DOI:
10.1039/C0AN00452A
(Paper)
Analyst, 2011,
136, 196-200
Anti-aggregation of gold nanoparticle-based colorimetric sensor for glutathione with excellent selectivity and sensitivity†
Received
29th June 2010
, Accepted 7th September 2010
First published on 7th October 2010
Abstract
For the widely used Au nanoparticles (AuNPs)-based colorimetric probe, AuNPs generally change from the dispersion to the aggregation state and corresponding colors turn from red to blue concomitantly. In previous studies, there are few probes based on the anti-aggregation of AuNPs though anti-aggregation of AuNPs is preferable to aggregation to achieve higher selectivity. In this manuscript, a fast and simple but sensitive and selective sensor suitable for on-site and real-time detection of glutathione (GSH) has been developed based on the anti-aggregation of AuNPs. The sensor has a LOD of 8 nM and excellent selectivity toward GSH by a factor of 200-fold or more relative to natural amino acids as well as homocysteine (Hcys) and glutathione disulfide (GSSG). The dynamic range of the sensor can be tuned simply by adjusting the amount of aggregation agent used.
1. Introduction
Glutathione (GSH), a thiol-containing tripeptide (γ-Glu-Cys-Gly), is known as the most abundant cellular thiol and plays a central role in maintaining redox homeostasis. It serves as an antioxidant, keeping the cysteine thiol group in proteins in the reduced state and protecting the cells from oxidative stress by trapping free radicals that damage DNA and RNA.1 Upon oxidation, GSH is transformed to glutathione disulfide (GSSG). The concentrations of GSH and GSSG and their molar ratio are indicators of cell functionality and oxidative stress. Thus, ever-increasing demands in various clinical states and medical applications are essentially required to conveniently monitor the GSH concentration, preferably in the nano- to micro-molar concentration range.2 Many strategies have been documented for the quantification of GSH in the literature.3–9 However, to a certain extent, there exist some drawbacks in terms of the actual applicability in most of these detection methods, including relatively time-consuming procedures, requiring complicated and expensive instrumentation, and few methods combine all the desired features for a rapid, reliable, sensitive and simple assay, especially for determining GSH while other structurally related interference biothiols like cysteine (Cys), homocysteine (Hcys) and GSSG are present. Therefore, the development of novel and facile routes that could improve the simplicity, selectivity and sensitivity of the GSH assay is highly desirable.
Colorimetric sensors have been realized as a promising analytical method since signaling the targeted event is through a visual color change in reaction media. Gold nanoparticles (AuNPs) are emerging as an important type of colorimetric reporter because of intrinsically exploitable properties of the high extinction coefficient and of the distinct variation in color associated with the transition of the nanoparticles from the dispersion to the aggregation state or vice versa.10 The AuNPs-based colorimetric sensors have been developed for the facile detection of biologically relevant thiol-containing amino acids biothiols (including Cys, Hcys and GSH) based on the color variation of AuNPs in the turnover process of the dispersion to the aggregation state induced by intermolecular zwitterionic interactions between the biothiols attached onto the gold nanoparticles/nanorods.3 It should be noted that these colorimetric sensors based on AuNPs aggregation processes are not selective among different biothiols because of the structural similarity (Chart 1), all incorporating thiol, carboxylic and amino groups. To our knowledge, only one colorimetric assay of GSH based on the disassembly of aggregated AuNPs has been reported, in which, however, sulfhydryl compounds (including Cys and Hcys) caused interference.11
 |
| | Chart 1 | |
Herein we demonstrate the design of an AuNPs colorimetric sensor for the facile, selective and sensitive detection of GSH based on the anti-aggregation of AuNPs through the competition replacement between GSH and the aggregation agent sodium piperazinebisdithiocarbamate (ppzdtc). The specific affinity of GSH for AuNPs combined with the high extinction coefficient of AuNPs substantially enable this sensor with a detection limit down to 8 nM and selectivity toward GSH by a factor of over 200-fold relative to 20 natural amino acids as well as biothiols Hcys and GSSG. The dynamic range of this assay can be conveniently adjusted based on the amount of ppzdtc used. Furthermore, this method remains much simpler than the existing methods, without the requirements of much instrumentation or designing and synthesizing complicated fluorophore or chromophore molecules to achieve the required sensitivity and selectivity.3–9
2. Experimental
2.1 Materials and instrumentation
All chemicals were obtained from commercial sources and used without further purification. Milli-Q water was used throughout. UV-vis spectra were obtained with a Shimadzu UV-2450 spectrophotometer. 1H NMR spectra were recorded on a Bruker SpectroSpin 400 MHz spectrometer. Transmission electron microscopy (TEM) images were taken on a JEOL JEM-1400 at an acceleration voltage of 100 kV. TEM samples were prepared by depositing a drop of dilute dispersion of nanoparticles on a copper grid coated with carbon film.
Caution: Carbon disulfide (CS2) is highly toxic and has to be handled under a fume hood.
2.2 Preparation of ppzdtc ligand
Sodium piperazinebisdithiocarbamate (ppzdtc) was prepared by a literature method.12 A 2-propanolic solution (100 ml) of piperazine, CS2 and NaOH in relative proportions 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was stirred for about four hours and then filtered. The colorless crystals were obtained through recrystallization from mixture of methanol and diethyl ether. 1H NMR (400 MHz, in D2O): δ 4.35 (t). The NMR spectrum is shown in Fig. S1.†
2 was stirred for about four hours and then filtered. The colorless crystals were obtained through recrystallization from mixture of methanol and diethyl ether. 1H NMR (400 MHz, in D2O): δ 4.35 (t). The NMR spectrum is shown in Fig. S1.†
2.3 Preparation of AuNPs
AuNPs (13 nm in diameter) were synthesized by sodium citrate reduction of HAuCl4 following a literature procedure.13 Briefly, trisodium citrate (5 mL, 38.8 mM) was rapidly added to a boiling solution of HAuCl4 (50 mL, 1 mM), and the solution was kept continually boiling for another 30 min to give a wine-red solution. After filtering the solution through a 0.45 mm Millipore syringe to remove the precipitate, the filtrate was stored at 4 °C.
2.4
Detection of
GSH
For the detection of GSH, different concentrations of GSH were mixed well with ppzdtc ligands in 350 μL of 2-[4-(2-hydroxyethyl)-1-piperazinyl]ethanesulfonic acid buffer (HEPES; 10 mM, pH 7.4) for 5 min, and then the ppzdtc-GSH solution was mixed with the AuNPs dispersion (300 μL). The total volume of the solution was 650 μL and the final concentration of AuNPs was 5 nM, ppzdtc ligand was 1.5 μM. Photographs and UV-Vis spectra were taken after ppzdtc-GSH was added to the AuNPs dispersion for 30 min.
3. Results and discussion
3.1 Aggregation and anti-aggregation of AuNPs induced by ppzdtc ligand and GSH, respectively
Scheme 1 illustrates the method for the detection of GSH. In the absence of GSH, the addition of ppzdtc to the AuNPs suspension leads to an aggregation of AuNPs and the color changes from the origin red to blue concomitantly with the increase of ppzdtc concentration. The photographic images and corresponding UV-vis spectra of AuNPs dependent on the concentration of ppzdtc are available in Fig. 1. The aggregation of AuNPs is derived from the bridge-linking coordination of ppzdtc molecules on the AuNPs surface that displace the original weakly surface-bound citrate encapsulation through the dithiocarbamate groups in both ends of the molecule, and finally cross-link the AuNPs. Thus the ppzdtc molecule is a kind of aggregation agent for AuNPs.
 |
| | Scheme 1 Schematic representation of GSH-induced anti-aggregation of AuNPs with the use of ppzdtc as aggregation agent. | |
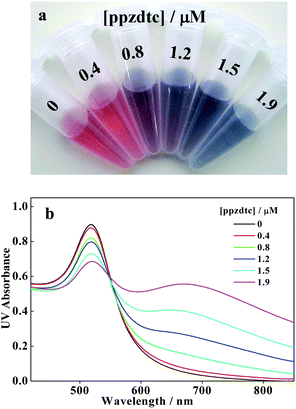 |
| | Fig. 1 Photographic images (a) and UV-vis spectra (b) of AuNPs suspensions under different concentrations of ppzdtc ligand. | |
Interestingly, when a ppzdtc solution with a concentration that can induce an observable aggregation of AuNPs is firstly treated with GSH and then mixed with the AuNPs suspension, the color change of AuNPs goes through a totally reverse process: with the increase of GSH concentration, the color changes from blue to purple, and finally to wine-red, which corresponds to AuNPs changing from an aggregation to a dispersion state. This clearly demonstrates that GSH takes the role of anti-aggregation agent. The anti-aggregation role of GSH toward AuNPs is derived from the stronger affinity of GSH (or referred to coordination priority) than that of the aggregation agent ppzdtc for AuNPs due to the multidentate anchor (e.g., –SH, –NH2 and –COO−) together with the specific steric structure existing in GSH. This renders the GSH molecule to encapsulate AuNPs in priority and leaving little or no chance for ppzdtc binding on the AuNPs, and thus the AuNPs turn from the aggregation to the dispersion state. TEM measurement clearly demonstrates the ppzdtc-induced aggregation (Fig. 2a) and GSH-induced anti-aggregation (Fig. 2b) of AuNPs. To further demonstrate the coordination priority determining the anti-aggregation process, we design the demonstration experiment as follows. It has been well established that the aggregation capacity for AuNPs is in the order of HCys ≫ Cys > GSH.3a Our experimental results indicated that 14 μM HCys can induce aggregation of citrated-capped 13 nm AuNPs (5 nM) in 10 min; while for GSH with the same concentration, the spectral change is not detectable in one day and for Cys it needs up to 4 h to detect a significant spectral change (Fig. 3). When a mixture of HCys and GSH or Cys and GSH (14 μM for each component) was added independently to the AuNPs suspension, the obtained results seem counter-intuitive. The rate for AuNPs aggregation was expected to be faster in comparison with the system containing 14 μM HCys alone. However, the experimental results showed that the rate for AuNPs aggregation has slowed down remarkably in the co-presence of HCys and GSH (Fig. 3). A similar result was also observed in the mixture of Cys and GSH compared with system containing Cys alone. These results clearly demonstrate that the coordination priority of GSH on AuNPs reduces the aggregation capacity of other reagents that co-exist.
 |
| | Fig. 2 The TEM images of (a) the aggregated AuNPs in solution containing 1.5 μM ppzdtc in the absence of GSH, and (b) the re-dispersed AuNPs with the presence of 1.5 μM ppzdtc and 0.25 μM GSH. | |
 |
| | Fig. 3 Temporal evolution of UV-vis spectra of an AuNPs (5 nM) suspension containing Hcys (14 μM, a), Cys (14 μM, b), Hcys + GSH (14 μM for each, c), or Cys + GSH (14 μM for each, d). (e) Summary of spectral variation illustrated by curves of A520/A640 (the ratio of absorbance at 640 nm to that at 520 nm) vs. time. It is noted that a lower A520/A640 ratio corresponds to a greater extent of aggregation of AuNPs. | |
3.2 Assay of GSH
The process of anti-aggregation of AuNPs induced by GSH can be visualized by the naked eye and also be monitored by UV-vis spectroscopy quantitatively (Fig. 4). As mentioned above, with the addition of the mixture solution containing ppzdtc (1.5 μM) and GSH to the as-prepared citrate-stabilized 13 nm AuNPs suspension (5 nM) in physiological pH values (10 mM HEPES buffer, pH 7.4), the solution color turned from blue to purple, and finally to red with the increase of GSH concentration from 0 to 250 nM. The dependence of the AuNPs suspension color together with the corresponding UV-vis spectra on the GSH concentration is shown in Fig. 4. In the case of the absence of GSH, there are two absorption peaks in the spectrum: one is centered at ∼520 nm, which is ascribed to the surface plasmon resonance (SPR) absorption corresponding to the dispersed AuNPs; the other is centered at ∼640 nm, which is ascribed to the absorption from the aggregated AuNPs.14 It is clear that with the increase of GSH concentration, the absorbance at 640 nm decreased systematically; while that at 520 nm increased contrarily. Such a change in the spectrum coincides with the change in solution color displayed in the inset of Fig. 4a. Even the addition of a nanomolar amount (8 nM) of GSH can lead to a change in the solution color that could be distinguished from that of the initial GSH blank solution. This demonstrates that this sensor could be used for the direct visualization of GSH down to the nanomolar level. When the GSH concentration was up to 250 nM, no further variation was observed in the spectrum and the absorption spectrum recovered almost completely to that of the plain AuNPs suspension. The ratio of the absorbance at 520 nm (A520) to that at 640 nm (A640) was used to monitor the color variation caused by different concentrations of GSH (Fig. 4b). A lower ratio is associated with aggregated AuNPs of blue color, while a higher ratio corresponded to dispersed AuNPs of red color. With the increase of the GSH concentration in the ppzdtc-AuNPs system, this ratio increased linearly (linear correlation R = 0.989) from 1.3 to 11.2 over the GSH concentration range of 8–250 nM with the linear detection limit of 8 nM. To our knowledge, this detection limit is among the lowest values reported for GSH detection.3–9 It should be noted that the selection of the peak position can only influence the value of the calculated absorbance ratio, but shows a similar trend in the dependence on the GSH concentration.
 |
| | Fig. 4 (a) UV-vis spectra of AuNPs suspension containing 1.5 μM ppzdtc in the presence of different GSH concentrations: (1) 0, (2) 8, (3) 30, (4) 50, (5) 100, (6) 150, (7) 200, and (8) 250 nM. The inset shows the corresponding images of color change. (b) Calibration curve of A520/A640vs.GSH concentration in the AuNPs suspension. The inset shows the linear dependence of A520/A640 on the GSH concentration. | |
3.3 High selectivity
As reported, the coordination chemistry of GSH usually involves two or more sites of the molecule (e.g., –SH and –COO−).15 Such chelating makes the GSH-metal atom interactions stronger than other thiols or amino acids with a single –SH or weak amine or carboxylate chelating site.15 Furthermore, the large steric hindrance effect created by GSH (greater than Cys and Hcys) always enhances the stability to coordinate to the metal atoms or ions.16 The strong affinity of GSH for metal nanoclusters is also embodied in its detoxification of heavy metals in plant cells.17 Recent studies also demonstrated that GSH is superior to other thiols as a capping ligand for semiconductor nanocrystals.18 As stated above, the detection mechanism of the reported sensor is based on the coordination priority of GSH in the competition with the aggregation agent ppzdtc, and it is reasonable to suppose that the tremendous difference in coordination capability and steric hindrance effects between the GSH and amino acids as well as other thiol-containing molecules ensures that this sensor system has a high selectivity for GSH detection. To evaluate the high selectivity of this sensor for GSH, 20 natural amino acids as well as some common bio-thiols such as Hcys and GSSG were examined under identical conditions. It was found that upon addition of these interfering molecules with concentrations up to 0.6 mM, the AuNPs remained in the aggregated state with the corresponding blue-purple color. However, the introduction of GSH with a concentration down to 250 nM could effectively induce an anti-aggregation of the AuNPs and rendered the AuNPs in the dispersed state with a red color (Fig. 5). This clearly demonstrates that all of the 20 natural amino acids as well as Hcys and GSSG could not produce the wrong signals even if present at 200-fold higher concentrations. The absorbance ratios of A520/A640 were calculated on the basis of an analyte concentration of 0.6 mM except for GSH at 0.25 μM to further estimate the selectivity of this sensor to GSH (Fig. 5). It is clearly seen that the most striking effect is observed for GSH, whereas 20 natural amino acids as well as Hcys and GSSG do not obviously influence the aggregation state of the AuNPs. This means that our sensor responds selectively towards GSH by a factor of 200-fold or more relative to the interfering molecules. Notably, the discrimination of GSH from Cys and Hcys is of particular importance since GSH are often difficult to be detected from them due to their similar response to many other sensors.3–6
 |
| | Fig. 5 Absorbance ratio A520/A640 of solutions containing AuNPs (5.0 nM), ppzdtc (1.5 μM), and various interferent molecules (each 0.6 mM, except for GSH at 250 nM). The inset shows the corresponding photographic images of the AuNPs suspensions. | |
In order to verify the performance of the sensor for the detection of GSH in practical applications, the GSH selectivity response in the presence of amino acids and bio-thiols as stated above was tested. The particular examples of non-interference in the co-presence of Cys and Hcys are illustrated in Fig. 6. Experimental results indicated that with the presence of a 3-fold concentration of Cys or Hcys relative to GSH in the sample system, the UV-Vis spectra (or solution color) of the AuNPs suspension gave an identical response in comparison with the system containing GSH alone. These results suggest that our AuNPs-based sensor has a high selectivity towards GSH and that the presence of other thiols or amino acids produced a negligible effect on the measurement. In addition, this sensor also showed tolerance to the pH variation. Fig. S3† showed that the pH value in the range of 5.5–8.5 had only a small perturbation on the measurement of the GSH content.
 |
| | Fig. 6 Photographic images (a) and corresponding UV-vis spectra (b) of AuNPs suspensions (5 nM) in the system containing 1.5 μM ppzdtc and 250 nM GSH with or without the presence of 750 nM Cys or 750 nM Hcys. | |
3.4 Tuning dynamic range
A tunable dynamic range is important for practical applications as the desirable concentration for the same target analyte can be different for various applications. Because the anti-aggregation of AuNPs is transferred from the competition replacement between ppzdtc by GSH, we believed that a higher concentration of GSH should be needed to achieve the anti-aggregation effect if a higher concentration of aggregation agent ppzdtc is used. To test this hypothesis, we carried out the same colorimetric sensing as stated above, except that the ppzdtc concentration was varied to 5.4 μM instead of 1.5 μM as stated above. Experimental results demonstrated that the dynamic range of GSH was shifted to 8.5–17 μM (Fig. S4†). It is clear that a wide detection range and application of this sensor can be realized with the facile adjustment of the aggregation agent ppzdtc concentration.
4. Conclusions
In summary, we have developed a facile but sensitive and selective colorimetric sensor for the quantitative detection of GSH at physiological pH conditions based on the anti-aggregation of AuNPs in the ppzdtc-AuNPs system. This sensor has a detection limit of 8 nM and selectivity toward GSH by a factor of over 200-fold relative to 20 natural amino acids as well as Hcys and GSSG. Moreover, the dynamic range of this sensor can be tuned simply by adjusting the amount of aggregation agent ppzdtc, facilitating its practical applications. This colorimetric sensing method provides a general platform for sensing species based on the anti-aggregation of AuNPs transferred from the competition replacement between target analytes and the aggregation agent, and further research along this line is currently in progress.
Acknowledgements
We thank the NSFC (20771037, 20871047), NCET-06-0417, Shuguang Project (06SG33), SRFDP (20070251014), the Fundamental Research Funds for the Central Universities, and the Program for Professor of Special Appointment at Shanghai Institutions of Higher Learning for financial support.
References
-
C. K. Mathews, K. E. van Holde and K. G. Ahem, Biochemistry, Addison Wesley Longman, San Fransisco, 3rd edn, 2000 Search PubMed.
- P. Monostori, G. Wittmann, E. Karg and S. Turi, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3331–3346 CrossRef.
-
(a) F. X. Zhang, L. Han, L. B. Israel, J. G. Daras, M. M. Maye, N. K. Ly and C. J. Zhong, Analyst, 2002, 127, 462–465 RSC;
(b) P. K. Sudeep, S. T. S. Joseph and K. G. Thomas, J. Am. Chem. Soc., 2005, 127, 6516–6517 CrossRef CAS;
(c) I.-I. S. Lim, W. Ip, E. Crew, P. N. Njoki, D. Mott, C. J. Zhong, Y. Pan and S. Zhou, Langmuir, 2007, 23, 826–833 CrossRef CAS;
(d) I.-I. S. Lim, D. Ott, W. Ip, P. N. Njoki, Y. Pan, S. Zhou and C. J. Zhong, Langmuir, 2008, 24, 8857–8863 CrossRef CAS;
(e) C. Wu and Q.-H. Xu, Langmuir, 2009, 25, 9441–9946 CrossRef CAS.
-
(a) Y.-H. Ahn, J.-S. Lee and Y.-T. Chang, J. Am. Chem. Soc., 2007, 129, 4510–4511 CrossRef CAS;
(b) Y. Fujikawa, Y. Urano, T. Komatsu, K. Hanaoka, H. Kojima, T. Terai, H. Inoue and T. Nagano, J. Am. Chem. Soc., 2008, 130, 14533–14543 CrossRef CAS;
(c) W. Lin, L. Yuan, Z. Cao, Y. Feng and L. Long, Chem.–Eur. J., 2009, 15, 5096–5103 CrossRef CAS;
(d) Y. Zhang, Y. Li and X.-P. Yan, Anal. Chem., 2009, 81, 5001–5007 CrossRef CAS;
(e) S. Banerjee, S. Kar, J. M. Perez and S. Santra, J. Phys. Chem. C, 2009, 113, 9659–9663 CrossRef CAS;
(f) Z. Yao, X. Feng, C. Li and G. Shi, Chem. Commun., 2009, 5886–5888 RSC;
(g) J. Liu, C. Bao, X. Zhong, C. Zhao and L. Zhu, Chem. Commun., 2010, 46, 2971–2973 RSC.
- T. Mourad, K. L. Min and J. P. Steghens, Anal. Biochem., 2000, 283, 146–152 CrossRef CAS.
- Y.-H. Ahn, J.-S. Lee and Y.-T. Chang, J. Am. Chem. Soc., 2007, 129, 4510–4511 CrossRef CAS.
-
(a) E. J. Pacsial-Ong, R. L. McCarley, W. Wang and R. M. Strongin, Anal. Chem., 2006, 78, 7577–7581 CrossRef CAS;
(b) D. Bhattacharyay, K. Dutta, S. Banerjee, A. P. F. Turner and P. Sarkara, Electroanalysis, 2008, 20, 1947–1952 CrossRef CAS;
(c) J. C. Ndamanisha, J. Bai, B. Qi and L. Guo, Anal. Biochem., 2009, 386, 79–84 CrossRef CAS.
-
(a) G. G. Huang, X. X. Han, M. K. Hossain and Y. Ozaki, Anal. Chem., 2009, 81, 5881–5888 CrossRef CAS;
(b) Y. Wang, Y. Xie, M. Bernier and I. W. Wainer, J. Chromatogr., A, 2009, 1216, 3533–3537 CrossRef CAS;
(c) J. Mendoza, T. Garrido, R. Riveros and J. Parada, Phytochem. Anal., 2009, 20, 114–119 CrossRef CAS.
-
(a) A. R. Ivanov, I. V. Nazimov and L. A. Baratova, J. Chromatogr., A, 2000, 870, 433–442 CrossRef CAS;
(b) Y.-F. Huang and H.-T. Chang, Anal. Chem., 2007, 79, 4852–4859 CrossRef CAS.
-
(a) M. C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS;
(b) H. Li, Q. Zheng and C. Han, Analyst, 2010, 135, 1360–1364 RSC.
- N. Uehara, K. Ookubo and T. Shimizu, Langmuir, 2010, 26, 6818–6825 CrossRef CAS.
- J.-P. Legros, D. T. and J. Galy, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 1984, 40, 801–804 CrossRef.
- J. J. Storhoff, R. Elghanian, R. C. Mucic, C. A. Mirkin and R. L. Letsinger, J. Am. Chem. Soc., 1998, 120, 1959–1964 CrossRef CAS.
- J. J. Storhoff, A. A. Lazarides, R. C. Mucic, C. A. Mirkin, R. L. Letsinger and G. C. Schatz, J. Am. Chem. Soc., 2000, 122, 4640–4650 CrossRef CAS.
- M. S. Diaz-Cruz, F. Mendieta, R. Tauler and M. Esteban, J. Inorg. Biochem., 1997, 66, 29–36 CrossRef CAS.
- B. Han, J. Yuan and E. Wang, Anal. Chem., 2009, 81, 5569–5573 CrossRef CAS.
- X. Michalet, F. F. Pinaud, L. A. Bentolila, J. M. Tsay, S. Doose, J. J. Li, G. Sundaresan, A. M. Wu, S. S. ambhir and S. Weiss, Science, 2005, 307, 538–544 CrossRef CAS.
-
(a) H. Qian, C. Dong, J. Weng and J. Ren, Small, 2006, 2, 747–751 CrossRef CAS;
(b) Y. Zheng, S. Gao and J. Y. Ying, Adv. Mater., 2007, 19, 376–380 CrossRef CAS;
(c) Z. Fang, Y. Li, H. Zhang, X. Zhong and L. Zhu, J. Phys. Chem. C, 2009, 113, 14145–14150 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 was stirred for about four hours and then filtered. The colorless crystals were obtained through recrystallization from mixture of methanol and diethyl ether. 1H NMR (400 MHz, in D2O): δ 4.35 (t). The NMR spectrum is shown in Fig. S1.†
2 was stirred for about four hours and then filtered. The colorless crystals were obtained through recrystallization from mixture of methanol and diethyl ether. 1H NMR (400 MHz, in D2O): δ 4.35 (t). The NMR spectrum is shown in Fig. S1.†







