Tris(2-mercapto-1-tert-butylimidazolyl)hydroborato gallium derivatives: synthesis of di- and trigallium compounds in a sulfur-rich coordination environment†
Kevin
Yurkerwich
,
Daniela
Buccella
,
Jonathan G.
Melnick
and
Gerard
Parkin
*
Department of Chemistry, Columbia University, New York, New York 10027, USA. E-mail: parkin@columbia.edu
First published on 25th May 2010
Abstract
A series of tris(2-mercapto-1-tert-butylimidazolyl)hydroborato gallium compounds have been synthesized. While GaI3 and GaCl3 afford mononuclear {[TmBut]Ga} compounds, namely {[TmBut]GaI}I, {[TmBut]GaCl}[GaCl4], and [κ2-TmBut]2GaI, the reactions of “GaI”, Ga[GaCl4] and (HGaCl2)2 yield compounds that feature Ga–Ga bonds, namely [TmBut]GaGaI3, [TmBut]GaGaCl3, {[TmBut]GaGa[TmBut]}I2, {[TmBut]GaGa[TmBut]}[GaCl4]2, {[TmBut]Ga(GaI2)Ga[TmBut]}I and {[TmBut]GaGa[TmBut]}{[μ-κ1,κ2-TmBut]GaI2GaI2GaI}2. These Ga–Ga bonded compounds may be formally regarded as donor–acceptor adducts between monovalent [TmBut]Ga and various trivalent moieties; for example, [TmBut]GaGaI3 may be described as an adduct of [TmBut]Ga and GaI3, while {[TmBut]Ga(GaI2)Ga[TmBut]}+ is an adduct between two molecules of [TmBut]Ga and [GaI2]+. Comparison of the structure of [TmBut]Ga→B(C6F5)3 with that of [TmBut]In→B(C6F5)3 indicates that [TmBut]Ga is a more effective Lewis base than is [TmBut]In.
Introduction
Tris(2-mercapto-1-R-imidazolyl)hydroborato ligands, [TmR], have found diverse applications with respect to compounds that feature metal centers with sulfur-rich coordination environments.1 However, despite their widespread use in both main group and transition metal chemistry, there are few studies concerned with the Group 13 metals2 and none pertaining to gallium. Here we describe the first application of the [TmBut] ligand to gallium chemistry, including the synthesis of a series of compounds that feature Ga–Ga bonds which have no precedent in the related indium system.Results and discussion
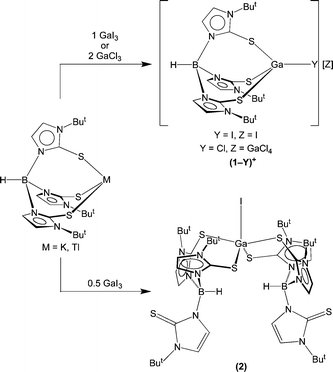
Mononuclear gallium compounds may be accessed by treatment of the thallium derivative [TmBut]Tl3 with either GaI3 or GaCl3 (Scheme 1). For example, [TmBut]Tl reacts with GaI3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio) to give a compound of composition [TmBut]GaI2, which has been shown by X-ray diffraction to exist in the solid state as an ion-pair, {[TmBut]GaI}I, (1-I)I. A related chloride derivative, {[TmBut]GaCl}[GaCl4], (1-Cl)[GaCl4], is likewise obtained via treatment of [TmBut]Tl with GaCl3 (1
1 molar ratio) to give a compound of composition [TmBut]GaI2, which has been shown by X-ray diffraction to exist in the solid state as an ion-pair, {[TmBut]GaI}I, (1-I)I. A related chloride derivative, {[TmBut]GaCl}[GaCl4], (1-Cl)[GaCl4], is likewise obtained via treatment of [TmBut]Tl with GaCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio). The ability of [TmBut] to bind in a facial manner to gallium via three sulfur donors contrasts with the applications of other [S3] donors that incorporate additional donors and bind in more encapsulating modes, as illustrated by tetradentate [S3N]4 and hexadentate [S3N3]5 ligands.
2 molar ratio). The ability of [TmBut] to bind in a facial manner to gallium via three sulfur donors contrasts with the applications of other [S3] donors that incorporate additional donors and bind in more encapsulating modes, as illustrated by tetradentate [S3N]4 and hexadentate [S3N3]5 ligands.
 | ||
| Scheme 1 | ||
In addition to {[TmBut]GaX}+ (X = Cl, I), which feature one [TmBut] ligand per gallium, the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex [κ2-TmBut]2GaI (2), which exhibits κ2 coordination of the [TmBut] ligands, has also been obtained from the reaction of [TmBut]K with GaI3 (2
1 complex [κ2-TmBut]2GaI (2), which exhibits κ2 coordination of the [TmBut] ligands, has also been obtained from the reaction of [TmBut]K with GaI3 (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio). The ability to isolate {[TmBut]GaX}+ and [κ2-TmBut]2GaI provides an interesting comparison with the related indium system. Specifically, the indium counterparts of {[TmBut]GaX}+ have not been reported, while the indium analogue of [κ2-TmBut]2GaI exists as an ion pair, {[TmBut]2In}I,2a in which the indium is octahedrally coordinated to the two [TmBut] ligands in a κ3 manner.6
1 molar ratio). The ability to isolate {[TmBut]GaX}+ and [κ2-TmBut]2GaI provides an interesting comparison with the related indium system. Specifically, the indium counterparts of {[TmBut]GaX}+ have not been reported, while the indium analogue of [κ2-TmBut]2GaI exists as an ion pair, {[TmBut]2In}I,2a in which the indium is octahedrally coordinated to the two [TmBut] ligands in a κ3 manner.6
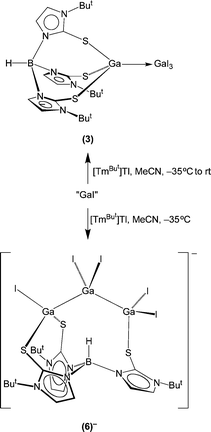
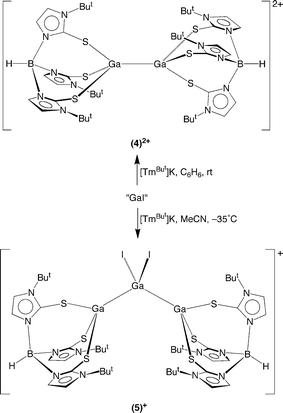
Another notable difference between the gallium and indium systems is that treatment of [TmBut]M (M = K, Tl) with “GaI”7,8 does not simply yield monovalent9 [TmBut]Ga, the analogue of [TmBut]In,2a but rather gives compounds that feature gallium–gallium bonds, namely (i) dinuclear [TmBut]GaGaI3 (3) and {[TmBut]GaGa[TmBut]}I2 (4)[I]210 and (ii) trinuclear {[TmBut]Ga(GaI2)Ga[TmBut]}I (5)[I] (Schemes 2 and 3), depending on the reaction conditions. In addition to these discrete dinuclear and trinuclear compounds, the ion pair {[TmBut]GaGa[TmBut]}{[μ-κ1,κ2-TmBut]GaI2GaI2GaI}2 that features both dinuclear and trinuclear moieties has been isolated, of which the anionic {[μ-κ1,κ2-TmBut]GaI2GaI2GaI}− (6)− species features an unprecedented [μ-κ1,κ2-TmBut] coordination mode in which the ligand bridges a chain of metal atoms.
 | ||
| Scheme 2 | ||
 | ||
| Scheme 3 | ||
The formation of each of these dinuclear and trinuclear compounds may be rationalized in terms of disproportion of “GaI” to gallium metal and GaI3,8 of which the latter would react with [TmBut]K to generate [TmBut]GaI2 (vide supra). Thus, dinuclear [TmBut]GaGaI3 may be formally regarded as a donor–acceptor adduct between monovalent [TmBut]Ga and trivalent GaI3,11 while {[TmBut]GaGa[TmBut]}2+ may be viewed as a donor–acceptor adduct between [TmBut]Ga and {[TmBut]Ga}2+. Similarly, trinuclear {[TmBut]Ga(GaI2)Ga[TmBut]}I is formally derived from a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of [TmBut]Ga and GaI3. It is also worth noting that the isolation of {[TmBut]Ga(GaI2)Ga[TmBut]}I is best achieved at low temperature (−35 °C), and that solutions in MeCN convert to a mixture comprising [TmBut]GaGaI3 and {[TmBut]GaGa[TmBut]}2+ at room temperature.
1 ratio of [TmBut]Ga and GaI3. It is also worth noting that the isolation of {[TmBut]Ga(GaI2)Ga[TmBut]}I is best achieved at low temperature (−35 °C), and that solutions in MeCN convert to a mixture comprising [TmBut]GaGaI3 and {[TmBut]GaGa[TmBut]}2+ at room temperature.
The molecular structures of [TmBut]GaGaI3, {[TmBut]GaGa[TmBut]}I2, {[TmBut]Ga(GaI2)Ga[TmBut]}I and {[TmBut]GaGa[TmBut]}{[μ-κ1,κ2-TmBut]GaI2GaI2GaI}2 have been determined by X-ray diffraction, as illustrated respectively in Fig. 1–4. The Ga–Ga bond lengths of [TmBut]GaGaI3 [2.4138(4) and 2.4254(3) Å],12 {[TmBut]GaGa[TmBut]}2+ [2.412(2) Å], {[TmBut]Ga(GaI2)Ga[TmBut]}+ [2.4586(5) Å] and {[μ-κ1,κ2-TmBut]GaI2GaI2GaI}− [2.406(3) and 2.412(2) Å] are comparable to twice the covalent radius of gallium (2.44 Å,13 2.48 Å14) and are typical for compounds with Ga–Ga single bonds.15
![Molecular structure of [TmBut]GaGaI3 (from benzene).](/image/article/2010/SC/c0sc00145g/c0sc00145g-f1.gif) | ||
| Fig. 1 Molecular structure of [TmBut]GaGaI3 (from benzene). | ||
![Molecular structure of {[TmBut]GaGa[TmBut]}I2 (only the cation is shown).](/image/article/2010/SC/c0sc00145g/c0sc00145g-f2.gif) | ||
| Fig. 2 Molecular structure of {[TmBut]GaGa[TmBut]}I2 (only the cation is shown). | ||
![Molecular structure of {[TmBut]Ga(GaI2)Ga[TmBut]}+.](/image/article/2010/SC/c0sc00145g/c0sc00145g-f3.gif) | ||
| Fig. 3 Molecular structure of {[TmBut]Ga(GaI2)Ga[TmBut]}+. | ||
![Molecular structure of {[μ-κ1,κ2-TmBut]GaI2GaI2GaI}−.](/image/article/2010/SC/c0sc00145g/c0sc00145g-f4.gif) | ||
| Fig. 4 Molecular structure of {[μ-κ1,κ2-TmBut]GaI2GaI2GaI}−. | ||
{[TmBut]GaGa[TmBut]}2+ and {[TmBut]Ga(GaI2)Ga[TmBut]}+ are of particular note because there are no other structurally characterized cationic dinuclear and trinuclear gallium compounds listed in the Cambridge Structural Database16 and catenation is not common for gallium.17 Furthermore, in view of the analogy between [TmR], tris(pyrazolyl)hydroborato [TpRR′] and cyclopentadienyl [CpR] ligands, it is interesting to note that the {[TpRR′]GaGa[TpRR′]}2+ and {[CpR]GaGa[CpR]}2+ counterparts of {[TmBut]GaGa[TmBut]}2+ have not been reported, even though such species are isoelectronic with the recently reported neutral zinc complexes CpRZnZnCpR,18 the first examples of compounds with Zn–Zn bonds.19
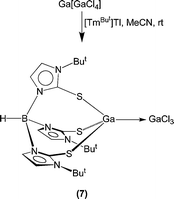
The chloride complexes Ga[GaCl4] and (HGaCl2)220 may also be used to synthesize compounds with Ga–Ga bonds. For example, Ga[GaCl4] reacts with [TmBut]Tl to give [TmBut]GaGaCl3 (7) (Scheme 4), while treatment of [TmBut]K with (HGaCl2)2 yields {[TmBut]GaGa[TmBut]}[GaCl4]210 (8) (Scheme 5), both of which have been structurally characterized by X-ray diffraction. While the mechanism for formation of {[TmBut]GaGa[TmBut]}2+ in the latter reaction is not known, it is pertinent to note that Lewis bases promote reductive dehydrogenation of (HGaCl2)2,20,21 which thereby provides a means to generate {[TmBut]GaGa[TmBut]}2+.
 | ||
| Scheme 4 | ||
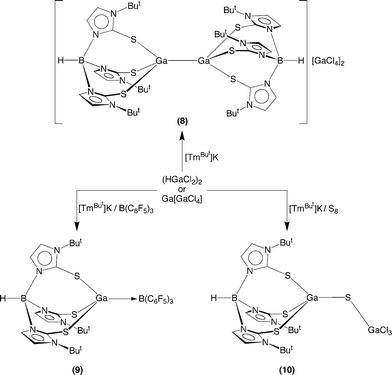 | ||
| Scheme 5 | ||
Since [TmBut]GaGaX3 (X = Cl, I) and {[TmBut]GaGa[TmBut]}2+ may be formally regarded as donor–acceptor adducts between monovalent [TmBut]Ga and the respective trivalent moiety, GaI3 and {[TmBut]Ga}2+, we sought evidence for the existence of [TmBut]Ga in this system.22 In this regard, support for the accessibility of [TmBut]Ga is provided by the observation that the simple adduct [TmBut]Ga→B(C6F5)3 (9) may be isolated via the reaction of [TmBut]K and (HGaCl2)2 in the presence of B(C6F5)3 (Scheme 5). Furthermore, the bridging sulfido complex [TmBut]GaSGaCl3 (10) may be obtained from the reaction of [TmBut]K with (HGaCl2)2 in the presence of sulfur (Scheme 5). In view of the fact that [TpBut2]Ga reacts with elemental chalcogens to give the terminal chalcogenido complexes [TpBut2]GaE (E = S, Se, Te),23 the formation of [TmBut]GaSGaCl3 is consistent with a sequence in which in situ generated [TmBut]Ga reacts with sulfur to give the terminal sulfido complex [TmBut]GaS, which is subsequently trapped by GaCl3, although other mechanisms are possible.24
The molecular structure of [TmBut]GaSGaCl3 has been determined by X-ray diffraction (Fig. 5), thereby demonstrating that the Ga–S bonds for the bridging sulfido ligand [2.2024(6) and 2.2153(6) Å] are shorter than those for the [TmBut] ligand (2.30–2.31 Å), an observation which is in accord with the L2X nature of [TmBut],25 such that the Ga–[TmBut] interaction is appropriately described as a composite of covalent and dative covalent bonds. The gallium sulfido bond lengths are, nevertheless, comparable to the values for other bridging sulfido complexes,26 but are considerably longer than that for the terminal sulfido complex [TpBut2]GaS [2.093(2) Å].23a The similarity of the two gallium–sulfido bond lengths is significant in view of the fact that the gallium centers in the isolated fragments, namely monovalent [TmBut]Ga and trivalent GaCl3, are electronically distinct. However, in view of the zwitterionic nature of the bonding within [TmBut]Ga(+)–S–Ga(−)Cl3, both gallium centers in the sulfido complex are trivalent and the Ga–S bond lengths are comparable.
![Molecular structure of [TmBut]GaSGaCl3.](/image/article/2010/SC/c0sc00145g/c0sc00145g-f5.gif) | ||
| Fig. 5 Molecular structure of [TmBut]GaSGaCl3. | ||
Comparison of the structure of [TmBut]Ga→B(C6F5)3 (Fig. 6) with that of the indium counterpart provides a means to assess the relative abilities of the [TmBut]Ga and [TmBut]In moieties to serve as a Lewis base. Specifically, the deviation of the B(C6F5)3 ligand from planarity, as indicated by the magnitude of Σ(C–B–C) provides a simple indication of the strength of the interaction with the Lewis base.27 On this basis, Σ(C–B–C) for [TmBut]Ga→B(C6F5)3 [342.2°]28 is smaller than that for [TmBut]In→B(C6F5)3 [347.9°],2a thereby indicating that the lone pair on gallium is more available for bonding than is that on indium, an observation which is in accord with the so-called “inert pair effect” being more prevalent for the heavier element.29 Support for the greater Lewis basicity of [TmBut]Ga, as implied by the structural differences between [TmBut]Ga→B(C6F5)3 and [TmBut]In→B(C6F5)3, is provided by DFT calculations which indicate that coordination of B(C6F5)3 to [TmBut]Ga (−23.7 kcal mol−1) is more exothermic than is coordination to [TmBut]In (−19.3 kcal mol−1).
![Molecular structure of [TmBut]GaB(C6F5)3.](/image/article/2010/SC/c0sc00145g/c0sc00145g-f6.gif) | ||
| Fig. 6 Molecular structure of [TmBut]GaB(C6F5)3. | ||
It is also pertinent to note that the 5.7° difference in Σ(C–B–C) for [TmBut]Ga→B(C6F5)3 and [TmBut]In→B(C6F5)3 is distinctly larger than the corresponding difference for ArGa→B(C6F5)3 and ArIn→B(C6F5)3 counterparts; for example, Σ(C–B–C) for (2,6-C6H3Trip2)Ga→B(C6F5)3 (337.5°)27b and (2,6-C6H3Trip2)In→B(C6F5)3 (339.3°)30 differ by only 1.9°. As such, this observation demonstrates that supporting ligands can exert differential effects on the relative Lewis basicities of monovalent gallium and indium centers.
Conclusions
In summary, the tris(2-mercapto-1-tert-butylimidazolyl)hydroborato ligand has been employed to synthesize the first cationic digallium and trigallium compounds, {[TmBut]GaGa[TmBut]}2+ and {[TmBut]Ga(GaI2)Ga[TmBut]}+, while comparisons between [TmBut]Ga→B(C6F5)3 and [TmBut]In→B(C6F5)3 indicate that [TmBut]Ga is a more effective Lewis base than is [TmBut]In.Acknowledgements
We thank the National Science Foundation (CHE-0749674) for support of this research and for the acquisition of an X-ray diffractometer (CHE-0619638).Notes and references
- (a) M. D. Spicer and J. Reglinski, Eur. J. Inorg. Chem., 2009, 1553–1574 CrossRef CAS; (b) G. Parkin, New J. Chem., 2007, 31, 1996–2014 RSC.
- For indium systems, see: (a) K. Yurkerwich, D. Buccella, J. G. Melnick and G. Parkin, Chem. Commun., 2008, 3305–3307 RSC; (b) C. A. Dodds, J. Reglinski and M. D. Spicer, Chem.–Eur. J., 2006, 12, 931–939 CrossRef CAS.
- D. J. Mihalcik, J. L. White, J. M. Tanski, L. N. Zakharov, G. P. A Yap, C. D. Incarvito, A. L. Rheingold and D. Rabinovich, Dalton Trans., 2004, 1626–1634 RSC.
- R. J. Motekaitis, A. E. Martell, S. A. Koch, J. W. Hwang, D. A. Quarless, Jr and M. J. Welch, Inorg. Chem., 1998, 37, 5902–5911 CrossRef CAS.
- D. A. Moore, P. E. Fanwick and M. J. Welch, Inorg. Chem., 1990, 29, 672–676 CrossRef CAS.
- [κ2-TmBut]2InCl (ref. 2a), nevertheless, has a structure that is analogous to that of [κ2-TmBut]2GaI.
- M. L. H. Green, P. Mountford, G. J. Smout and S. R. Speel, Polyhedron, 1990, 9, 2763–2765 CrossRef CAS.
- R. J. Baker and C. Jones, Dalton Trans., 2005, 1341–1348 RSC.
- G. Parkin, J. Chem. Educ., 2006, 83, 791–799 CrossRef CAS.
- 1H NMR spectroscopic studies suggest that, in solution, the counterions bind to gallium such that the [TmBut] ligand adopts a κ2-coordination mode. In this regard, [TmBut]2InX compounds have been shown to adopt two structural types, namely [κ2-TmBut]2InX (X = Cl, N3) and {[κ3-TmBut]2In}+X− (X = Cl, I, [InCl4]). See ref. 2a.
- In this regard, it is pertinent to note that the synthesis of [TpBut2]Ga is also accompanied by the formation of [TpBut2]GaGaI3. See ref. 7.
- Values for two different crystalline forms.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC.
- P. Pyykkö and M. Atsumi, Chem.–Eur. J., 2009, 15, 186–197 CrossRef.
- See, for example: (a) W. Uhl, Adv. Organomet. Chem., 2004, 51, 53–108 CrossRef CAS; (b) R. J. Baker, H. Bettentrup and C. Jones, Eur. J. Inorg. Chem., 2003, 2446–2451 CrossRef CAS; (c) A. M. Valean, S. Gómez-Ruiz, P. Lönnecke, I. Silaghi-Dumitrescu, L. Silaghi-Dumitrescu and E. Hey-Hawkins, New J. Chem., 2009, 33, 1771–1779 RSC.
- For neutral and anionic variants, see ref. 15 and: (a) A. Schnepf, C. Doriat, E. Möllhausen and H. Schnöckel, Chem. Commun., 1997, 2111–2112 RSC; (b) A. Kempter, C. Gemel and R. A. Fischer, Inorg. Chem., 2008, 47, 7279–7285 CrossRef CAS; (c) R. J. Baker, C. Jones, M. Kloth and J. A. Platts, Angew. Chem., Int. Ed., 2003, 42, 2660–2663 CrossRef CAS; (d) G. Linti, A. Rodig and W. Köstler, Z. Anorg. Allg. Chem., 2001, 627, 1465–1476 CrossRef CAS; (e) N. Wiberg, T. Blank, K. Amelunxen, H. Nöth, J. Knizek, T. Habereder, W. Kaim and M. Wanner, Eur. J. Inorg. Chem., 2001, 1719–1727 CrossRef CAS.
- M. S. Hill, P. B. Hitchcock and R. Pongtavornpinyo, Science, 2006, 311, 1904–1907 CrossRef CAS.
- A. Grirrane, I. Resa, A. Rodríguez and E. Carmona, Coord. Chem. Rev., 2008, 252, 1532–1539 CrossRef CAS.
- G. Parkin, Science, 2004, 305, 1117–1118 CrossRef CAS.
- (a) S. Nogai and H. Schmidbaur, Inorg. Chem., 2002, 41, 4770–4774 CrossRef CAS; (b) S. G. Alexander and M. L. Cole, Eur. J. Inorg. Chem., 2008, 4493–4506 CrossRef CAS.
- S. D. Nogai and H. Schmidbaur, Organometallics, 2004, 23, 5877–5880 CrossRef CAS.
- In this regard, it is pertinent to note that tris(pyrazolyl)borato counterparts have been isolated. See: (a) M. C. Kuchta, J. B. Bonanno and G. Parkin, J. Am. Chem. Soc., 1996, 118, 10914–10915 CrossRef CAS; (b) H. V. R. Dias and W. Jin, Inorg. Chem., 2000, 39, 815–819 CrossRef CAS.
- (a) M. C. Kuchta and G. Parkin, J. Chem. Soc., Dalton Trans., 1998, 2279–2280 RSC; (b) M. C. Kuchta and G. Parkin, Inorg. Chem., 1997, 36, 2492–2493 CrossRef CAS.
- For example, it is also possible that sulfur could insert directly into the Ga–Ga bond of [TmBut]GaGaCl3, or react initially with (HGaCl2)2.
- (a) M. L. H. Green, J. Organomet. Chem., 1995, 500, 127–148 CrossRef CAS; (b) G. Parkin, in Comprehensive Organometallic Chemistry III, ed. R. H. Crabtree and D. M. P. Mingos, Elsevier, Oxford, 2006, vol. 1, ch. 1 Search PubMed.
- S. D. Nogai and H. Schmidbaur, Dalton Trans., 2003, 2488–2495 RSC.
- (a) E. Rivard and P. P. Power, Inorg. Chem., 2007, 46, 10047–10064 CrossRef CAS; (b) N. J. Hardman, R. J. Wright, A. D. Phillips and P. P. Power, J. Am. Chem. Soc., 2003, 125, 2667–2679 CrossRef CAS.
- It is also worth noting that Σ(C–B–C) for [TmBut]Ga→B(C6F5)3 [342.2°] is comparable to that for Cp*Ga→B(C6F5)3 [342.2° 28a and 342.3° 28b]. Other donor–acceptor adducts of Cp*Ga are also known.28c–e (a) N. J. Hardman, P. P. Power, J. D. Gorden, C. L. B. Macdonald and A. H. Cowley, Chem. Commun., 2001, 1866–1867 RSC; (b) P. Jutzi, B. Neumann, G. Reumann, L. O. Schebaum and H.-G. Stammler, Organometallics, 2001, 20, 2854–2858 CrossRef CAS; (c) S. Schulz, A. Kuczkowski, D. Schuchmann, U. Flörke and M. Nieger, Organometallics, 2006, 25, 5487–5491 CrossRef CAS; (d) A. H. Cowley, Chem. Commun., 2004, 2369–2375 RSC; (e) P. W. Roesky, Dalton Trans., 2009, 1887–1893 RSC.
- F. A. Cotton, G. Wilkinson, C. A. Murillo, and M. Bochmann, Advanced Inorganic Chemistry, 6th edn, Wiley, 1999, p. 175 Search PubMed.
- R. J. Wright, A. D. Phillips, N. J. Hardman and P. P. Power, J. Am. Chem. Soc., 2002, 124, 8538–8539 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and crystallographic data. CCDC reference numbers 763457–763470. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00145g |
| This journal is © The Royal Society of Chemistry 2010 |
