DOI:
10.1039/C0SC00125B
(Edge Article)
Chem. Sci., 2010,
1, 206-209
Selective accumulation of rhodacyanine in plasmodial mitochondria is related to the growth inhibition of malaria parasites†
Received
25th January 2010
, Accepted 12th February 2010
First published on
25th May 2010
Abstract
Fluorescent rhodacyanines, which display antimalarial activity in vitro and in vivo, stain plasmodial parasites at the erythrocytic stage. A good correlation between the antimalarial activity and the accumulation of the dyes is observed. A fused-rhodacyanine, which displays a strong fluorescent property itself, was designed as a new probe for plasmodial parasites. It appears to be specifically localized in the parasitic mitochondria.
Introduction
Malaria, which is caused by the infection of Plasmodium spp., is one of the most serious tropical diseases. The gravest problem is that the parasites rapidly develop resistance to antimalarial drugs.1 Particularly, the efficacy of clinically available antimalarials, such as chloroquine (CQ), primaquine, and pyrimethamine, is dramatically decreasing. The development of new antimalarials with novel molecular skeletons and displaying different mechanisms of action against plasmodial parasites to existing drugs is required.2,3 Recently, we reported that rhodacyanines 14 (Fig. 1), which are structurally unrelated to those compounds commonly used as chemotherapeutics for malaria treatment, show a strong in vitro antimalarial activity against P. falciparum.5 Further studies have revealed that they are highly active against chloroquine-resistant strains and that several of them display good in vivo efficacy (P. berghei mouse models).6 It is noteworthy that some of the in vivo active compounds showed no significant acute toxicity. However, no information on the biological mode of action of antimalarial rhodacyanines has been reported. We describe herein the intracellular behavior of rhodacyanines in the malaria-infected erythrocytes. Furthermore, we report on the good correlation between the antimalarial activity of the rhodacyanines and their accumulation effect in the parasitic mitochondrion.
 |
| | Fig. 1 Rhodacyanines: general structure (left) and representative compound 1a (right). | |
Results and discussion
At the outset of our study, the intracellular distribution of 1a4 (EC50 = 21 nM against P. falciparum K1 strain, selective index = >5000 in vitro) was examined. Rhodacyanine 1a itself shows weak fluorescence in response to irradiation with visible light (λex = 495 nm, λem = 516 nm, Φ = 2.1 × 10−4 in MeOH). Upon the addition of 1a (final concentration: 5 × 10−6 M) onto cultured human erythrocytes infected with P. falciparum and after stirring for 2 h, the cells were fixed as a thin-layered smear on a glass plate and stained using the Diff-Quik® method. In the bright-field image, mature parasites in malaria-infected erythrocytes could be recognized by the optically dense, and brown-black staining of hemozoin in their digestive vacuoles (Fig. 2a). In contrast, fluorescent microscopic observation (through an FITC filter) reveals that 1a, which is visualized as a red spot, is selectively accumulated in malaria parasites (Fig. 2b). Non-infected host erythrocytes are not stained by 1a. Additionally, we noted that the selective accumulation can be clearly observed in the living cells (unfixed erythrocytes on the glass plate). Essentially, the accumulation is irreversible; the fluorescent localization is maintained after the treatment with 1a in infected erythrocytes, after washing with PBS (phosphate buffered saline) several times.
 |
| | Fig. 2 Microscopic images of the intracellular distribution of 1a in P. falciparum-infected erythrocytes. (a) Bright-field image; parasitic hemozoins (black arrows). (b) Fluorescent image through an FITC filter; 1a (white arrows). | |
The fluorescence distribution of several rhodacyanines and their analogues in the malaria-infected erythrocytes was examined using P. berghei. In accordance with the results found for P. falciparum, 1a selectively localizes in the same specific subcellular sites (organelles) of the parasites (Fig. 3a, b).7 Rhodacyanines displaying strong antimalarial activity, such as 1b (EC50 = 22 nM against P. falciparum) also exhibited specific localization. Their fluorescence can be observed as sharp spots (Fig. 3c, d). In contrast, less active compounds like 1d (EC50 = 680 nM) do not show specific concentration into an organelle and instead leach away into the parasitic cytoplasm (Fig. 3g, h), although they still remain in the plasmodial cells (no fluorescence was observed in the erythrocyte cytoplasm). Compound 1c (EC50 = 78 nM against P. falciparum) with an intermediate activity shows less specific localization (Fig. 3e, f) compared with 1a and 1b. No specific accumulation was observed for much less active compounds like 2 (EC50 = 4000 nM; Fig. 3i, j). These observations indicate that there is a good correlation between antimalarial activity and specific accumulation.
 |
| | Fig. 3 Accumulation of 1a (a, b), 1b (c, d), 1c (e, f), 1d (g, h) and 2 (i, j). The final concentration of the rhodacyanines was 5.0 × 10−6 M. Bright-field images (a, c, e, g, i) and fluorescent images through an FITC filter (b, d, f, h, j). | |
Next, we attempted to determine the plasmodial subcellular sites into which the rhodacyanines were selectively accumulated. A multiple-stain experiment with 1a, DAPI (4′,6-diamino-2-phenylindole; selective staining agent for nuclei) and Diff-Quik® showed that 1a accumulated in all of the parasitic organelles, except for the nucleus and food vacuoles. However, further investigation could not be carried out owing to the weak fluorescence and/or staining abilities of 1a.
In order to shed light on the more detailed mechanism, stronger fluorescent probe candidates should be synthesized. We envisaged that fixation of flexible bonds to rhodacyanines would give compounds of improved fluorescent ability due to suppression of energy loss from the excited state to the ground state by vibrational transitions. In this regard, we designed four classes of rhodacyanines 3–6, which have a fused ring skeleton. The synthetic route to a series of rhodacyanines is shown in Scheme 1.8 Preparation of 3 started from cyclization of alcohol 7, followed by condensation with 9 and successive ion exchange. Rhodacyanine 4, which possesses a pyrrolothiazolinone skeleton as a central core, was synthesized through a 7 step sequence synthesis from 7. Rhodacyanine 5, in which rhodanine and pyridine rings are interlocked by an ethylene tether, were synthesized from 14 in 7 steps. Compound 6 was synthesized from ethyl 2-methylnicotinate (18). Reaction of 3-aminomethyl-2-methylpyridine (19), which was prepared from 18 in three steps, yields rhodanine 20 on treatment with carbon disulfide and methyl bromoacetate in the presence of triethylamine. Then 20 was coupled with methylthiobenzothiazolium salt (12) to give 21 in a 69% yield (2 step yield, from 19). N-Methylation of 21 with methyl iodide followed by treatment with methyl triflate in the presence of triethylamine and an ion exchange process afforded fused-rhodacyanine 6 in 42% yield (3 steps).
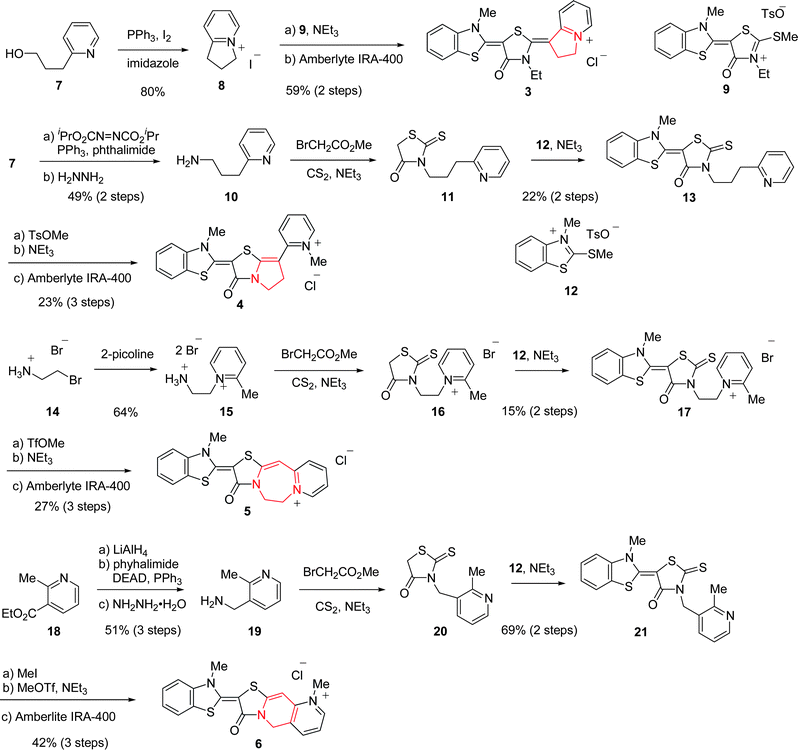 |
| | Scheme 1 Synthesis of fused-rhodacyanines 3–6. | |
The antimalarial activity and fluorescent properties of fused-rhodacyanines 3–6 are summarized in Table 1. In vitro antimalarial activities (EC50) of 3–6 were shown to be comparable to that of the original rhodacyanine 1a. Their selective toxicities were excellent as well. As expected, the fluorescence intensities of the new probes are improved over that of 1a. Especially, compounds 5 and 6 show a 70-fold increase in fluorescence intensity compared to 1a, and a significant red shift of the fluorescent emission was observed (excitation: λex = 495 nm).
Table 1
In vitro antimalarial activity and fluorescent properties of tested compounds
| Probe |
EC50/nMa |
Selectivityb |
λ
em/nmc |
Φ
F
|
|
In vitro antiplasmodial activity against P. falciparum K1.
Selective toxicity, EC50 value for L-6/EC50 for P. falciparum. Cytotoxicity was evaluated using rat skeletal myoblast L-6 cells representing a model of a host.
Maximum with highest wavelength of emission spectra (excitation: λex = 495 nm) in MeOH (1.0 × 10−6 M) at 20 °C.
Determined relative to fluorescein.
|
|
1a
|
21 |
>5000 |
516 |
2.1 × 10−4 |
|
3
|
46 |
3500 |
548 |
4.7 × 10−4 |
|
4
|
64 |
2300 |
516 |
2.9 × 10−3 |
|
5
|
46 |
>5000 |
576 |
1.4 × 10−2 |
|
6
|
65 |
>5000 |
563 |
1.4 × 10−2 |
With the probes 3–6 in hand, their fluorescent accumulation effects were examined using the P. berghei strain. The results are shown in Fig. 4. It is noteworthy that the fluorescence localization of 6 into parasitic organelles can be clearly detected, even upon treatment with a 100-fold less amount of 6 (final concentration; 5 × 10−8 M) compared to the original rhodacyanine 1a. Double stain experiments of the P. berghei-infected erythrocytes co-incubated with 6 and a selective fluorescent marker of subcellular organelle, were performed. As markers of nuclei and mitochondria, DAPI and MitoTracker Red CMXRos® were used, respectively. Microscopic images are shown in Fig. 5 and 6. The studies indicated that rhodacyanine 6 and DAPI were selectively accumulated in different organelles, respectively (Fig. 5). In contrast, fluorescence localization of 6 is clearly consistent with that of CMXRos (Fig. 6). Thus, we concluded that rhodacyanines selectively accumulate in the plasmodial mitochondria. Fused-rhodacyanine 6 could be used as a visible diagnostic probe for plasmodial mitochondria.9
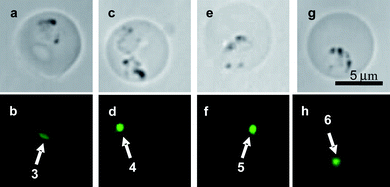 |
| | Fig. 4 Accumulation of fused rhodacyanines 3 (a, b), 4 (c, d), 5 (e, f) and 6 (g, h). Their final concentrations were 5.0 × 10−6 M (3, 4), 1.0 × 10−6 M (5) and 5.0 × 10−8 M (6). Bright-field images (a, c, e, g) and fluorescent images through an FITC filter (b, d, f, h). | |
 |
| | Fig. 5 Fluorescent microscopic images of the intracellular distribution of 6 and DAPI in P. berghei-infected erythrocytes, (a) through an FITC filter; 6 (green spot); (b) though a DAPI filter, parasitic nucleus stained by DAPI; (c) superimposed. | |
 |
| | Fig. 6 Fluorescent microscopic images of the intracellular distribution of 6 and CMXRos® in P. berghei-infected erythrocytes, (a) through an FITC filter; 6 (green spots); (b) through a Y7 filter, mitochondria (MT) stained by CMXRos® (red spots); (c) superimposed. | |
The selective uptake of rhodacyanines in mitochondria is consistent with the DLC (π-delocalized lipophilic cation) hypothesis,10 which is our starting point for the drug design. The conceptual term DLC was originally proposed by Chen et al. in their anticancer research work. It has subsequently been reported that several DLC compounds exhibit selective antitumor activity due to their selective accumulation in the mitochondria of carcinoma cells. This accumulation depends on the mitochondrial membrane potential (negative inside). Our results in this study suggest the DLC hypothesis would also be applicable to antimalarial drug design.11 It is noteworthy that rhodacyanines are found to display strong antileishmanial activity against Leishmania spp.,12 which are related parasitic protozoa to Plasmodium spp. The uptake of rhodacyanines in mitochondria might be a key role in the inhibition of related protozoal diseases.
Conclusions
In summary, we have found that antimalarial rhodacyanines selectively accumulate in erythrocytic plasmodial mitochondria. Furthermore, there is good correlation between their accumulation and antimalarial activity. Thus, the accumulation effect will have a dominant influence on the inhibition of plasmodial growth. It is noteworthy that the newly synthesized rhodacyanine 6 could be applied as a probe for plasmodial parasites as well as their mitochondria since it displays strong fluorescent properties and almost no cytotoxicity. Further studies are undergoing to understand the biological mechanism of action of rhodacyanines in detail.
Acknowledgements
We thank Mr Marcel Kaiser and Prof. Reto Brun for the determination of the EC50 values of the synthetic compounds against P. falciparum K1. This work was financially supported by Grants-in-Aid from the Intelligent Cosmos Foundation and Japan Science and Technology Agency (JST).
Notes and references
-
(a) W. Peters, Br. Med. Bull., 1982, 38, 187 Search PubMed;
(b) W. H. Wernsdorfer and D. Payne, Pharmacol. Ther., 1991, 50, 95 CrossRef CAS.
-
P. J. Rosenthal and L. H. Miller, in Antimalarial Chemotherapy, ed. P. J. Rosenthal, Humana Press, Totowa, 2001, pp. 3–15 Search PubMed.
-
(a) M.-L. Go, Med. Res. Rev., 2003, 23, 456 CrossRef CAS;
(b) R. G. Ridley, Nature, 2002, 415, 686 CrossRef CAS;
(c) A. M. Thayer, Chem. Eng. News, 2005, 83(43), 69.
- M. Kawakami, K. Koya, T. Ukai, N. Tatsuta, A. Ikegawa, K. Ogawa, T. Shishido and L. B. Chen, J. Med. Chem., 1998, 41, 130 CrossRef CAS.
- K. Takasu, H. Inoue, H.-S. Kim, M. Suzuki, T. Shishido, Y. Wataya and M. Ihara, J. Med. Chem., 2002, 45, 995 CrossRef CAS.
-
(a) K. Takasu, K. Pudhom, M. Kaiser, R. Brun and M. Ihara, J. Med. Chem., 2006, 49, 4795 CrossRef CAS;
(b) K. Pudhom, K. Kasai, H. Terauchi, H. Inoue, M. Kaiser, R. Brun, M. Ihara and K. Takasu, Bioorg. Med. Chem., 2006, 14, 8550 CrossRef CAS;
(c) K. Pudhom, J.-F. Ge, C. Arai, M. Yang, M. Kaiser, S. Wittlin, R. Brun, I. Itoh and M. Ihara, Heterocycles, 2009, 77, 207 CrossRef CAS.
- After verifying similar accumulation effects, we mainly used rodent malaria, P. berghei, instead of human malaria, P. falciparum, for safety reasons.
- Syntheses of fused-rhodacyanines 3–5 have been reported in a preliminary communication: K. Takasu, D. Morisaki, M. Kaiser, R. Brun and M. Ihara, Heterocycles, 2005, 66, 161 Search PubMed.
- Although several low-molecular weight compounds have been reported to be visible diagnostic agents for plasmodial parasites, almost all of those stain the parasitic nucleus or cytoplasm. To the best of our knowledge, it has been reported that parasitic mitochondria can be stained only by rhodamine 123, see:
(a) K. Tanabe, J. Protozool., 1983, 30, 707 Search PubMed;
(b) A. A. Divo, T. G. Geary, J. B. Jensen and H. Ginsburg, J. Protozool., 1985, 32, 442 Search PubMed.
- L. B. Chen, Annu. Rev. Cell Biol., 1988, 4, 155 Search PubMed.
- K. Takasu, T. Shimogama, C. Saiin, H.-S. Kim, Y. Wataya, R. Brun and M. Ihara, Chem. Pharm. Bull., 2005, 53, 653 CrossRef CAS.
-
(a) K. Takasu, H. Terauchi, H. Inoue, M. Takahashi, S. Sekita and M. Ihara, Heterocycles, 2004, 54, 215;
(b) M. Yang, C. Arai, A. Baker Md, J. Lu, J.-F. Ge, K. Pudhom, K. Takasu, K. Kasai, M. Kaiser, R. Brun, V. Yardley, I. Itoh and M. Ihara, J. Med. Chem., 2010, 53, 368 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Color graphics of the accumulation study and characterization data for all new compounds. See DOI: 10.1039/c0sc00125b/ |
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. 






