The potential of a cyclo-As5 ligand complex in coordination chemistry†
Hannes
Krauss
,
Gabor
Balázs
,
Michael
Bodensteiner
and
Manfred
Scheer
*
Institute of Inorganic Chemistry, University of Regensburg, D-93040, Regensburg, Germany. E-mail: manfred.scheer@chemie.uni-regensburg.de; Fax: +49 941 9434439; Tel: +49 941 9434441
First published on 15th June 2010
Abstract
The reaction of [Cp*Fe(η5-As5)] (1) with CuI halides leads to the formation of the 1D-polymeric compounds [{Cu(μ-X)}3(CH3CN){Cp*Fe(η5:η2:η2:η2-As5)}]n (X = Cl (2), Br (3)), [{Cu(μ3-I)}2{Cp*Fe(η5:η2:η2-As5)}]n (4) and [{Cu(μ-I)}3{CuI}{Cp*Fe(η5:η2:η2:η1:η1-As5)}{Cp*Fe(η5:η5:η2-As5)}]n (5). The polymers are built up by the π-coordination of the cyclo-As5 ring to (CuX)n moieties forming discrete units, which are additionally linked by weak intermolecular As⋯Cu σ-interactions. Only polymer 4 is an exception revealing a (CuI)n ladder which is alternately coordinated by molecules of 1. In a side arm of polymer 5 a novel η5-coordination of a Cu atom below the cyclo-As5 ring is found, showing an unprecedented heteroleptic triple-decker sandwich complex with a polyarsenic middle-deck. All products are characterised by single crystal X-ray structure analysis. To define the differences in the coordination behaviour of 1 and its phosphorus analogue [Cp*Fe(η5-P5)] (1a), DFT calculations are described.
Introduction
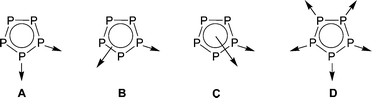
The self-organisation of discrete units to form supramolecular aggregates and networks has become one of the foremost areas of current research.1 Contrary to established principles in this field which make almost exclusively use of N- and O-donor-containing ligands to connect different metal centres, we developed an alternative concept using Pn ligand complexes as linking moieties. The concept is based on the use of different organometallic Pn ligand moieties in the reaction with Lewis acidic CuI and AgI complex units to obtain inorganic polymers and large spherical oligomers.2 Note that the first attempts in this direction were carried out by the groups of Stoppioni and Peruzzini using [(triphos)M(η3-P3)] (M = Co, Rh, or Ir; triphos = 1,1,1-tris(diphenylphosphinomethyl)ethane) as starting material.3,4 We have already shown that the reaction of the tetrahedral complex [{CpMo(CO)2}2(μ,η2-P2)] with CuI and AgI salts yields dimeric and polymeric coordination compounds.5 Also the use of the cyclo-P3 ligand complex [CpMo(CO)2(η3-P3)] results in the formation of novel 1D polymers.6However, the most progress is obtained using the pentafold-symmetric molecule [CpRFe(η5-P5)] (CpR = Cp*, C5Me4Et) containing a cyclo-P5 ligand as a building block. Thus, in the reaction of [CpRFe(η5-P5)] with CuI,7 AgI8 and TlI9 starting materials, one dimensional (1D) and two dimensional (2D) polymers are formed, in which the 1,2- (type A), η2:η1- (type B) and η5:η1-coordination modes (type C) are found. By applying special reaction conditions and stoichiometries, the reaction with CuI halides leads to the formation of soluble spherical aggregates with fullerene-like topology,10 in which all P atoms coordinate with their lone pairs (type D). Furthermore, these systems react in a template controlled reaction to form large spherical clusters by encapsulating guest molecules such as C60 or o-carborane.11 Besides pentaphosphaferrocene, so far, only the cyclo-P4 ligand complex [Cp′′Ta(CO)2(η4-P4)] (Cp′′ = η5-C5H3tBu2) forms a fullerene-like spherical cluster revealing a C32 topology.12
In view of the versatility of Pn ligand complexes as linking moieties in coordination and supramolecular chemistry we are interested in exploring the potential of Asn ligand complexes.13 Recently, novel results have been reported based e.g. on the generation of AsP3.14 Although the reactivity of Asn ligand complexes has been investigated in organometallic chemistry to some extent,15 no examples of supramolecular aggregates featuring these compounds as connecting moieties are known. The first attempts with [Cp*Mo(CO)2(η3-As3)]16 as a starting material in coordination chemistry towards CuI halides have demonstrated the formation of discrete dimers which are connected by very weak Cu⋯As interactions to incoherent polymeric assemblies.17 However, the coordination behaviour of [Cp*Fe(η5-As5)] (1)18 is of particular interest. Here the question is raised as to whether similar coordination modes A–D of the [Cp*Fe(η5-P5)] (1a) are to be designed for the cyclo-As5 ligand complex 1 by using mainly the lone pairs of the group 15 element atoms. Furthermore, the ability of 1 to form spherical aggregates with fullerene-like topology is of special interest. We report herein on the first results using the cyclo-As5 ligand complex 1 in coordination chemistry, which differs significantly from the coordination behaviour of the cyclo-P5 homologue.
Results and discussion
DFT calculations
Based on the relatively similar behaviour of organophosphorus and organoarsenic compounds in general one would expect a similar reactivity of Asn ligand complexes as is observed for Pn ligand complexes. To gain insight into the potential reactivity of 1 and [Cp*Fe(η5-P5)] (1a) towards Lewis acids, their electronic structures were modelled by DFT calculations using the hybrid B3LYP functional.†19 The geometries of 1 and 1a were optimised in the staggered conformation of the C5v symmetry. The isosurfaces of the frontier molecular orbitals of 1 and 1a are depicted in Fig. 1.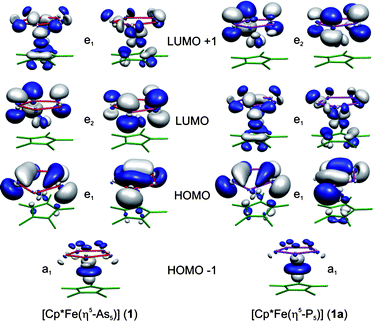 | ||
| Fig. 1 Isosurfaces of the frontier molecular orbitals in 1 and 1a. | ||
The degenerate e1 orbital pairs represent the highest occupied molecular orbital (HOMO) and are mainly localised on the cyclo-As5 and cyclo-P5 ligands. The a1 symmetric HOMO−1 orbital is composed of the iron dz2 orbital with almost no ligand orbital contribution. The energies of the HOMO and HOMO−1 in 1 lie 0.33 eV and 0.27 eV higher than in 1a. Furthermore, differences in the electronic structures of 1 and 1a can also be observed in the lowest unoccupied molecular orbital (LUMO) region. The LUMO in 1 is doubly degenerate (e2 symmetry) involving mainly π orbital contribution from the cyclo-As5 ligand, and the LUMO+1 (e1 symmetry) contains metal and Cp* as well as cyclo-As5 ligand contributions. In 1a the relative energies of the unoccupied e2 and e1 orbitals are reversed.† However, it has to be noted that the energy difference between the unoccupied orbitals e1 and e2 is very narrow (1: 0.07 eV and 1a: 0.11 eV). The HOMO–LUMO gap in 1 (4.16 eV) is slightly smaller than that in 1a (4.48 eV) which is caused by the higher energy of the occupied frontier molecular orbitals in 1 in comparison to 1a. The natural charge distribution shows a negative charge accumulation on the iron atom (−1.33 in 1 and −1.11 in 1a) and a positive charge accumulation on the arsenic and phosphorus atoms (0.19 in 1 and 0.14 in 1a). This and the relative energy of the HOMO in 1 in comparison to 1a suggest a somewhat more electron releasing character of the cyclo-As5 unit in 1 compared to the cyclo-P5 moiety in 1a. Accordingly, one would expect that 1 should be a weaker ligand than 1a. Due to the small differences in the ground state electronic structure, a similar coordination behaviour might be expected. Surprisingly, the experimental results show a considerable difference.
Synthesis and characterisation of the products
The reactions of complex 1 with copper halides CuX were carried out in CH2Cl2/CH3CN solutions using layering techniques (Scheme 1). In the cases of copper chloride and bromide the isostructural compounds [{Cu(μ-X)}3(CH3CN){Cp*Fe(η5:η2:η2:η2-As5)}]n (X = Cl (2), Br(3)) were obtained in good yields. In contrast, the reaction with copper iodide yields the products [{Cu(μ3-I)}2{Cp*Fe(η5:η2:η2-As5)}]n (4) and [{Cu(μ-I)}3{CuI}{Cp*Fe(η5:η2:η2:η1:η1-As5)}{Cp*Fe(η5:η5:η2-As5)}]n (5), comprising the same stoichiometric composition but showing different structural arrangements.† By varying the stoichiometry of the reactions no change in the nature of the products is observed. As mentioned before, the reaction of the phosphorus analogue 1a with copper halides yields 1D and 2D polymeric compounds,10 whereas under diluted reaction conditions or in the presence of templates this reaction proceeds to form spherical aggregates.11 Surprisingly, by applying the latter reaction conditions to the arsenic derivative 1 (reactions in diluted solutions or in the presence of e.g. C60 as a template), the polymeric compounds 2–5 are exclusively formed. This behaviour of the pentaarsaferrocene was unexpected at a first glance; however, it demonstrates the extended π coordination behaviour of 1 in contrast to a more σ donation reactivity pattern of the pentaphosphaferrocene.![Reaction behaviour of [Cp*Fe(η5-As5)] (1) with different Cu(i) halides. Depicted are the repeating units of the products 2–5.](/image/article/2010/SC/c0sc00254b/c0sc00254b-s1.gif) | ||
| Scheme 1 Reaction behaviour of [Cp*Fe(η5-As5)] (1) with different Cu(I) halides. Depicted are the repeating units of the products 2–5. | ||
The products are stable in the solid state under an atmosphere of nitrogen and are insoluble in common solvents without decomposition. This is also reflected in the ESI mass spectra.† In CH3CN solutions no peaks of molecular units of the polymers are detected. Peaks of low intensity could be assigned to aggregates formed by two molecules of 1 and one Cu+ cation or a fragment of (Cu2X)+ (X = Cl, Br, I). Furthermore, a peak of 1 in combination with one Cu+ cation and one solvent molecule CH3CN is identified. This indicates that small amounts of depolymerised material are present in the solutions of the solids at ambient temperatures.
The molecular structures of compounds 2–5 were determined by single crystal X-ray diffraction.‡ The 1D-polymeric compounds 2 and 3 are isostructural; their molecular structure is shown in Fig. 2, and the interaction between the monomeric building blocks forming the 1D polymeric chain is depicted in Fig. 3. Table 1 contains selected bond lengths and angles.
 | ||
| Fig. 2 Section of the 1D polymeric structure of 2 (X = Cl) and 3 (X = Br) revealing the repeating units. Hydrogen atoms are omitted for clarity. | ||
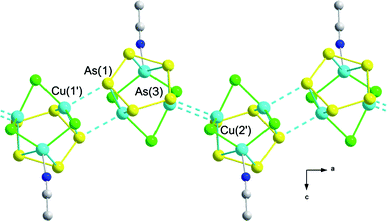 | ||
| Fig. 3 Orientation of the repeating units of 2 and 3 along the crystallographic a-axis. Hydrogen atoms and Cp*Fe fragments are omitted for clarity. | ||
| 2 (X = Cl) | 3 (X = Br) | |
|---|---|---|
| As(1)–As(2) | 2.3911(10) | 2.3880(9) |
| As(1)–As(5) | 2.3613(11) | 2.3671(9) |
| As(2)–As(3) | 2.3751(10) | 2.3793(8) |
| As(3)–As(4) | 2.4107(10) | 2.4100(9) |
| As(1)–Cu(1) | 2.4733(10) | 2.4698(11) |
| As(2)–Cu(1) | 2.4692(10) | 2.4809(11) |
| As(3)–Cu(2) | 2.4534(10) | 2.4577(11) |
| As(4)–Cu(2) | 2.5647(11) | 2.5513(11) |
| As(4)–Cu(3) | 2.5677(11) | 2.5783(11) |
| As(3)–Cu(2′) | 2.8372(13) | 2.9653(11) |
| As(1)–Cu(1′) | 2.9075(13) | 2.9608(11) |
| Fe(1)–As(3) | 2.4660(11) | 2.4664(10) |
| Fe(1)–As(4) | 2.5538(11) | 2.5621(11) |
| Cu(1)–X(1) | 2.3105(17) | 2.4260(11) |
| Cu(2)–X(3) | 2.2756(16) | 2.3944(11) |
| Cu(3)–X(3) | 2.4005(17) | 2.5283(10) |
The main structural motif of 2 and 3 is a monomer-like building block, consisting of the pentaarsaferrocene moiety coordinated by all five arsenic atoms to the three copper atoms of a six-membered (CuX)3 ring in a novel η2:η2:η2 coordination mode. The (CuX)3 ring is centred below the cyclo-As5 ring and shows a distorted chair conformation. The formation of such copper halide rings is known in the literature,20,21 but usually, they are sections of a polymeric (CuX)n network, in contrast to the discrete (CuX)3 rings found in 2 and 3. The Cu–X (X = Cl, Br) distances are comparable to those found in the literature21–23 ranging from 2.276(2) Å to 2.401(2) Å in 2 and 2.394(1) to 2.528(1) Å in 3, respectively. Interestingly, the distance between Cu(2) and Cu(3) is remarkably short (2.669(1) Å (2); 2.667(1) Å (3), respectively), indicating the presence of Cu⋯Cu interactions.24 Interactions of this kind have been well documented and often cause photoluminescence properties.25,26 However, preliminary investigations for 2 and 3 show no luminescence.
Due to the π-coordination of the As–As edges towards the copper atoms, all As–As distances in the cyclo-As5 unit are elongated in comparison to 1 (2.312(2)–2.319(2) Å)18 and vary in a bigger range. The shortest bond is found between As(1)–As(5) (2.361(1) Å (2); 2.367(1) Å (3)) and the longest is found between the doubly coordinating atom As(4) and its neighbours (e.g. As(3)–As(4): 2.411(1) Å (2); 2.410(1) Å (3)). A further consequence of the double As⋯Cu interaction of As(4) is the distortion of the planar cyclo-As5 unit to form a more envelope-like shape, dragging As(4) about 0.26 Å out of the cyclo-As5 plane towards the copper atoms and elongating the Fe(1)–As(4) bond to 2.554(1) Å (2) and 2.562(1) Å (3) when compared e.g. to the Fe(1)–As(3) bond (2.466(1) Å (2) and 2.466(1) Å (3)). The Cu–As distances (2.453(1)–2.568(1) (2); 2.458(1)–2.578(1) (3)) of the arsenic atoms (As(1) and As(3), respectively) of the cyclo-As5 ring and the copper atoms of the (CuX)3 ring are in the range of those reported.17,27 Besides these interactions, one copper atom is additionally coordinated by a solvent molecule of acetonitrile. The most intriguing feature of 2 and 3 is the additional end-on coordination of two copper atoms by arsenic atoms of neighbouring molecules of 1, leading to an infinite aggregation of the monomeric building blocks along the crystallographic a axis. In this way, a sinusoidal undulated 1D polymer is formed (Fig. 3). The distances between these atoms (2.837(1) to 2.908(1) Å for 2 and 2.961(1) to 2.965(1) Å for 3) are shorter than the sum of the van der Waals radii of As and Cu (3.25 Å)28 and indicate a weak interaction. However, they are responsible for the low solubility of these 1D polymers. Similar weak interactions are found in [{Cu(μ-X)[Cp*(CO)2Mo(μ,η3:η2-As)]}2] (X = Cl, Br, I)17 and demonstrate the capability of arsenic to realize higher coordination numbers when compared to its lighter homologue phosphorus. Bearing in mind the π-coordination mode of the As–As edges, one could propose a distorted trigonal-planar environment for the copper atoms, with one vertex in the middle of the As–As bond and two vertices occupied by the halide atoms (alternatively, a square pyramidal environment regarding both As atoms). The copper atoms are slightly pulled out of this plane by the additional coordination from the acetonitrile molecule or the σ-coordination of the neighbouring arsenic atom of the next molecule of 1 in the polymeric strand.
A section of the 1D polymeric structure of 4 is depicted in Fig. 4; selected bond lengths and angles are summarized in Table 2.‡ Compound 4 shows a (CuI)n ladder as the main structural feature, of which only a few examples are known in the literature.20 However, in 4 the copper atoms of this stair-like strand are coordinated by molecules of 1, alternately placed on both sides of the CuI chain. The arsenic atoms of the cyclo-As5 moiety adopt a η2:η2 coordination mode which always has two arsenic atoms coordinated side-on to one copper atom, similar to 2 and 3. Also, all of the As–As bonds are elongated relative to 1 due to the additional coordination revealing longer bonds between As(2)–As(3) (2.392(2) Å) and shorter bonds between As(3)–As(4) (2.346(2) Å). In contrast to 2 and 3 a unique arsenic atom shows no further coordination and also the planarity of the cyclo-As5 ring is better maintained. The Cu–As distances are between 2.474(2) and 2.521(2) Å and are comparable to those found in the literature.17,27 Taking into account the π-coordination mode of the As–As edges, the Cu atoms are in a tetrahedral environment, spanned by the three halogen atoms and the centre of the As–As bond. The 1D polymeric (CuI)n strand shows a slightly distorted stair-like structure, which is similar to the coordination polymer [(CuI)2(Quin)]n.29a In contrast, in 4 one molecule of 1 bridges one “step” of the (CuI)n chain, causing a narrow Cu(1)–I(2)–Cu(2′′) angle of 88.44(5)° compared to the unbridged “step” with a wider Cu(2)–I(1)–Cu(1′′) angle of 120.51(6)°. The Cu–I distances (2.671(2)–2.715(2) Å) are within the expected range.21,22,29
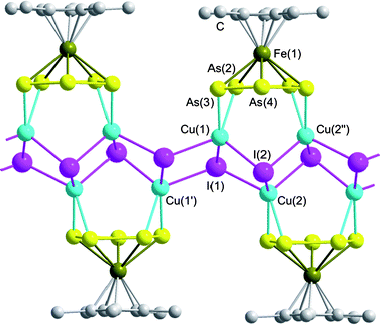 | ||
| Fig. 4 Section of the polymeric structure of 4. Hydrogen atoms are omitted for clarity. | ||
| As(2)–As(3) | 2.392(2) | As(2)–Cu(1)–As(3) | 57.29(6) |
| As(3)–As(4) | 2.346(2) | I(1)–Cu(1)–I(1′) | 92.25(5) |
| As(3)–Cu(1) | 2.512(2) | Cu(1)–I(2)–Cu(2′′) | 88.44(5) |
| Fe(1)–As(3) | 2.519(2) | Cu(2)–I(1)–Cu(1′) | 120.51(6) |
| I(2)–Cu(2′′) | 2.671(2) | ||
| Cu(1)–I(1) | 2.715(2) |
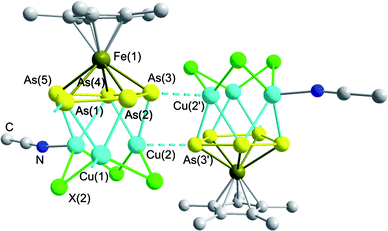
The solid state structure of 5 is shown in Fig. 5; selected bond lengths and angles are summarized in Table 3.‡ Despite the fact that 4 and 5 contain pentaarsaferrocene and CuI in the same stoichiometric ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, the latter compound shows a strikingly different structure in the solid state, combining structural motifs of 2, 3, and 4 along with new coordination modes. At a first glance, the central pentaarsaferrocene moiety reveals the same coordination mode as in 4, adopting a η2:η2 coordination towards two copper atoms. The coordinating As–As edges are slightly elongated (As(2)–As(3): 2.385(1) Å) in contrast to the non-coordinating bonds (As(1)–As(2): 2.336(1) Å) similar to 4. However, the 1D polymer is not formed by a (CuI)n chain as in 4. Instead a further end-on coordination of two arsenic atoms towards copper atoms is observed, as is found in 2 and 3. However in 5, the σ-coordination is much stronger, giving a short Cu–As distance (As(2)–Cu(1′′): 2.569(1) Å) which is even shorter than the distance to the π-coordinated Cu atom (As(2)–Cu(1): 2.601(1) Å). Finally, this leads to a novel η2:η2:η1:η1 coordination mode of the cyclo-As5 ring. The atom Cu(1) is situated in a tetrahedral environment and coordinated by two iodide atoms, one σ-coordinating As atom and one π-coordinating As–As edge. The two copper atoms below the cyclo-As5 ring belong to a (CuI)3 ring, revealing a distorted chair structure similar to compounds 2 and 3. The Cu–I distances are within the expected range (2.591(1)–2.622(1) Å) and are comparable to those found in the literature.21,29 An interesting structural feature is introduced by the additional coordination of the third copper atom of the six-membered CuI ring. This copper atom is side-on coordinated by two arsenic atoms of a second molecule of 1, revealing a slightly elongated As–Cu bond (As(4)–Cu(2): 2.663(2) Å). Furthermore, this cyclo-As5 ring coordinates in an η5 fashion to another Cu atom of a dumbbell shaped CuI located nearly perpendicular above the cyclo-As5 plane (angle between the cyclo-As5 plane and the CuI-dumbbell ≈ 79°). This structural motif is reminiscent of a triple-decker sandwich complex which exists for cyclo-As5 complexes only in a few examples in a pure homoleptic environment.15a However, here the first example of a heteroleptic arrangement is found. The copper atom is not ideally centred above the cyclo-As5 ring; this is deduced from the differences in the As–Cu distances, decreasing from 2.696(2) Å (As(4)–Cu(3)) to 2.479(2) (As(6)–Cu(3)). Within the cyclo-As5 ring, similar As–As distances are found (As(4)–As(5): 2.370(1) Å; As(5)–As(6): 2.368(1) Å) with a slightly elongated bond (As(4)–As(4′): 2.389(1) Å) due to the additional π-coordination. The CuI dumbbell is attached to the (CuI)3 ring through a Cu(2)⋯I(3) interaction of 2.740(2) Å and a very short Cu⋯Cu interaction of 2.563(2) Å; this is in the range of distances usually found in copper metal (2.56 Å).24,26
2, the latter compound shows a strikingly different structure in the solid state, combining structural motifs of 2, 3, and 4 along with new coordination modes. At a first glance, the central pentaarsaferrocene moiety reveals the same coordination mode as in 4, adopting a η2:η2 coordination towards two copper atoms. The coordinating As–As edges are slightly elongated (As(2)–As(3): 2.385(1) Å) in contrast to the non-coordinating bonds (As(1)–As(2): 2.336(1) Å) similar to 4. However, the 1D polymer is not formed by a (CuI)n chain as in 4. Instead a further end-on coordination of two arsenic atoms towards copper atoms is observed, as is found in 2 and 3. However in 5, the σ-coordination is much stronger, giving a short Cu–As distance (As(2)–Cu(1′′): 2.569(1) Å) which is even shorter than the distance to the π-coordinated Cu atom (As(2)–Cu(1): 2.601(1) Å). Finally, this leads to a novel η2:η2:η1:η1 coordination mode of the cyclo-As5 ring. The atom Cu(1) is situated in a tetrahedral environment and coordinated by two iodide atoms, one σ-coordinating As atom and one π-coordinating As–As edge. The two copper atoms below the cyclo-As5 ring belong to a (CuI)3 ring, revealing a distorted chair structure similar to compounds 2 and 3. The Cu–I distances are within the expected range (2.591(1)–2.622(1) Å) and are comparable to those found in the literature.21,29 An interesting structural feature is introduced by the additional coordination of the third copper atom of the six-membered CuI ring. This copper atom is side-on coordinated by two arsenic atoms of a second molecule of 1, revealing a slightly elongated As–Cu bond (As(4)–Cu(2): 2.663(2) Å). Furthermore, this cyclo-As5 ring coordinates in an η5 fashion to another Cu atom of a dumbbell shaped CuI located nearly perpendicular above the cyclo-As5 plane (angle between the cyclo-As5 plane and the CuI-dumbbell ≈ 79°). This structural motif is reminiscent of a triple-decker sandwich complex which exists for cyclo-As5 complexes only in a few examples in a pure homoleptic environment.15a However, here the first example of a heteroleptic arrangement is found. The copper atom is not ideally centred above the cyclo-As5 ring; this is deduced from the differences in the As–Cu distances, decreasing from 2.696(2) Å (As(4)–Cu(3)) to 2.479(2) (As(6)–Cu(3)). Within the cyclo-As5 ring, similar As–As distances are found (As(4)–As(5): 2.370(1) Å; As(5)–As(6): 2.368(1) Å) with a slightly elongated bond (As(4)–As(4′): 2.389(1) Å) due to the additional π-coordination. The CuI dumbbell is attached to the (CuI)3 ring through a Cu(2)⋯I(3) interaction of 2.740(2) Å and a very short Cu⋯Cu interaction of 2.563(2) Å; this is in the range of distances usually found in copper metal (2.56 Å).24,26
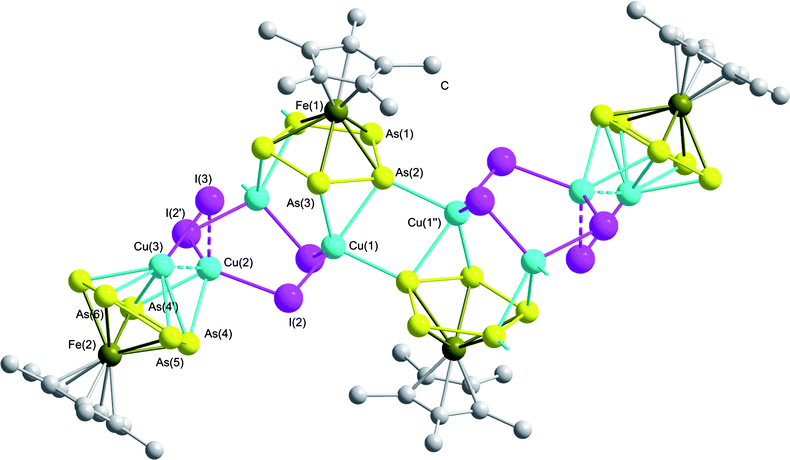 | ||
| Fig. 5 Section of the polymeric structure of 5. Hydrogen atoms are omitted for clarity. | ||
| As(1)–As(2) | 2.3358(10) | Cu(1)–I(1) | 2.5908(12) |
| As(2)–As(3) | 2.3852(9) | Cu(1)–I(2) | 2.6222(11) |
| As(4)–As(5) | 2.3696(12) | Cu(2)⋯I(3) | 2.7404(16) |
| As(5)–As(6) | 2.3675(12) | Cu(3)–I(3) | 2.5237(17) |
| As(4)–As(4′) | 2.3890(13) | Cu(2)–Cu(3) | 2.563(2) |
| As2–Cu(1) | 2.6007(12) | As(2)–Cu(1)–As(3) | 55.72(3) |
| As(2)–Cu(1′′) | 2.5694(14) | As(4)–Cu(2)–As(4′) | 53.30(4) |
| As(4)–Cu(2) | 2.6631(15) | As(4)–Cu(3)–As(4′) | 52.61(4) |
| As(4)–Cu(3) | 2.6956(18) | Cu(1)–I(2)–Cu(2) | 106.80(4) |
| As(5)–Cu(3) | 2.5968(14) | I(2)–Cu(2)–I(2′) | 112.15(5) |
| As(6)–Cu(3) | 2.4794(18) | Cu(2)–I(3)–Cu(3) | 58.10(5) |
Conclusions
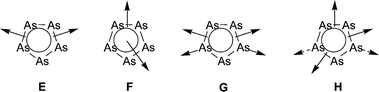
The results show that the cyclo-As5 ligand complex 1, surprisingly, reveals a preferred edge-bridging coordination mode to form π-coordination as found in 4 (type E), 5 (type F and G) and in a maximum manner in 2 and 3 (type H).This is in contrast to the preferred σ-coordination behaviour of the cyclo-P5 complex homologue [Cp*Fe(η5-P5)]. In addition, σ-coordination modes of two As atoms of the ring are found, revealing intermolecular interactions responsible for forming 1D polymeric structures. Whereas this feature is present in H (compounds 2 and 3) to give rather weak interactions, this behaviour is more pronounced in G (compound 5) to result in short As–Cu bonds. However, the most striking observation is the formation of a heteroleptic triple-decker-like sandwich complex motif in 5 in which Fe and Cu are incorporated into the multiple-decker arrangement. These surprising results prove the intriguing new coordination features of the arsenic complexes and extend our knowledge on the variety of the palette of coordination modes and compounds formed by ligand complexes of the group 15 elements.
Notes and references
- (a) J.-M. Lehn, Supramolecular Chemistry: Concepts and Perspectives, VCH, Weinheim, 1995 Search PubMed; (b) R. W. Saalfrank and B. Demleitner, in Transition Metals in Supramolecular Chemistry, ed. J.-P. Sauvage, Wiley-VCH, Weinheim, 1999, vol. 5, pp. 1–51 Search PubMed; (c) M. M. Conn and J. Rebek, Jr., Chem. Rev., 1997, 97, 1647–1668 CrossRef CAS; (d) C. J. Jones, Chem. Soc. Rev., 1998, 27, 289–299 RSC; (e) G. F. Swiegers and T. J. Malefetse, Chem. Rev., 2000, 100, 3483–3537 CrossRef CAS; (f) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853–908 CrossRef CAS; (g) M. Ruben, J. Rojo, F. J. Romero-Salguero, L. H. Uppadine and J.-M. Lehn, Angew. Chem., 2004, 116, 3728–3747 ( Angew. Chem., Int. Ed. , 2004 , 43 , 3644–3662 ) CrossRef; (h) M. Fujita, M. Tominaga, A. Hori and B. Therrien, Acc. Chem. Res., 2005, 38, 369–378 CrossRef CAS; (i) T. Kreickmann and F. E. Hahn, Chem. Commun., 2007, 1111–1120 RSC.
- M. Scheer, Dalton Trans., 2008, 4372 RSC.
- (a) M. Di Vaira, M. P. Ehses, M. Peruzzini and P. Stoppioni, Polyhedron, 1999, 18, 2331–2336 CrossRef CAS; (b) M. Di Vaira, P. Stoppioni and M. Peruzzini, J. Chem. Soc., Dalton Trans., 1990, 109–113 RSC.
- (a) M. F. Cecconi, C. A. Ghilardi, S. Midollini and A. Orlandini, J. Chem. Soc., Chem. Commun., 1982, 229–230 RSC; (b) F. Cecconi, C. A. Ghilardi, S. Midollini and A. Orlandini, Angew. Chem., 1983, 95, 554–555 ( Angew. Chem., Int. Ed. Engl. , 1983 , 22 , 554–555 ) CAS.
- (a) J. Bai, E. Leiner and M. Scheer, Angew. Chem., 2002, 114, 820–823 ( Angew. Chem., Int. Ed. , 2002 , 41 , 783–786 ) CrossRef; (b) M. Scheer, L. Gregoriades, J. Bai, M. Sierka, G. Brunklaus and H. Eckert, Chem.–Eur. J., 2005, 11, 2163–2169 CrossRef CAS; (c) M. Scheer, L. J. Gregoriades, M. Zabel, M. Sierka, L. Zhang and H. Eckert, Eur. J. Inorg. Chem., 2007, 2775 CrossRef CAS; (d) L. J. Gregoriades, G. Balázs, E. Brunner, C. Gröger, J. Wachter, M. Zabel and M. Scheer, Angew. Chem., 2007, 119, 6070 ( Angew. Chem., Int. Ed. , 2007 , 46 , 5966 ) CrossRef; (e) M. Scheer, L. J. Gregoriades, M. Zabel, J. Bai, I. Krossing, G. Brunklaus and H. Eckert, Chem.–Eur. J., 2008, 14, 282 CrossRef CAS.
- L. J. Gregoriades, B. K. Wegley, M. Sierka, E. Brunner, C. Gröger, E. V. Peresypkina, A. V. Virovets, M. Zabel and M. Scheer, Chem.–Asian J., 2009, 4, 1578–1587 CrossRef CAS.
- J. Bai, A. V. Virovets and M. Scheer, Angew. Chem., 2002, 114, 1808–1811 ( Angew. Chem., Int. Ed. , 2002 , 41 , 1737–1740 ) CrossRef.
- M. Scheer, L. J. Gregoriades, A. V. Virovets, W. Kunz, R. Neueder and I. Krossing, Angew. Chem., 2006, 118, 5818 ( Angew. Chem., Int. Ed. , 2006 , 45 , 5689 ) CrossRef.
- S. Welsch, L. J. Gregoriades, M. Sierka, M. Zabel, A. V. Virovets and M. Scheer, Angew. Chem., 2007, 119, 9483 ( Angew. Chem., Int. Ed. , 2007 , 46 , 9323 ) CrossRef.
- (a) J. Bai, A. V. Virovets and M. Scheer, Science, 2003, 300, 781–783 CrossRef CAS; (b) M. Scheer, J. Bai, B. P. Johnson, R. Merkle, A. V. Virovets and C. E. Anson, Eur. J. Inorg. Chem., 2005, 4023–4026 CrossRef CAS; (c) M. Scheer, A. Schindler, J. Bai, B. P. Johnson, R. Merkle, R. Winter, A. V. Virovets, E. V. Peresypkina, V. A. Blatov, M. Sierka and H. Eckert, Chem.–Eur. J., 2010, 16, 2092–2107 CrossRef CAS.
- (a) M. Scheer, A. Schindler, C. Gröger, A. V. Virovets and E. V. Peresypkina, Angew. Chem., 2009, 121, 5148 CrossRef; (b) M. Scheer, A. Schindler, R. Merkle, B. P. Johnson, M. Linseis, R. Winter, C. E. Anson and A. V. Virovets, J. Am. Chem. Soc., 2007, 129, 13386 CrossRef CAS.
- B. P. Johnson, F. Dielmann, G. Balázs, M. Sierka and M. Scheer, Angew. Chem., 2006, 118, 2533 ( Angew. Chem., Int. Ed. , 2006 , 45 , 2473 ) CrossRef.
- For examples of supramolecular aggregates based on [{CpMo(CO)2}2(μ,η2-Sb2)], see: H. V. Ly, M. Parvez and R. Roesler, Inorg. Chem., 2006, 45, 345–351 Search PubMed.
- (a) B. M. Cossairt, M.-C. Diawara and C. C. Cummins, Science, 2009, 323, 602 CrossRef CAS; (b) H. A. Spinney, N. A. Piro and C. C. Cummins, J. Am. Chem. Soc., 2009, 131, 16233–16243 CrossRef CAS; (c) B. M. Cossairt and C. C. Cummins, J. Am. Chem. Soc., 2009, 131, 15501–15511 CrossRef CAS; (d) J. J. Curley, N. A. Piro and C. C. Cummins, Inorg. Chem., 2009, 48, 9599–9601 CrossRef CAS.
- Review articles: (a) O. J. Scherer, Acc. Chem. Res., 1999, 32, 751–762 CrossRef CAS; (b) K. H. Whitmire, Adv. Organomet. Chem., 1998, 42, 1–145 CAS; (c) O. J. Scherer, Angew. Chem., 1990, 102, 1137–1155 ( Angew. Chem., Int. Ed. Engl. , 1990 , 29 , 1104–1122 ) CAS; (d) M. Scheer and E. Herrmann, Z. Chem., 1990, 30, 41–55 CAS.
- I. Bernal, H. Brunner, W. Meier, H. Pfisterer, J. Wachter and M. L. Ziegler, Angew. Chem., 1984, 96, 428–429 ( Angew. Chem., Int. Ed. Engl. , 1984 , 23 , 438–439 ).
- L. J. Gregoriades, H. Krauss, J. Wachter, A. V. Virovets, M. Sierka and M. Scheer, Angew. Chem., 2006, 118, 4295–4298 ( Angew. Chem., Int. Ed. , 2006 , 45 , 4189–4192 ) CrossRef.
- O. J. Scherer, C. Blath and G. Wolmershäuser, J. Organomet. Chem., 1990, 387, C21–C24 CrossRef CAS.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789 CrossRef CAS.
- Review article: R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1–18 Search PubMed.
- A. Biegerl, E. Brunner, C. Gröger, M. Scheer, J. Wachter and M. Zabel, Chem.–Eur. J., 2007, 13, 9270–9276 CrossRef CAS.
- A. Schindler, M. Zabel, J. F. Nixon and M. Scheer, Z. Naturforsch., B: Chem. Sci., 2009, 64, 1429–1437 CAS.
- W. S. Sheldrick and T. Häusler, Z. Anorg. Allg. Chem., 1994, 620, 334–342 CrossRef CAS.
- P. Pyykkö, Chem. Rev., 1997, 97, 597–636 CrossRef; H. L. Hermann, G. Boche and P. Schwerdtfeger, Chem.–Eur. J., 2001, 7, 5333–5342 CrossRef CAS.
- P. C. Ford, E. Cariati and J. Bourassa, Chem. Rev., 1999, 99, 3625–3647 CrossRef CAS.
- N. P. Rath, E. M. Holt and K. Tanimura, Inorg. Chem., 1985, 24, 3934–3938 CrossRef CAS.
- J. Besinger, J. Treptow and D. Fenske, Z. Anorg. Allg. Chem., 2002, 628, 512–515 CrossRef CAS.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- (a) P. M. Graham, R. D. Pike, M. Sabat, R. D. Bailey and W. T. Pennington, Inorg. Chem., 2000, 39, 5121–5132 CrossRef CAS; (b) D. M. Haddleton, D. J. Duncalf, A. J. Clark, M. C. Crossman and D. Kukulj, New J. Chem., 1998, 22, 315–317 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental, analytical, crystallographic and computational details. CCDC reference numbers 772393–772396. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00254b |
‡ Crystal data for 2: C12H18As5Cl3Cu3FeN, M = 903.72, monoclinic, space group P21/c (no. 14), a = 11.660(3) Å, b = 21.626(6) Å, c = 8.490(2) Å, β = 90.00(2)°, V = 2140.8(10) Å3, Z = 4, μ = 11.647 mm−1, F(000) = 1704, T = 123(1) K, 24053 reflections measured, 6531 unique (Rint = 0.0929), R1 = 0.0427, wR2 = 0.1015 for I > 2σ(I). CCDC 772393. Crystal data for 3: C12H18As5Br3Cu3FeN, M = 1037.07, monoclinic, space group P21/c (no. 14), a = 11.8229(4) Å, b = 21.7908(8) Å, c = 8.6497(2) Å, β = 90.118(2)°, V = 2228.42(12) Å3, Z = 4, μ = 22.890 mm−1, F(000) = 1920, T = 123(1) K, 15185 reflections measured, 3774 unique (Rint = 0.0816), R1 = 0.0381, wR2 = 0.0840 for I > 2σ(I). CCDC 772394. Crystal data for 4: C10H15As5Cu2FeI2, M = 946.57, triclinic, space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 8.4256(6) Å, b = 10.9119(8) Å, c = 11.4820(9) Å, α = 71.256(7)°, β = 81.825(6)°, γ = 78.273(6)°, V = 975.42(13) Å3, Z = 2, μ = 42.587 mm−1, F(000) = 860, T = 123(1) K, 4805 reflections measured, 2111 unique (Rint = 0.0375), R1 = 0.0337, wR2 = 0.0711 for I > 2σ(I). CCDC 772395. Crystal data for 5·nCH2Cl2: C11H17As5Cl2Cu2FeI2, M = 1031.50, orthorhombic, space group Pnma (no. 62), a = 15.6513(1) Å, b = 11.7371(3) Å, c = 24.5300(5) Å, V = 4506.18(16) Å3, Z = 8, μ = 39.105 mm−1, F(000) = 3776, T = 123(1) K, 10949 reflections measured, 3650 unique (Rint = 0.0367), R1 = 0.0322, wR2 = 0.0741 for I > 2σ(I). CCDC 772396. (no. 2), a = 8.4256(6) Å, b = 10.9119(8) Å, c = 11.4820(9) Å, α = 71.256(7)°, β = 81.825(6)°, γ = 78.273(6)°, V = 975.42(13) Å3, Z = 2, μ = 42.587 mm−1, F(000) = 860, T = 123(1) K, 4805 reflections measured, 2111 unique (Rint = 0.0375), R1 = 0.0337, wR2 = 0.0711 for I > 2σ(I). CCDC 772395. Crystal data for 5·nCH2Cl2: C11H17As5Cl2Cu2FeI2, M = 1031.50, orthorhombic, space group Pnma (no. 62), a = 15.6513(1) Å, b = 11.7371(3) Å, c = 24.5300(5) Å, V = 4506.18(16) Å3, Z = 8, μ = 39.105 mm−1, F(000) = 3776, T = 123(1) K, 10949 reflections measured, 3650 unique (Rint = 0.0367), R1 = 0.0322, wR2 = 0.0741 for I > 2σ(I). CCDC 772396. |
| This journal is © The Royal Society of Chemistry 2010 |