change of water protons between dispersion and aggregation of functional magnetic nanoparticles by the binding with analyte, and it has been used to detect a variety of targets ranging from small molecules such as DNA, peptide, metal ion to macromolecules such as protein, virus, bacteria and cell.13 It is suitable for the detection in complex media because the assay is based on the magnetic properties.12,14,15 In MRS, a common phenomenon called the prozone effect,16 means the redispersion of formed aggregation by excess analytes would lead to reversible T2 change and interfere with the detection (Fig. S1)†. Herein, a strategy of MRS was designed to solve this problem. As a proof of concept, a1-acid glycoprotein (AGP), an acute phase protein in human serum that functions as the indicator of tissue injury, inflammation, infection (HIV)17 and other diseases18 was chosen as the target molecule to verify the idea. As shown in Fig. 1, superparamagnetic iron oxide (SPIO) nanoparticles aggregated and decreased T2 with concanavalin A (Con A) by the binding of Con A and the surface dextran of SPIO. Subsequently, the analyte AGP was added and redispersed the aggregation, reincreasing T2 by the interaction of AGP with Con A for its high carbohydrate content (45%). The assay can avoid the prozone effect because T2 would not decrease again with excess AGP, and it was an easy method for glycoprotein detection which only includes two mixing steps and one measurement step without cumbersome pretreatments.
 |
| | Fig. 1 Rational strategy of magnetic relaxation switches for glycoprotein sensing. | |
Experimental
Materials
Bovine serum albumin (BSA), PSA, Con A and AGP were purchased from Sigma-Aldrich (St. Louis, MO, USA). Human a2-HS glycoprotein (AHSG) was purchased from ProSpec (Rehovot, Israel). Dextran (Mw = 10T) was acquired from Amresco (USA). All other chemicals were of analytical grade and obtained from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, P. R. China). Deionized water was used for all aqueous solutions in the experiment.
Synthesis and characterization of SPIO
SPIO
nanoparticles were prepared by coprecipitation method as described.19Dynamic light scattering (DLS) measurements were conducted on a Zetasizer Nano (Malvern Instruments S.A., Worcester, United Kingdom) at 25 °C. The hysteresis loop was measured by VSM 7400 (Lakeshore, Westerville, Ohio, USA). Inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements were performed on a Thermo Elemental IRIS Intrepid spectrometer.
Relaxation time measurements
All the relaxation time measurements were performed on a 0.47 T NMI20-Analyst (Niumag Corp., Shanghai, P. R. China) at 25 °C or 38 °C. Longitudinal relaxation time (T1) and T2 were measured by an inversion recovery pulse sequence and a Carr–Purcell–Meiboom–Gill pulse sequence respectively. 1000 μL SPIO aqueous solution (25 μM Fe3+) was mixed with Con A in various concentrations (0∼10 μg mL−1) at room temperature, and then the solution was diluted to 2000 μL before measurement. In the detection of glycoproteins, 1000 μL SPIO aqueous solution (25 mM Fe3+) was first mixed with 50 μL Con A (100 μg mL−1) for 20 min, then AGP and control proteins in various concentrations were added and mixed with the above solution. Finally, the solution was diluted to 2000 μL before measurement.
Results and discussion
Characterization of SPIO
In order to realize the assay, SPIO was first synthesized with Fe2+/Fe3+ in the presence of dextran by coprecipitation method. The resulting SPIO showed an average hydrodiameter of about 50 nm and superparamagnetic characteristic (Fig. 2). The magnetite content was about 49.85% according to the results of ICP-OES. The longitudinal (r1) and transverse (r2) relaxivity of SPIO at 38 °C and 20 MHz was 18.51 mM−1s−1 and 269.2 mM−1s−1 respectively.
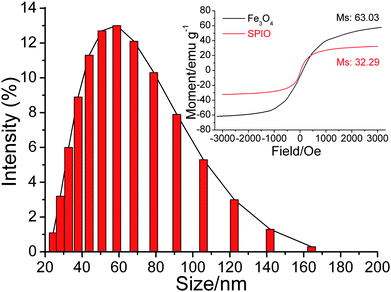 |
| | Fig. 2 Size distribution of SPIO. Inset: magnetization curves of SPIO (red) and Fe3O4 (black). | |
Aggregation of SPIO with Con A
The key step of this assay was the formation of stable aggregation of SPIO with Con A. As shown in Fig. 3, T2 of SPIO (25 μM Fe3+) decreased with increasing Con A concentration immediately after the addition of Con A, and a dramatic decrease was observed in 0∼5 μg mL−1. In 5∼10 μg mL−1 Con A, T2 of SPIO reincreased with the increase of measurement time. T2 of 10 μg mL−1 Con A was closed to T2 of 1 μg mL−1 Con A, 60 min after mixing. Two mechanisms were proposed for the reincrease of T2: (i) aggregation redispersed with excess Con A; (ii) aggregation formed larger cluster with excess Con A. Dynamic light scattering (DLS) results confirmed the second mechanism. The average size of aggregation further increased with 5∼10 μg mL−1 Con A (Fig. 4). The reincrease of T2 can be explained by outer-sphere relaxation theory13 which was not applied in the case of larger cluster.20,21 However, SPIO kept the aggregated state in 0∼4 μg mL−1 Con A with steady T2 for 60 min. Therefore, 2.5 μg mL−1 was chosen as the concentration of Con A for the following assays. Because relaxation time is temperature dependent, the effect of temperature on T2 change was also studied. Temperature with larger T2 change would be preferred because it is easier to be detected. In Fig. 5, variation of T2 (δT2) of SPIO at 38 °C was larger than that at 25 °C (0 min), which showed two distinct linear concentration ranges of 0∼5 and 5∼10 μg mL−1. As shown in the inset, the slope of 0∼5 μg mL−1 was larger than that of 5∼10 μg mL−1, suggesting a more dramatic T2 change in low concentration, which was consistent with the result obtained at 25 °C. In order to perform more sensitive detection, 38 °C was selected as the detection temperature.
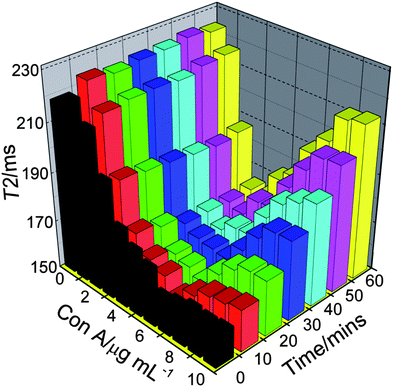 |
| | Fig. 3 Concentration and time dependence on T2 of SPIO with 0∼10 μg ml−1 Con A at 25 °C and 20 MHz. | |
 |
| | Fig. 4 Size distribution of SPIO with 0∼10 μg mL−1 Con A at 25 °C. | |
 |
| | Fig. 5 Concentration dependence on T2 of SPIO with 0∼10 μg mL−1 Con A at 38 °C and 20 MHz. Inset: The linear fit of δT2 with 0∼5 and 5∼10 μg mL−1 Con A. T2 data were the average of 3 measurements. | |
Redispersion of SPIO-Con A with AGP
The detection of target molecule AGP with SPIO-Con A was also performed. In Fig. 6, T2 of SPIO-Con A showed neglectable change just after the addition of AGP, but a distinguishable increase was observed after 30 min, and T2 showed inconspicuous change with subsequent increase of measurement time (data not shown). The increase of T2 was more pronounced with 0∼2.5 μg mL−1AGP, while a concentration of AGP higher than 2.5 μg mL−1 would not increase T2 further. Corresponding DLS result showed the redispersion of SPIO-Con A with AGP (Fig. 6 inset). The average size of aggregation was decreased from 221.3 nm to 186.6 nm with 2.5 μg mL−1AGP, and the size did not change further with subsequent increase of AGP concentration. Although the carbohydrate content of AGP was closed to that of SPIO, the addition of AGP could not reverse SPIO-Con A back to the monodisperse state. A possible reason is the different carbohydrates between AGP and SPIO. AGP is one of the few serum glycoproteins that contain di-antennary as well as tri- and tetra-antennary N-linked glycans. The terminal sugar units of AGP are associated with the diversity of glycans, which are composed of a series of carbohydrates such as mannose, N-acetylglucosamine, fucose, galactose, and neuraminidase (sialic acid), among which, sialic acid is the most common terminal sugar (10∼12%), leading to a very low pI of 2.8∼3.8.22 In contrast, the surface of SPIO is dextran, which is composed of glucoses. Most of all, Con A used here is a lectin that binds preferentially to the oligosaccharides such as mannose or glucose, probably resulting in its different avidity to SPIO and AGP.23 However, Con A have much high avidity to oligomannan structures in N-glycans than monosaccharides such as mannose or glucose, which can lead to a different result. The real reason for its different avidity between SPIO and AGP needs a full study of the effect for each carbohydrate component on the avidity.
 |
| | Fig. 6 T2 of SPIO-Con A with 0∼10 μg mL−1AGP in 0 (open square, black) and 30 min (open circle, red) after mixing at 38 °C and 20 MHz. Inset: Size distribution of SPIO-Con A with 0∼10 μg mL−1AGP. | |
Finally, three kinds of protein with different carbohydrate content, BSA (trace), PSA (8.3%) and AHSG (22.9%) were chosen as control to verify the specificity of SPIO-Con A to AGP (Fig. 7). In contrast with AGP, T2 of SPIO-Con A showed a relatively small change with 0∼0.3 μg mL−1 AHSG, but neglectable change with PSA and BSA in the same concentration, indicating the carbohydrate content was the most important factor in the assay presented here. The sensitivity of the assay was also assessed (Fig. 7 inset). According to 3σ rule, the detection limit was 28.4 ng ml−1 (0.66 nM) in the linear concentration range of 0∼0.3 μg mL−1, which was far lower than the normal concentration of AGP in human plasma (0.6∼1.2 mg mL−1).17 Because the base of the assay was the binding of Con A with carbohydrates, one problem in the practical application such as serum assay would be the interferences by other glycoproteins and carbohydrates. In order to perform specific detection, dextran should be replaced with more specific lectins such as whole germ agglutinin, which recognizes selectively GlcNAc and sialic acid.
 |
| | Fig. 7 T2 of SPIO-Con A with 0∼0.3 μg mL−1AGP (open square, black), AHSG (open circle, red), PSA (open triangle, green) and BSA (open star, blue) at 38 °C and 20 MHz. Inset: The linear fit for T2 of SPIO-Con A with 0∼0.3 μg mL−1AGP. T2 data were the average of 3 measurements. | |
Conclusions
In summary, a rational strategy of magnetic relaxation switches was proposed to detect AGP, an acute phase a-globulin plasma glycoprotein. The detection was based on the relaxation time change between the aggregation of SPIO with Con A and the redispersion of SPIO-Con A with AGP. Prozone effect, the common phenomenon of MRS can be avoided because T2 of the sample would not change reversibly with excess AGP. Furthermore, the assay was an easy and efficient method for rapid detection of glycoprotein. Recently, glyco-nanoparticles were used to detect and differentiate cancer cells with magnetic resonance imaging.24 Therefore, the magnetic-based assay described here held great promise in the detection of a variety of glyco-based target molecules such as glucides, glycolipids and the carbohydrate receptors on cell surface.
Acknowledgements
This work was supported by Shanghai nano project (0852nm03800), Shanghai Leading Academic Discipline Project (B109) and the National Natural Science Foundation of China (No. 20805009, 20890022).
Notes and references
- P. M. Rudd, T. Elliott, P. Cresswell, I. A. Wilson and R. A. Dwek, Science, 2001, 291, 2370–2376 CrossRef.
- A. Helenius and M. Aebi, Science, 2001, 291, 2364–2369 CrossRef CAS.
- L. Wells, K. Vosseller and G. W. Hart, Science, 2001, 291, 2376–2378 CrossRef CAS.
- G. Durand and N. Seta, Clin. Chem., 2000, 46, 795–805 CAS.
- J. Roth, Chem. Rev., 2002, 102, 285–303 CrossRef CAS.
- J. S. Rohrer, G. A. Cooper and R. R. Townsend, Anal. Biochem., 1993, 212, 7–16 CrossRef CAS.
- A. Dell and H. R. Morris, Science, 2001, 291, 2351–2356 CrossRef CAS.
- J. Q. Gao, D. J. Liu and Z. X. Wang, Anal. Chem., 2008, 80, 8822–8827 CrossRef CAS.
- C. H. Liang, C. C. Wang, Y. C. Lin, C. H. Chen, C. H. Wong and C. Y. Wu, Anal. Chem., 2009, 81, 7750–7756 CrossRef CAS.
- S. Lee and V. H. Perez-Luna, Anal. Chem., 2005, 77, 7204–7211 CrossRef CAS.
- C. C. Huang, C. T. Chen, Y. C. Shiang, Z. H. Lin and H. T. Chang, Anal. Chem., 2009, 81, 875–882 CrossRef CAS.
- J. M. Perez, L. Josephson, T. O'Loughlin, D. Hogemann and R. Weissleder, Nat. Biotechnol., 2002, 20, 816–820 CAS.
- I. Koh and L. Josephson, Sensors, 2009, 9, 8130–8145 CrossRef CAS.
- J. M. Perez, F. J. Simeone, Y. Saeki, L. Josephson and R. Weissleder, J. Am. Chem. Soc., 2003, 125, 10192–10193 CrossRef CAS.
- H. Lee, E. Sun, D. Ham and R. Weissleder, Nat. Med., 2008, 14, 869–874 CrossRef CAS.
- G. Y. Kim, L. Josephson, R. Langer and M. J. Cima, Bioconjugate Chem., 2007, 18, 2024–2028 CrossRef CAS.
- S. Colombo, T. Buclin, L. A. Decosterd, A. Telenti, H. Furrer, B. L. Lee, J. Biollaz and C. B. Eap, Clin. Pharmacol. Ther., 2006, 80, 307–318 CrossRef CAS.
- T. Hochepied, F. G. Berger, H. Baumann and C. Libert, Cytokine Growth Factor Rev., 2003, 14, 25–34 CrossRef CAS.
- L. Josephson, C. H. Tung, A. Moore and R. Weissleder, Bioconjugate Chem., 1999, 10, 186–191 CrossRef CAS.
- T. Atanasijevic, M. Shusteff, P. Fam and A. Jasanoff, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14707–14712 CrossRef CAS.
- S. Laurent, D. Forge, M. Port, A. Roch, C. Robic, L. V. Elst and R. N. Muller, Chem. Rev., 2008, 108, 2064–2110 CrossRef CAS.
- T. Fournier, N. Medjoubi-N and D. Porquet, Biochim. Biophys. Acta, Protein Struct. Mol. Enzymol., 2000, 1482, 157–171 Search PubMed.
- H. Lis and N. Sharon, Chem. Rev., 1998, 98, 637–674 CrossRef CAS.
- K. El-Boubbou, D. C. Zhu, C. Vasileiou, B. Borhan, D. Prosperi, W. Li and X. F. Huang, J. Am. Chem. Soc., 2010, 132, 4490–4499 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1. See DOI: 10.1039/c0an00362j |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 






