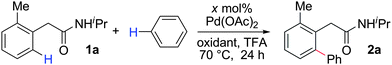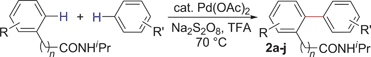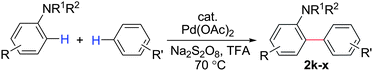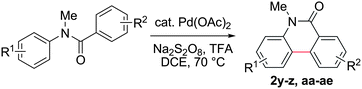Pd-catalyzed ortho-arylation of phenylacetamides, benzamides, and anilides with simple arenes using sodium persulfate†
Charles S.
Yeung
,
Xiaodan
Zhao
,
Nadine
Borduas
and
Vy M.
Dong
*
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario, Canada M5S 3H6. E-mail: vdong@chem.utoronto.ca
First published on 18th June 2010
Abstract
We report a mild and efficient Pd-catalyzed ortho-arylation of phenylacetamides, benzamides, and anilides with a range of simple arenes using sodium persulfate (Na2S2O8). This green strategy generates biaryl C–C bonds from two unactivated sp2 hybridized C–H bonds. Electron-rich and electron-neutral arenes underwent oxidative arylation under our optimized reaction conditions. In substrates bearing two reactive ortho C–H bonds, selective diarylation via quadruple C–H bond functionalization was possible. The same reaction conditions were extended to an intramolecular cross-coupling for preparing lactams. The synthesis of relevant trifluoroacetate-bridged bimetallic Pd complexes derived from anilides and their stoichiometric reactivity were investigated.
Introduction
A range of functional molecules, from antibiotics to light-emitting devices, contain the biaryl motif.1 This key scaffold can be accessed by cross-coupling simple arenes, an emerging strategy for making C–C biaryl linkages.2,3 By using arenes bearing a directing group, high levels of regiocontrol are possible. Sanford has achieved oxidative ortho-arylation with pyridines2c and Chang with pyridine N-oxides,2g while Shi and Buchwald independently demonstrated cross-coupling with anilides.2e,f Our group recently reported an oxidative cross-coupling with O-phenylcarbamates using sodium persulfate (Na2S2O8), a non-toxic, environmentally benign, and easy-to-handle oxidant.4 Due to the efficiency and practicality of these reaction conditions, we aimed to expand the application and understanding of this ortho-arylation. Herein, we report an intermolecular oxidative arylation that includes phenylacetamides, benzamides, and anilides as coupling partners (Fig. 1) and an extension to an intramolecular variant.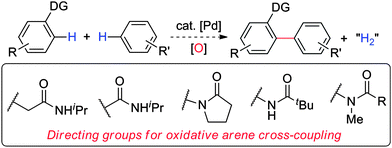 | ||
| Fig. 1 Substrates for oxidative arene cross-couplings. | ||
Results and discussion
Initial studies
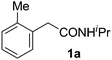
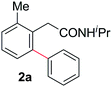
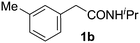
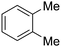
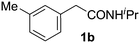
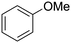
Only a few accounts of directed C–H functionalization of phenylacetic acid derivatives exist.5 Because of their importance6 and inaccessibility via directed ortho-metalation,5a,7 our initial study focused on coupling benzene with N-isopropyl-2-o-tolylacetamide (1a). By surveying various reaction parameters, we obtained key results shown in Table 1. No biaryl 2a was observed in the absence of Pd catalysts and/or trifluoroacetic acid (TFA) (entries 1 and 2).8 Desired product 2a was formed, however, in the presence of catalytic Pd(OAc)2, TFA (5 equiv.), oxidant, and benzene at 70 °C with excellent regioselectivity. Reaction efficiency depended on the oxidant; Cu(OAc)22e,i afforded 28% yield and O22f gave 73% of the desired product (entries 3 and 4). Na2S2O84 gave the most promising result (entry 5, 99%) and was thus chosen for further studies.8Synthetic scope
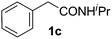
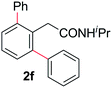
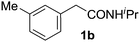
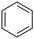
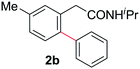
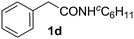
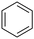
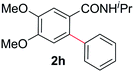
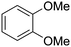
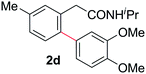
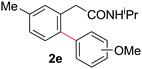
With this protocol,‡ a wide range of 2-arylphenylacetamides (2a–g) could be synthesized from simple arene cross-coupling partners (Table 2, entries 1–7, 67% to 99%).9 Substrates bearing substituents at the ortho- or meta-positions underwent selective monoarylation with benzene, o-dimethylbenzene, o-dimethoxybenzene, and anisole in high reaction efficiencies and regioselectivities on both coupling partners (2a–e, 81–99%). In contrast to O-phenylcarbamate arylation,4 electron deficient arenes such as o-dichlorobenzene were ineffective. Subjecting N-isopropyl-2-phenylacetamide (1c) and N-cyclohexyl-2-phenylacetamide (1d), substrates bearing two reactive ortho C–H bonds, to our optimized conditions resulted in selective formation of the difunctionalization products 2f and 2g (67–68%). This particular transformation involves a rare quadruple C–H bond activation.2b,3|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Arene | Product | Yield (%) | Entry | Substrate | Arene | Product | Yield (%) |
a Conditions: Substrate, 0.2 mmol; arene, 1 mL; Pd(OAc)2, 10 mol%; Na2S2O8, 3 equiv.; TFA, 5 equiv.; 24–61 h.
b After 30 h, an additional 5 mol% Pd(OAc)2 was added.
c
2c was isolated as a mixture of two isomeric products in a ratio of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.
d
2d was isolated as a mixture of three isomeric products in a ratio of o 7.
d
2d was isolated as a mixture of three isomeric products in a ratio of o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p = 9 p = 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 78.
e Pd(OAc)2, 5 mol%, 60 °C. 78.
e Pd(OAc)2, 5 mol%, 60 °C.
|
|||||||||
| 1 |
|
|
|
81b | 6 |
|
|
|
67 |
| 2 |
|
|
|
99 | 7 |
|
|
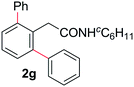
|
68 |
| 3 |
|
|
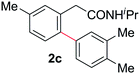
|
95c | 8 |
|
|
|
78e |
| 4 |
|
|
|
99 | 9 |
|
|
|
83e |
| 5 |
|
|
|
99d | 10 |
|
|
|
61e |
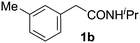
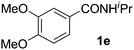
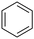
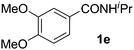
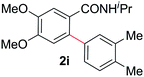
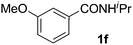
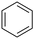
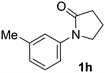
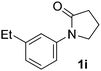
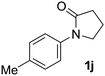
Next, we examined benzamides as substrates for oxidative arene cross-coupling (Table 2, entries 8–10, 61–83%).10 We observed that N-isopropyl-3-methylbenzamide was unreactive under our optimized conditions. However, the more electron-rich N-isopropyl-3,4-dimethoxybenzamide (1e) coupled with benzene and o-dimethylbenzene, respectively, to produce biaryls 2h (78%) and 2i (83%). Both biphenyl products were obtained as single regioisomers under especially mild conditions (5 mol% Pd(OAc)2, 60 °C, Table 2). N-Isopropyl-3-methoxybenzamide (1f) also underwent phenylation to produce 2j (61%).
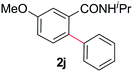
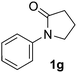
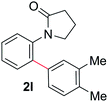
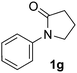
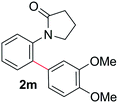
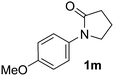
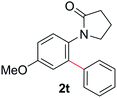
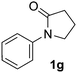
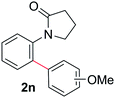
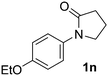
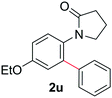
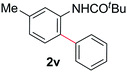
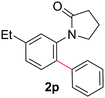
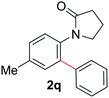
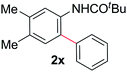
During this study, we found that anilides2e,f were also reactive toward simple arenes (Table 3, 70–99%). While phenylacetamides and O-phenylcarbamates4 can undergo diarylation, 1-phenyl-2-pyrrolidone (1g) reacts with benzene to form the biphenyl product 2k, exclusively (Table 3). Notably, this oxidative coupling was carried out on a one-gram scale to produce 1.30 g of 1-(biphenyl-2-yl)pyrrolidin-2-one (2k, 88%). Various arenes (e.g., o-dimethylbenzene, o-dimethoxybenzene, anisole) could be used for this transformation (2l–n, 70–89%). Additionally, substitution patterns on the aromatic backbone had minimal effect on reaction efficiency (2o–u, 80–99%). Anilides bearing free N–H bonds were also tolerated (2v–x, 82–99%).2f
|
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Substrate | Arene | Product | Yield (%) | Entry | Substrate | Arene | Product | Yield (%) |
a Conditions: Substrate, 0.2 mmol; arene, 1 mL; Pd(OAc)2, 10 mol%; Na2S2O8, 3 equiv.; TFA, 5 equiv.; 24–61 h.
b On a one-gram scale; on a 0.2 mmol scale, product 2k was isolated in 83% yield.
c 0.5 mL o-dimethoxybenzene, 90 °C.
d
2n was isolated as a mixture of three isomeric products in a ratio of o![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) m m![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) p = 4 p = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85. 85.
|
|||||||||
| 1 |
|
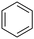
|
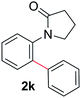
|
88b (1.30 g) | 8 |
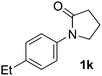
|
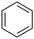
|
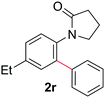
|
80 |
| 2 |
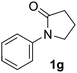
|
|
|
89 | 9 |
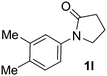
|
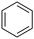
|
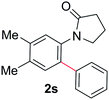
|
98 |
| 3 |
|
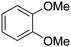
|
|
73c | 10 |
|
|
|
99 |
| 4 |
|
|
|
70d | 11 |
|
|
|
84 |
| 5 |
|
|
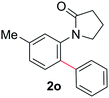
|
99 | 12 |
|
|
|
99 |
| 6 |
|
|
|
97 | 13 |
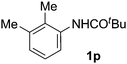
|
|
|
82 |
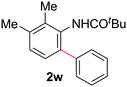
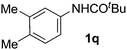
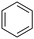
| 7 |
|
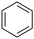
|
|
94 | 14 |
|
|
|
94 |
Three different types of substrates bearing amide directing groups (i.e., phenylacetamides, benzamides and anilides) successfully underwent the desired oxidative ortho-arylation with simple arenes.
Intramolecular coupling
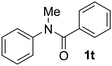
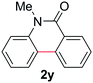
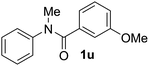
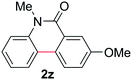
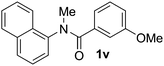
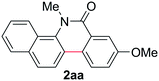
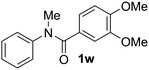
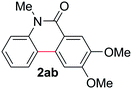
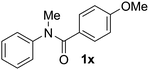
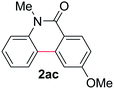
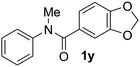
Known reaction conditions for oxidative cross-couplings have been thus far limited to either intermolecular2 or intramolecular11 variants, and not applicable to both. Based on known cyclopalladations,4,12 we proposed that N-methyl-N-phenylbenzamide (1t) would undergo the ring-closing oxidative cross-coupling to generate a six-membered lactam product. By subjecting 1t to the same reaction conditions, we observed formation of 5-methylphenanthridin-6(5H)-one (2y) in 60% yield (Table 4, entry 1). Using this strategy, a number of phenanthridin-6(5H)-one derivatives could be prepared in modest to good yields (Table 4, entries 1–6, 30–77%).13 In accordance with the oxidative ortho-arylation of benzamides (Table 2, entries 8–10), the presence of electron-donating groups on the benzamide portion of the substrates resulted in more efficient transformations (entries 2–4, 60–77%). The position of the electron-rich substituent played a critical role in reaction efficiency. 3-Methoxy-N-methyl-N-phenylbenzamide (1u) underwent lactam formation in 77% yield, while 4-methoxy-N-methyl-N-phenylbenzamide (1x) cyclized in a modest 33% yield (with 66% recovered starting material). The less electron rich N-methyl-N-phenylbenzo[d][1,3]dioxole-5-carboxamide (1y) generated N-methylcrinasiadine (2ad), a natural product isolated from Hippeastrum equestre and Lapiedramartinezii.14,15 Our strategy provides a direct route to 2ad in three steps from simple and inexpensive building blocks (i.e., N-methylaniline and piperonylic acid) in 30% overall yield.|
|
|||
|---|---|---|---|
| Entry | Substrate | Product | Yield (%) |
a Conditions: Substrate, 0.2 mmol; Pd(OAc)2, 10 mol%; Na2S2O8, 4 equiv.; TFA, 5 equiv.; DCE, 1 mL; 15–96 h.
b
2aa was isolated as a mixture of two isomeric products in a ratio of 2.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For more details, see ESI.1
c
1x was recovered in 66% yield. 1. For more details, see ESI.1
c
1x was recovered in 66% yield.
|
|||
| 1 |
|
|
60 |
| 2 |
|
|
77 |
| 3 |
|
|
63b |
| 4 |
|
|
60 |
| 5 |
|
|
33c |
| 6 |
|
|
30 |
Stoichiometric studies
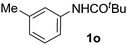
On the basis of the regioselectivity observed, we expected that the first C–H bond activation would occur via the well-accepted cyclopalladation mechanism.4,12 Indeed, cyclopalladates 3a and 3b could be prepared by treating N-(m-tolyl)pivalamide (1o) and 1-phenylpyrrolidin-2-one (1g), respectively, with Pd(OAc)2 in the presence of TFA (Fig. 2). The molecular geometries of 3a and 3b were determined by X-ray crystallography following recrystallization (Fig. 3 and 4). Both complexes exhibit a weak Pd–Pd interaction.16 Additionally, the N-(m-tolyl)pivalamide and 1-phenylpyrrolidin-2-one ligands are situated in a head-to-tail orientation. Efforts toward isolating Pd complexes derived from phenylacetamides and benzamides were unsuccessful.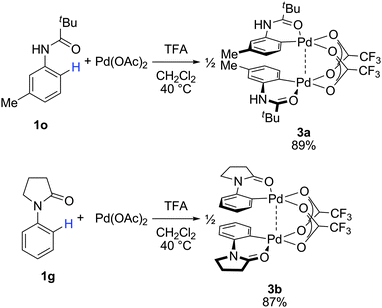 | ||
| Fig. 2 Preparation of dimeric Pd complexes 3a and 3b. | ||
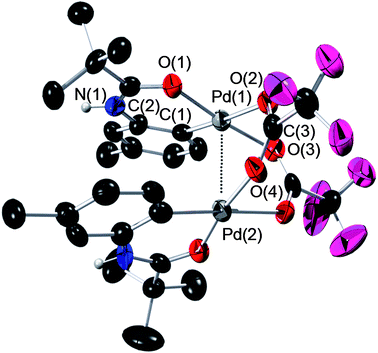 | ||
| Fig. 3 ORTEP plot of dimeric Pd complex 3a. All H atoms (except for the amide H) have been omitted for clarity. The complex contains two independent molecules in the asymmetric unit exhibiting minor geometric differences. For more details, see ESI.† Anisotropic displacement ellipsoids are shown at the 50% probability level. Selected bond lengths (Å) and angles (°) for 3a: Pd(1)–Pd(2) = 2.9233(9), Pd(1)–O(1) = 1.997(6), Pd(1)–C(1) = 1.947(9), C(2)–O(1) = 1.271(9), C(2)–N(1) = 1.312(10), Pd(1)–O(2) = 2.201(5), Pd(1)–O(3) = 2.058(6), N(1)–C(2)–O(1) = 121.4(8), C(1)–Pd(1)–O(1) = 91.6(3), C(1)–Pd(1)–O(3) = 91.9(3), O(2)–Pd(1)–O(3) = 90.0(2), O(2)–Pd(1)–O(1) = 86.3(2), O(2)–C(3)–O(4) = 130.1(8). In the second structure, the Pd–Pd bond distance was determined to be 2.9515(9). | ||
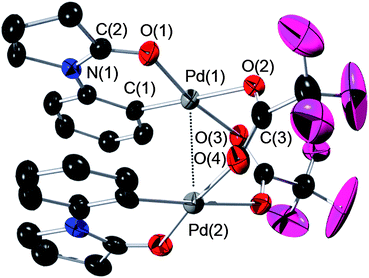 | ||
| Fig. 4 ORTEP plot of dimeric Pd complex 3b. All H atoms have been omitted for clarity. Anisotropic displacement ellipsoids are shown at the 50% probability level. Selected bond lengths (Å) and angles (°) for 3b: Pd(1)–Pd(2) = 2.8779(5), Pd(1)–O(1) = 1.994(4), Pd(1)–C(1) = 1.967(5), C(2)–O(1) = 1.257(6), C(2)–N(1) = 1.338(7), Pd(1)–O(2) = 2.178(3), Pd(1)–O(3) = 2.068(3), N(1)–C(2)–O(1) = 126.5(5), C(1)–Pd(1)–O(1) = 91.51(18), C(1)–Pd(1)–O(3) = 93.80(18), O(2)–Pd(1)–O(3) = 87.11(14), O(2)–Pd(1)–O(1) = 87.76(14), O(2)–C(3)–O(4) = 131.0(5). | ||
We previously reported that a bimetallic Pd complex derived from an O-phenylcarbamate undergoes smooth arylation to generate the corresponding ortho-arylation product in excellent yields, in the absence of oxidants or external additives. This result provided support for a second C–H bond activation via a Pd(II) intermediate.4 In contrast, no desired arylation product 2v could be detected when Pd complex 3a was subjected to benzene at 70 °C. Likewise, subjecting Pd complex 3b to the simple arene failed to generate the corresponding arylation product 2k. External additives, such as CH2Cl2, TFA, and DMSO did not promote the desired oxidative arylation process.
However, we found that addition of Na2S2O8 and TFA resulted in smooth transformation of both Pd complexes 3a and 3b with benzene to their corresponding ortho-arylation products, 2v and 2k, respectively (Fig. 5). Based on the high redox potential of Na2S2O8 (2.01 eV),17 it is possible that Pd(II) can undergo oxidation to generate either bimetallic Pd(III)18 or Pd(IV)19 complexes. Sanford and Wang have independently reported that persulfate salts can promote direct C–H bond acetoxylation presumably under Pd(II/IV) catalysis.20 Additionally, the Michael group has suggested arylation at Pd(IV).21 Nonetheless, oxidative ortho-arylation of anilides has been achieved using Cu salts2e or O22f as the terminal oxidant presumably via a Pd(0/II) mechanism.2f
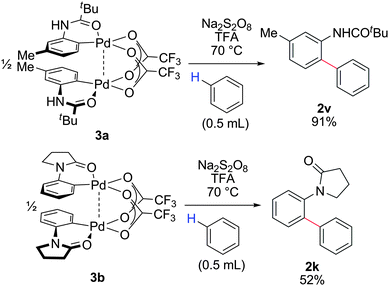 | ||
| Fig. 5 Direct phenylation of dimeric Pd complexes 3a and 3b in the presence of Na2S2O8 and TFA. Conditions: Na2S2O8, 2 equiv.; TFA, 1.1 equiv. | ||
These results suggest a reaction mechanism that varies greatly depending on the directing group (i.e., ligand) as well as the oxidant chosen (e.g., Na2S2O8,4 O2,2f Ag2CO32d). We are pursuing further studies to elucidate the mechanism of these catalytic cross-couplings in more detail.
Conclusions
We have developed an ortho-arylation of phenylacetamides, benzamides, and anilides using simple arenes as the cross-coupling partner and Na2S2O8 as a convenient oxidant. Application of the same reaction conditions to intramolecular variants provides a simple and direct route to phenanthridin-6(5H)-one cores, structural motifs prevalent in a number of biologically relevant molecules. Cyclopalladates derived from anilide substrates were found to be bimetallic, exhibiting weak Pd–Pd interactions; arylation of this Pd(II) species required the addition of Na2S2O8. These results will lead to future advances in oxidative cross-coupling, including development of new coupling partners, oxidants, catalysts, and strategies for controlling chemoselectivity.Acknowledgements
Funding is provided by the University of Toronto, Canada Foundation for Innovation, Ontario Research Fund, Boehringer Ingelheim Ltd. (Canada), and the Natural Sciences and Engineering Research Council (NSERC) of Canada. VMD is grateful for an Alfred P. Sloan Fellowship; CSY and NB are grateful for the NSERC André Hamer Award and the Julie Payette Research Scholarship.Notes and references
-
(a) J. Hassan, M. Sévignon, C. Gozzi, E. Schulz and M. Lemaire, Chem. Rev., 2002, 102, 1359 CrossRef CAS
; (b) J.-P. Corbet and G. Mignani, Chem. Rev., 2006, 106, 2651 CrossRef CAS
.
- For examples of oxidative cross-coupling involving twofold C–H functionalization, see:
(a) D. R. Stuart and K. Fagnou, Science, 2007, 316, 1172 CrossRef CAS
; (b) D. R. Stuart, E. Villemure and K. Fagnou, J. Am. Chem. Soc., 2007, 129, 12072 CrossRef CAS
; (c) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2007, 129, 11904 CrossRef CAS
; (d) K. L. Hull and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 9651 CrossRef CAS
; (e) B.-J. Li, S.-L. Tian, Z. Fang and Z.-J. Shi, Angew. Chem., Int. Ed., 2008, 47, 1115 CrossRef CAS
; (f) G. Brasche, J. García-Fortanet and S. L. Buchwald, Org. Lett., 2008, 10, 2207 CrossRef CAS
; (g) S. H. Cho, S. J. Hwang and S. Chang, J. Am. Chem. Soc., 2008, 130, 9254 CrossRef CAS
; (h) T. A. Dwight, N. R. Rue, D. Charyk, R. Josselyn and B. DeBoef, Org. Lett., 2007, 9, 3137 CrossRef CAS
; (i) S. Potavathri, A. S. Dumas, T. A. Dwight, G. R. Naumiec, J. M. Hammann and B. DeBoef, Tetrahedron Lett., 2008, 49, 4050 CrossRef CAS
; (j) J.-B. Xia and S.-L. You, Organometallics, 2007, 26, 4869 CrossRef CAS
; (k) R. Li, L. Jiang and W. Lu, Organometallics, 2006, 25, 5973 CrossRef CAS
. For a review on other oxidative couplings, see: (l) J. A. Ashenhurst, Chem. Soc. Rev., 2010, 39, 540 RSC
.
- For reviews on direct arylation, see:
(a) D. Alberico, M. E. Scott and M. Lautens, Chem. Rev., 2007, 107, 174 CrossRef CAS
; (b) B.-J. Li, S.-D. Yang and Z.-J. Shi, Synlett, 2008, 949 CAS
; (c) L. Ackermann, R. Vicente and A. R. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef CAS
; (d) G. P. McGlacken and L. M. Batemann, Chem. Soc. Rev., 2009, 38, 2447 RSC
.
- X. Zhao, C. S. Yeung and V. M. Dong, J. Am. Chem. Soc., 2010, 132, 5837 CrossRef CAS
.
- For examples of C–H functionalization of phenylacetic acid derivatives, see:
(a) D.-H. Wang, T.-S. Mei and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 17676 CrossRef CAS
; (b) R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14082 CrossRef CAS
; (c) M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 14058 CrossRef CAS
.
- For selected accounts on the bioactivity of phenylacetic acid derivatives, see:
(a) W. R. Hudgins, S. Shack, C. E. Myers and D. Samid, Biochem. Pharmacol., 1995, 50, 1273 CrossRef CAS
; (b) H. Martínez-Blanco, A. Reglero and J. M. Luengo, J. Ind. Microbiol., 1994, 13, 144 CrossRef CAS
; (c) V. K. Chamberlain and R. L. Wain, Ann. Appl. Biol., 1971, 69, 65 Search PubMed
; (d) R. M. Muir, T. Fujita and C. Hansch, Plant Physiol., 1967, 42, 1519 CrossRef CAS
. For phenylacetamide derivatives, see: (e) J. G. Samaritoni, L. Arndt, T. J. Bruce, J. E. Dripps, J. Gifford, C. J. Hatton, W. H. Hendrix, J. R. Schoonover, G. W. Johnson, V. B. Hegde and S. Thornburgh, J. Agric. Food Chem., 1997, 45, 1920 CrossRef CAS
; (f) P. K. Yonan, R. L. Novotney, C. M. Woo, K. A. Prodan and F. M. Hershenson, J. Med. Chem., 1980, 23, 1102 CrossRef CAS
.
- For a review on directed ortho-metallation, see M. P. Sibi and V. Snieckus, J. Org. Chem., 1983, 48, 1935 Search PubMed
.
- TFA facilitates formation of Pd(II) σ-aryl intermediates from direct electrophilic palladation of benzene; our results suggest that TFA can also promote directing group assisted C–H bond functionalizations by cyclopalladation. See
(a) C. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura and Y. Fujiwara, Science, 2000, 287, 1992 CrossRef CAS
; (b) Ref. 2k .
- 2-Arylacetamides bearing no free N–H bonds, such as 2-phenyl-1-(pyrrolidin-1-yl)ethanone (1r), did not undergo the desired oxidative arylation.
- For examples of C–H functionalization of benzamides, see:
(a) Y. Kametani, T. Satoh, M. Miura and M. Nomura, Tetrahedron Lett., 2000, 41, 2655 CrossRef CAS
; (b) D. Shabashov and O. Daugulis, Org. Lett., 2006, 8, 4947 CrossRef CAS
.
- For examples of intramolecular oxidative arylations, see:
(a) B. Liégault, D. Lee, M. P. Huestis, D. R. Stuart and K. Fagnou, J. Org. Chem., 2008, 73, 5022 CrossRef CAS
; (b) T. Watanabe, S. Oishi, N. Fujii and H. Ohno, J. Org. Chem., 2009, 74, 4720 CrossRef CAS
; (c) V. Sridharan, M. A. Martín and J. C. Menéndez, Eur. J. Org. Chem., 2009, 4614 CrossRef CAS
; (d) H. Hagelin, J. D. Oslob and B. Åkermark, Chem.–Eur. J., 1999, 5, 2413 CrossRef CAS
; (e) Ref. 2h .
- For a review on palladacycles, see: J. Dupont, C. S. Consorti and J. Spencer, Chem. Rev., 2005, 105, 2527 Search PubMed
.
- N-Arylbenzamides bearing free N–H bonds, such as N-phenylbenzamide (1aa), did not undergo the desired oxidative arylation. The presence of an OMe group on the anilide portion was not tolerated, in contrast to the efficient phenylation of 1m (Table 3, entry 10, 99%).
- For reports on isolation of N-methylcrinasiadine (2ad), see:
(a) W. Doepke, H. P. Lam, E. Gruendemann, M. Bartoszek and S. Flatau, Pharmazie, 1995, 50, 511 CAS
; (b) R. Suau, A. I. Gómez and R. Rico, Phytochemistry, 1990, 29, 1710 CrossRef CAS
.
- For total syntheses of N-methylcrinasiadine (2ad), see:
(a) R. Sanz, Y. Fernandez, M. P. Castroviejo, A. Perez and F. J. Fananas, Eur. J. Org. Chem., 2007, 62 CrossRef CAS
; (b) G. Cahiez, A. Moyeux, J. Buendia and C. Duplais, J. Am. Chem. Soc., 2007, 129, 13788 CrossRef CAS
; (c) T. Harayama, Y. Kawata, C. Nagura, T. Sato, T. Miyagoe, H. Abe and Y. Takeuchi, Tetrahedron Lett., 2005, 46, 6091 CrossRef CAS
; (d) G. Cahiez, C. Chaboche, F. Mahuteau-Betzer and M. Ahr, Org. Lett., 2005, 7, 1943 CrossRef CAS
; (e) M. G. Banwell, B. D. Bissett, S. Busato, C. J. Cowden, D. C. R. Hockless, J. W. Holman, R. W. Read and A. W. Wu, J. Chem. Soc., Chem. Commun., 1995, 2551 RSC
; (f) M. G. Banwell and C. J. Cowden, Aust. J. Chem., 1994, 47, 2235
; (g) W. R. Bowman, H. Heaney and B. M. Jordan, Tetrahedron, 1991, 47, 10119 CrossRef CAS
; (h) J. Grimshaw, R. Hamilton and J. Trocha-Grimshaw, J. Chem. Soc., Perkin Trans. 1, 1982, 229 RSC
; (i) R. K.-Y. Zee-Cheng, S.-J. Yan and C. C. Cheng, J. Med. Chem., 1978, 21, 199 CrossRef CAS
; (j) A. Mondon and K. Krohn, Chem. Ber., 1972, 105, 3726 CAS
; (k) H. S. Forrest, R. D. Haworth, A. R. Pinder and T. S. Stevens, J. Chem. Soc., 1949, 1311 RSC
.
- The internuclear distance between the two Pd atoms in 3a was determined to be 2.9233(9) Å and 2.9515(9) Å for the two independent molecules in the asymmetric unit, while for 3b, the bond distance was 2.8779(5) Å. In comparison to other Pd complexes bearing bridging trifluoroacetate ligands, our bimetallic Pd complex 3 exhibits slightly shorter Pd–Pd bonds:
(a) P. L. Ruddock, D. J. Williams and P. B. Reese, Steroids, 2004, 69, 193 CrossRef CAS
; (b) T. A. Stromnova, D. V. Paschenko, L. I. Boganova, M. V. Daineko, S. B. Katser, A. V. Chukarov, L. G. Kuz'mina and J. A. K. Howard, Inorg. Chim. Acta, 2003, 350, 283 CrossRef CAS
; (c) I. Schwarz, J. Rust, C. W. Lehmann and M. Braun, J. Organomet. Chem., 2000, 605, 109 CrossRef CAS
; (d) R. Goddard, C. Krüger, R. Mynott, M. Neumann and G. Wilke, J. Organomet. Chem., 1993, 454, C20 CrossRef CAS
. For a Pd·TFA complex exhibiting a similar Pd–Pd bond distance, see: (e) Ref. 4 ; (f) T. A. Stromnova, S. T. Orlova, D. N. Kazyul'kin, I. P. Stolyarov and I. L. Eremenko, Russ. Chem. Bull., 2000, 49, 150 Search PubMed
.
- P. K. Killian, C. J. Bruell, C. Liang and M. C. Marley, Soil Sediment Contam., 2007, 16, 523 Search PubMed
.
- For seminal work on high oxidation state bimetallic Pd species, see:
(a) D. C. Powers and T. Ritter, Nat. Chem., 2009, 1, 302 Search PubMed
; (b) D. C. Powers, M. A. L. Geibel, J. E. M. N. Klein and T. Ritter, J. Am. Chem. Soc., 2009, 131, 17050 CrossRef CAS
; (c) N. R. Deprez and M. S. Sanford, J. Am. Chem. Soc., 2009, 131, 11234 CrossRef CAS
.
- For a review on Pd(II/IV) catalysis, see L.-M. Xu, B.-J. Li, Z. Yang and Z.-J. Shi, Chem. Soc. Rev., 2010, 39, 712 Search PubMed
.
-
(a) L. V. Desai, H. A. Malik and M. S. Sanford, Org. Lett., 2006, 8, 1141 CrossRef CAS
; (b) G.-W. Wang, T.-T. Yuan and X.-L. Wu, J. Org. Chem., 2008, 73, 4717 CrossRef CAS
.
-
(a) C. F. Rosewall, P. A. Sibbald, D. V. Liskin and F. E. Michael, J. Am. Chem. Soc., 2009, 131, 9488 CrossRef CAS
; (b) P. A. Sibbald, C. F. Rosewall, R. D. Swartz and F. E. Michael, J. Am. Chem. Soc., 2009, 131, 15945 CrossRef CAS
.
Footnotes |
| † Electronic supplementary information (ESI) available: General procedures for oxidative arylation, bimetallic palladacycle synthesis, and spectroscopic and crystallographic data. CCDC reference numbers 770196–770197. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00231c |
| ‡ General Procedure for Oxidative ortho-Arylation: In a one-dram vial equipped with a Teflon cap was added the substrate (0.2 mmol), Pd(OAc)2 (0.02 mmol, 10 mol%), Na2S2O8 (0.6 mmol), and the simple arene (1 mL). Subsequently, trifluoroacetic acid (1 mmol) was added. The vial was stirred on a heating block at 70 °C for the indicated length of time. The reaction mixture was cooled to room temperature, diluted in EtOAc and washed with sat'd. NaHCO3. Subsequently, the aqueous phase was re-extracted with EtOAc. The combined organic extracts were dried over Na2SO4, concentrated in vacuo and the resulting residue was purified by silica gel column chromatography or preparatory thin-layer chromatography (eluent: hexanes/CH2Cl2 or CH2Cl2/EtOAc) to afford the pure arylation products as single regioisomers. For more details, see ESI.† General Procedure for Intramolecular Oxidative Arylation: In a one-dram vial equipped with a Teflon cap was added the substrate (0.2 mmol), Pd(OAc)2 (0.02 mmol, 10 mol%), Na2S2O8 (0.8 mmol), and 1,2-dichloroethane (1 mL). Subsequently, trifluoroacetic acid (1 mmol) was added. The vial was stirred on a heating block at 70 °C for the indicated length of time. The reaction mixture was cooled to room temperature, diluted in EtOAc and washed with sat'd. NaHCO3. Subsequently, the aqueous phase was re-extracted with EtOAc. The combined organic extracts were concentrated in vacuo and the resulting residue was purified by preparatory thin-layer chromatography (eluent: CH2Cl2/MeOH) to afford the pure cyclization products. For more details, see ESI.† General Procedure for Synthesis of Bimetallic Palladacycles: In a one-dram vial equipped with a Teflon cap was added the substrate (0.1 mmol), Pd(OAc)2 (0.1 mmol), and dichloromethane (1 mL). Subsequently, trifluoroacetic acid was added (0.105 mmol). The vial was stirred on a heating block at 40 °C for 3 h. The reaction mixture was cooled to room temperature and concentrated in vacuo. The resulting residue was suspended in a mixture of hexanes and CHCl3 and filtered. The residue was washed with dichloromethane and the wash solution was subsequently collected and concentrated in vacuo to afford the bimetallic palladacycle as a yellow solid. Recrystallization from CH2Cl2/hexanes gives single crystals suitable for X-ray analysis. CCDC reference numbers 770196–770197. For more details, see ESI.† |
| This journal is © The Royal Society of Chemistry 2010 |