Size-controlled synthesis of dendrimer-stabilized silver nanoparticles for X-ray computed tomography imaging applications†
Hui
Liu
ab,
Han
Wang
c,
Rui
Guo
b,
Xueyan
Cao
b,
Jinglong
Zhao
c,
Yu
Luo
b,
Mingwu
Shen
b,
Guixiang
Zhang
*c and
Xiangyang
Shi
*ab
aState Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University, Shanghai, 201620, P. R. China
bCollege of Chemistry, Chemical Engineering and Biotechnology, Donghua University, Shanghai, 201620, P. R. China. E-mail: xshi@dhu.edu.cn; Fax: +0086-21-67792306-804; Tel: +0086-21-67792656
cDepartment of Radiology, Affiliated Shanghai First People's Hospital, Medical College, Shanghai Jiaotong University, Shanghai, 200080, P. R. China. E-mail: guixiangzhang@sina.com
First published on 22nd September 2010
Abstract
We report a facile size-controlled synthesis of dendrimer-stabilized silver nanoparticles (Ag DSNPs) for X-ray computed tomography (CT) imaging applications. Amine-terminated generation 5 poly(amidoamine) dendrimers were used as templates to complex Ag(I) ions for subsequent reductive formation of dendrimer-entrapped Ag nanoparticles. Following a one-step acetylation reaction to transform dendrimer terminal amine to acetyl groups, Ag DSNPs can be formed. The formed Ag DSNPs were characterized using 1H NMR, UV-Vis spectrometry, transmission electron microscopy, and ζ-potential measurements. We show that through the variation of the dendrimer/Ag salt molar ratio, the size of Ag DSNPs can be controlled at the range of 8.8–23.2 nm. The formed Ag DSNPs are stable not only in water, PBS buffer, and fetal bovine serum, but also at different pH conditions (pH 5–8) and temperatures (20–50 °C). X-Ray absorption coefficient measurements show that the attenuation of Ag DSNPs is size-dependent, and the Ag DSNPs with a diameter of 16.1 nm display an X-ray attenuation intensity close to that of a clinically used iodine-based contrast agent (Omnipaque) at the same molar concentration of the active element (Ag versus iodine). This suggests that Ag DSNPs with an appropriate size have a great potential to be used as a CT imaging contrast agent, although the atomic number of Ag is lower than that of iodine. Furthermore, CT scanning showed prolonged enhancement at the point of mice injected subcutaneously with Ag DSNPs, rendering them as a promising contrast agent in CT imaging applications.
Introduction
Noble metal nanoparticles (NPs) continue to receive immense scientific and technological interest in applications including but not limited to optoelectronics,1,2 sensing,3–5 biomedicine,6–10 and catalysis.11–13 In particular, silver (Ag) NPs (AgNPs) have been proved to be promising candidates for use in catalysis and biomedicine.3,14–16 For biomedical applications, the concerns over the toxicity of AgNPs can be usually resolved by appropriate surface modification.17,18 In general, most of the potential applications related to AgNPs require that the synthesized AgNPs should be size-tunable and colloidally stable under different conditions, which still remains a great challenge.Among many synthetic methodologies developed to synthesize AgNPs,14,19–21 the approaches to using dendrimers as templates or stabilizers have been proved to be quite promising. Dendrimers are a family of highly branched, monodispersed, synthetic macromolecules with well-defined composition and architecture.22,23 The unique structural features allow them to be employed as either templates or stabilizers to form AgNPs.24–28 In general, two types of dendrimer-protected AgNPs can be formed: (1) dendrimer-entrapped AgNPs (Ag DENPs) and (2) dendrimer-stabilized AgNPs (Ag DSNPs). Ag DENPs with a size generally smaller than 5 nm are formed using dendrimers as templates under fast reduction and nucleation chemistry, having a structure where each AgNP is entrapped within each dendrimer molecule,24,26,27,29 while Ag DSNPs with a size usually larger than 5 nm are formed using dendrimers as stabilizers under mild reduction conditions, having a structure where each AgNP is surrounded by multiple dendrimer molecules on its surface.7,14,27,28,30–32
For biological applications, it is ideal for Ag DENPs or DSNPs to have a neutral surface charge in order to avoid non-specific binding and toxicities. In this context, Ag DENPs or DSNPs formed using amine-terminated dendrimers as templates or stabilizers should be functionalized to neutralize the dendrimer terminal amines. Literature data have shown that acetylation is one of the key steps to form biocompatible and multifunctional gold DENPs and DSNPs for biomedical applications.5,10,33–35 For the case of Ag DENPs or DSNPs, we show that acetylation of Ag DENPs formed using generation 5 (G5) poly(amidoamine) (PAMAM) dendrimers as templates (with dendrimer/Ag salt molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.2) could significantly change the size and size distribution of the particles.27 The size of partially acetylated Ag DENPs displays a bimodal distribution (2.9 nm and 11.0 nm), whereas the pristine amine-terminated Ag DENPs and the completely acetylated Ag DENPs are relatively monodispersed with a size of 2.9 nm and 11.0 nm, respectively. This indicates that Ag DSNPs can be formed by complete acetylation of the amine-surfaced Ag DENPs. This previous study leads us to hypothesize that size-controlled synthesis of Ag DSNPs may be realized by varying the initial dendrimer/Ag salt molar ratio using a similar synthetic protocol.
51.2) could significantly change the size and size distribution of the particles.27 The size of partially acetylated Ag DENPs displays a bimodal distribution (2.9 nm and 11.0 nm), whereas the pristine amine-terminated Ag DENPs and the completely acetylated Ag DENPs are relatively monodispersed with a size of 2.9 nm and 11.0 nm, respectively. This indicates that Ag DSNPs can be formed by complete acetylation of the amine-surfaced Ag DENPs. This previous study leads us to hypothesize that size-controlled synthesis of Ag DSNPs may be realized by varying the initial dendrimer/Ag salt molar ratio using a similar synthetic protocol.
One important biological application of noble metal NPs is to use them as contrast agents for X-ray computed tomography (CT) imaging. Recent advances of nanotechnology in medical applications show that gold NPs display much higher X-ray attenuation than the clinically used iodine-based contrast agent (Omnipaque) and can be used for CT imaging of cells and tissue/organs.36–40 The major advantage of using metal NPs as CT imaging contrast agents is that the NPs have long blood circulation times, thereby enabling prolonged imaging of tissues/organs. In contrast, iodine-based small molecular agents are quickly metabolized after injection, unable to prolong the imaging time. Since AgNPs have structural and crystaline similarity to gold NPs, it is expected that Ag DSNPs with tunable sizes can be used for CT imaging applications.
In this present study, utilizing a similar approach reported by our group,27 we report the size-controlled synthesis of Ag DSNPs for CT imaging applications. The formed Ag DSNPs with different sizes were characterized by nuclear magnetic resonance (NMR) spectroscopy, UV-Vis spectrometry, transmission electron microscopy (TEM), and ζ-potential measurements. The stability of the Ag DSNPs under different pH and temperature conditions was evaluated by UV-Vis spectrometry. Finally, the X-ray attenuation properties and CT imaging capability were compared with a clinically used iodine-based contrast agent, Omnipaque. To our knowledge, this is the first report related to size-controlled synthesis of surface neutralized Ag DSNPs for CT imaging applications. Findings from this study are expected to provide a basis for rational design of functionalized NPs for medical imaging applications.
Experimental section
Materials
Ethylenediamine core amine-terminated G5 PAMAM dendrimers (G5.NH2) with a polydispersity index less than 1.08 were purchased from Dendritech (Midland, MI). All other chemicals were obtained from Aldrich and used as received. The water used in all the experiments was purified using a Milli-Q Plus 185 water purification system (Millipore, Bedford, MA) with resistivity higher than 18 MΩ cm. Regenerated cellulose dialysis membranes (molecular weight cut-off, MWCO = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were acquired from Fisher.
000) were acquired from Fisher.
Synthesis of Ag DSNPs
Following a similar protocol to that reported in our previous work,27 Ag DSNPs with tunable sizes were synthesized (Fig. 1). In the preparation procedure, Ag DENPs were first prepared by NaBH4 reduction of Ag(I) ions complexed with G5.NH2 dendrimers with different dendrimer/Ag salt molar ratios (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, and 1
75, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100). This was followed by complete acetylation of the dendrimer terminal amines, forming Ag DSNPs with a neutral surface charge. In a typical synthesis of Ag DSNPs with dendrimer/Ag salt molar ratio of 1
100). This was followed by complete acetylation of the dendrimer terminal amines, forming Ag DSNPs with a neutral surface charge. In a typical synthesis of Ag DSNPs with dendrimer/Ag salt molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, an aqueous AgNO3 solution (83.1 μL, 118.6 mM) was added to G5.NH2 aqueous solution (1 mL, 0.394 mM) under vigorous stirring. After 30 min, a water–methanol mixture solution (v/v = 2
25, an aqueous AgNO3 solution (83.1 μL, 118.6 mM) was added to G5.NH2 aqueous solution (1 mL, 0.394 mM) under vigorous stirring. After 30 min, a water–methanol mixture solution (v/v = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of NaBH4 (192.6 μL, 153.5 mM) was dropwise added to the dendrimer/Ag salt mixture solution while stirring. The stirring was continued for 2 h to complete the reaction. The reaction mixture was extensively dialyzed against water (6 times, 4 L) for 3 days to remove the excess reactants and by-products, followed by lyophilization to give [(Ag0)25-G5.NH2] DENPs.
1) of NaBH4 (192.6 μL, 153.5 mM) was dropwise added to the dendrimer/Ag salt mixture solution while stirring. The stirring was continued for 2 h to complete the reaction. The reaction mixture was extensively dialyzed against water (6 times, 4 L) for 3 days to remove the excess reactants and by-products, followed by lyophilization to give [(Ag0)25-G5.NH2] DENPs.
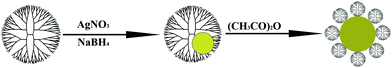 | ||
| Fig. 1 A schematic illustration of the preparation of Ag DSNPs. | ||
The acetylation reaction procedure used to modify Ag DENPs with acetamide groups is similar to that described in our previous work.27 Taking the acetylation of [(Ag0)25-G5.NH2] DENPs as an example, triethylamine (36.6 μL) was added to an aqueous solution of [(Ag0)25-G5.NH2] DENPs (11.3 mg, 5 mL). After 2 h stirring, acetic anhydride (20.7 μL) was added to the Ag DENPs/triethylamine mixture solution while stirring, and the mixture was allowed to react for 24 h. The crude product was extensively dialyzed against PBS buffer (3 times, 4 L) and water (3 times, 4 L) for 3 days to remove the excess of reactants and by-products, followed by lyophilization to get [(Ag0)25-G5.NHAc] DSNPs. [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs were obtained with the same procedure.
Characterization techniques
1H NMR spectra of Ag DSNPs were recorded on a Bruker DRX 400 nuclear magnetic resonance spectrometer. Samples were dissolved in D2O before measurements. ζ-Potential measurements were performed using a Malvern Zetasizer Nano ZS model ZEN3600 (Worcestershire, UK) equipped with a standard 633 nm laser. UV-Vis spectra were collected using a Lambda 25 UV-Vis spectrometer (Perkin-Elmer, United States). Samples were dissolved in water before the experiments. TEM was performed with a JEM 2100 analytical electron microscope operating at 200 kV. Five microlitres of an aqueous solution of Ag DSNPs (1 mg mL−1) were dropped onto a carbon-coated copper grid and air dried before measurements. The size distribution histogram of Ag DSNPs was analyzed using ImageJ software (http://rsb.info.nih.gov/ij/download.html). For each sample, 300 NPs from different images were randomly selected to analyze the size and size distribution.X-Ray CT imaging
Ag DSNPs or iohexol 300 (Omnipaque 300 mg I/mL, GE Healthcare) solutions with different concentrations were prepared in 100 μL Eppendorf tubes and placed in a self-designed scanning holder. CT scans were performed using a Micro-CT imaging system (eXplore Locus, GE) with 80 kV, 450 μA, and a slice thickness of 45 μm. Evaluation of the contrast enhancement of Ag DSNPs was carried out by loading the digital CT images in a standard display program and then selecting a uniform round region of interest on the resultant CT image for each sample. Contrast enhancement was determined in Hounsfield units (HU) for each concentration of Ag DSNPs with different compositions and Omnipaque.ICR mice (20–25 g, Shanghai Laboratory Animal Center) were anesthetized by intraperitoneal injection of 0.3 mL of 3% pentobarbital sodium (12 mL kg−1). [(Ag0)75-G5.NHAc] (50 μL, Ag concentration = 0.1 mol L−1) dispersed in PBS buffer was then subcutaneously injected into the mouse's back on the left. For a control experiment, Omnipaque (50 μL, iodine concentration = 0.1 mol L−1) was injected into the mouse's back on the right. CT scans were performed at 5 min and 25 min post injection by a Micro-CT imaging system with a tube voltage of 80 kV, an electrical current of 450 μA, and a slice thickness of 92 μm. Images were reconstructed by GEHC microView software based on voxels of 45 μm × 45 μm × 45 μm.
Results and discussion
Synthesis and characterization of Ag DSNPs
Metal DSNPs are often formed under mild reduction conditions, including the use of mild reducing agents (e.g., hydrazine)41 and physical treatments (e.g., UV-irradiation7,25 and thermo-treatment).32,42 Our previous studies have shown that acetylation of Ag DENPs formed with a G5 dendrimer/Ag salt molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 51.2 could readily convert Ag DENPs to Ag DSNPs.27 By varying the molar ratio between G5.NH2 dendrimers and Ag salt, it is expected that the size of the formed Ag DSNPs could be controlled. We showed that when the molar ratio of G5.NH2/Ag salt was beyond 1
51.2 could readily convert Ag DENPs to Ag DSNPs.27 By varying the molar ratio between G5.NH2 dendrimers and Ag salt, it is expected that the size of the formed Ag DSNPs could be controlled. We showed that when the molar ratio of G5.NH2/Ag salt was beyond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the formed particles precipitated out at the bottom of the vials. Therefore, the molar ratios of G5.NH2/Ag salt were selected as 1
100, the formed particles precipitated out at the bottom of the vials. Therefore, the molar ratios of G5.NH2/Ag salt were selected as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1
25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1
50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, and 1
75, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100.
100.
The complete acetylation of all Ag DENPs was confirmed by 1H NMR. In the 1H NMR spectra of all the formed Ag DSNPs (Fig. S1†), the appearance of the proton signal at 1.87 ppm related to the –CH3 protons of the acetyl group clearly indicates the formation of acetamide groups on the dendrimer surface, similar to the acetylated G5 dendrimers without AgNPs. These results were in agreement with literature.27,43 The complete acetylation of dendrimer terminal amines to acetamide groups was also verified by ζ-potential measurements (Table S1). It is clear that all Ag DSNPs have a surface potential close to zero after the acetylation reaction, in agreement with the literature.27,34
The optical properties of Ag DSNPs were investigated using UV-Vis spectrometry. Fig. 2a shows the UV-Vis spectra of Ag DSNPs with different G5 dendrimer/Ag atom molar ratios. The surface plasma resonance (SPR) peak27 around 412 nm clearly indicates the existence of Ag NPs in solutions. Under similar molar concentration of Ag DSNPs, the absorbance increased with the increase of Ag content. When the G5 dendrimer/Ag atom molar ratio reached up to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the absorbance value decreased, and a broad band of absorbance at the region of 500 to 900 nm appeared, indicating a relatively larger size or a certain degree of aggregation of Ag DSNPs formed in the solution. From the photograph of the [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs (Fig. 2b), it is clear that the solution color deepens with the Ag content. The [(Ag0)25-G5.NHAc] DSNPs had a pale yellow color, whilst the [(Ag0)50-G5.NHAc] and [(Ag0)75-G5.NHAc] DSNPs were quite yellow. For [(Ag0)100-G5.NHAc] DSNPs, a deep brown color was shown. The gradually deepened color of Ag DSNPs is ascribed to the gradual increase of the Ag content.
100, the absorbance value decreased, and a broad band of absorbance at the region of 500 to 900 nm appeared, indicating a relatively larger size or a certain degree of aggregation of Ag DSNPs formed in the solution. From the photograph of the [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs (Fig. 2b), it is clear that the solution color deepens with the Ag content. The [(Ag0)25-G5.NHAc] DSNPs had a pale yellow color, whilst the [(Ag0)50-G5.NHAc] and [(Ag0)75-G5.NHAc] DSNPs were quite yellow. For [(Ag0)100-G5.NHAc] DSNPs, a deep brown color was shown. The gradually deepened color of Ag DSNPs is ascribed to the gradual increase of the Ag content.
![(a) UV-Vis spectra and (b) photographs of the aqueous solution of Ag DSNPs with G5 dendrimer/Ag atom molar ratios of 1 : 25, 1 : 50, 1 : 75, and 1 : 100. In (b), the vials from left to right show the solution of [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs.](/image/article/2010/PY/c0py00218f/c0py00218f-f2.gif) | ||
Fig. 2 (a) UV-Vis spectra and (b) photographs of the aqueous solution of Ag DSNPs with G5 dendrimer/Ag atom molar ratios of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25, 1 25, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 1 50, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75, and 1 75, and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. In (b), the vials from left to right show the solution of [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs. 100. In (b), the vials from left to right show the solution of [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs. | ||
The size and morphology of Ag DSNPs with different compositions were characterized with TEM (Fig. 3). The diameter of [(Ag0)25-G5.NHAc], [(Ag0)50-G5.NHAc], [(Ag0)75-G5.NHAc], and [(Ag0)100-G5.NHAc] DSNPs was 8.8, 12.4, 16.1, and 23.2 nm, respectively. This implies that by simply varying the dendrimer/Ag salt molar ratios, the size of Ag DSNPs can be controlled. The diameter of [(Ag0)50-G5.NHAc] DSNPs (12.4 nm) is very close to that of [(Ag0)51.2-G5.100Ac] (11.0 nm) reported in our previous studies.27 In addition, all particles had a relatively narrow size distribution except [(Ag0)100-G5.NHAc] DSNPs. A small portion of aggregated particles appeared in the size range of 50 to 70 nm (Fig. 3d), in agreement with the UV-Vis spectrometry data (Fig. 2a). It is worth noting that the TEM images shown in Fig. 3 are representative images for NPs with each composition. However, the size distribution histograms of the Ag DSNPs were based on the measurement of 300 NPs in different TEM images. In this case, the selected TEM images for NPs with each composition may not fully reflect the statistical results of the size distribution.
![TEM images and size distribution histograms of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs, respectively.](/image/article/2010/PY/c0py00218f/c0py00218f-f3.gif) | ||
| Fig. 3 TEM images and size distribution histograms of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs, respectively. | ||
The crystalline nature of the Ag DSNPs was confirmed by selected area electron diffraction (SAED). Fig. 4 shows a typical SAED pattern of [(Ag0)25-G5.NHAc] DSNPs. The (111), (200), (220), and (311) rings in the SAED pattern clearly indicate the face-centered cubic (fcc) crystal structure of Ag DSNPs.
![A typical SAED pattern for [(Ag0)25-G5.NHAc] DSNPs.](/image/article/2010/PY/c0py00218f/c0py00218f-f4.gif) | ||
| Fig. 4 A typical SAED pattern for [(Ag0)25-G5.NHAc] DSNPs. | ||
Stability of Ag DSNPs
The stability of Ag DSNPs is of paramount importance for their biological applications. Literature data show that the aggregation degree of metal nanoparticles could be effectively reflected by changes of the absorption characteristics, especially the SPR peak shifting in the UV-Vis spectrum.38,44 In this work, we used UV-Vis spectroscopy to monitor the stability of Ag DSNPs under different pH and temperature conditions. Fig. 5 shows the UV-Vis spectra of Ag DSNPs dispersed in water with different pHs at room temperature (25 °C). HCl or NaOH (0.1 mol L−1) was employed to adjust the pH value of the particle suspension. We showed that at the pH range of 5 to 8, the SPR peak position did not appreciably change in the spectra of all Ag DSNPs with different compositions, suggesting that all the formed Ag DSNPs are colloidally stable within this range of pH.![UV-Vis spectra of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs under different pH conditions.](/image/article/2010/PY/c0py00218f/c0py00218f-f5.gif) | ||
| Fig. 5 UV-Vis spectra of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs under different pH conditions. | ||
The stability of Ag DSNPs dispersed in water at different temperatures was also investigated (Fig. 6). Similarly, in the UV-Vis spectra of Ag DSNPs with different compositions, the SPR peak position did not have any significant changes at the temperatures of 25, 37, and 50 °C. This implies that all Ag DSNPs have a good colloidal stability and do not form aggregates when temperature ranges from 25 °C to 50 °C. We noted that the SPR peak intensity had a slight change for some cases of Ag DSNPs under different pH and temperature conditions; however, the SPR peak positions did not change significantly under similar conditions. The SPR peak intensity changes only reflected the slight concentration differences of the Ag DSNPs.
![UV-Vis spectra of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs at different temperatures.](/image/article/2010/PY/c0py00218f/c0py00218f-f6.gif) | ||
| Fig. 6 UV-Vis spectra of [(Ag0)25-G5.NHAc] (a), [(Ag0)50-G5.NHAc] (b), [(Ag0)75-G5.NHAc] (c), and [(Ag0)100-G5.NHAc] (d) DSNPs at different temperatures. | ||
Furthermore, the colloidal stability of Ag DSNPs remained identical when they were dispersed into water, PBS buffer, and fetal bovine serum (Fig. S2†). No aggregation could be found even after 4 months of storage at room temperature. Thus, the Ag DSNPs prepared here possess good stability not only in water, PBS buffer, and fetal bovine serum but also under different pH and temperature conditions. The stability of all Ag DSNPs is essential for their biological applications.
X-Ray CT imaging
Gold NPs have good X-ray attenuation properties, enabling them to be used as contrast agents in CT imaging.38,39 The X-ray attenuation properties of gold NPs are demonstrated to be both size- and concentration-dependent.38,39,45 Due to the crystalline and structural similarity, AgNPs may be another choice of CT contrast agents. Theoretically, Ag element has a lower X-ray absorption coefficient than iodine because of its lower atomic number. However, the nanometre-sized AgNPs allow them to be cleared from the blood much more slowly than commercial iodine-based small molecular CT contrast agents, permitting longer imaging times.In this study, we investigated the effect of the size of Ag DSNPs on the X-ray absorption characteristics. X-Ray absorption coefficient measurements revealed that Ag DSNPs with the same Ag concentration and different sizes (8.8–23.2 nm) had different X-ray attenuation intensity (Fig. 7a). The X-ray attenuation intensity enhanced with the size of the Ag DSNPs. This may be caused by the gradual increase of the local concentration of Ag with the size of colloidally stable Ag DSNPs within a certain range. When the size of Ag DSNPs increased to 23.2 nm ([(Ag0)100-G5.NHAc] DSNPs), the intensity of X-ray attenuation decreases. This suggests that the dependence of the X-ray attenuation intensity on the particle sizes falls into a certain range. When the particle size is beyond the threshold (23.2 nm), the Ag DSNPs display larger size dispersity and more aggregation, which is not beneficial for enhancing the effect of the X-ray attenuation. This suggests that the colloidal stability of the particles is important for particles with good X-ray attenuation properties. In this case, [(Ag0)75-G5.NHAc] DSNPs with a diameter of 16.1 nm had the strongest X-ray attenuation intensity.
![(a) X-Ray attenuation (HU) as a function of the size of Ag DSNPs at the same Ag concentration (0.05 mol L−1); (b) X-ray attenuation (HU) of [(Ag0)75-G5.NHAc] DSNPs and Omnipaque as a function of the molar concentration of active element (Ag or iodine).](/image/article/2010/PY/c0py00218f/c0py00218f-f7.gif) | ||
| Fig. 7 (a) X-Ray attenuation (HU) as a function of the size of Ag DSNPs at the same Ag concentration (0.05 mol L−1); (b) X-ray attenuation (HU) of [(Ag0)75-G5.NHAc] DSNPs and Omnipaque as a function of the molar concentration of active element (Ag or iodine). | ||
In order to compare the X-ray attenuation properties of Ag DSNPs with a clinically used iodine-based CT contrast agent (Omnipaque), we selected [(Ag0)75-G5.NHAc] DSNPs for comparison (Fig. 7b). With the increase of the molar concentration of the active element (i.e., Ag or iodine), the attenuation coefficient of both [(Ag0)75-G5.NHAc] DSNPs and Omnipaque increased due to the concentration-dependent effect caused by the change in mass ratio between Ag (or iodine) and water molecules. At the concentration below 0.10 mol L−1, [(Ag0)75-G5.NHAc] DSNPs displayed approximately similar X-ray attenuation coefficients to Omnipaque. This implies that Ag DSNPs with an appropriate size have an approximately similar X-ray attenuation intensity to omnipaque with iodine concentration similar to Ag, although the atomic number of Ag is lower than that of iodine.
To further explore the potential to use Ag DSNPs as CT contrast agents, [(Ag0)75-G5.NHAc] DSNPs (50 μL, [Ag] = 0.1 mol L−1) was subcutaneously injected into a mouse's back on the left and the cross-sectional CT image was collected (Fig. 8). In CT images, parenchyma, including muscles and blood vessels, are gray and even darker, while bones, such as the mouse's spondyle in our case, are white due to their higher density and the resultant stronger X-ray absorption. Because AgNPs have a much higher attenuation coefficient than parenchyma, the existence of AgNPs within the parenchyma can be easily visualized in the CT image at 5 min and 25 min post-injection (arrows in Fig. 8a and 8b). Thus, AgNPs can greatly boost CT image contrast, providing that the administered AgNPs are enriched in particular areas or tissues. In Fig. 8a and 8b, the arrow points to the Ag DSNP injection region at 5 min and 25 min post injection, respectively. The inject region is displayed as a narrow bright strip in the CT image due to the limited diffusion and localized distribution of Ag DSNPs. In contrast, in the inject area of Omnipaque with iodine concentration similar to Ag on the mouse's back on the right (arrow head in Fig. 8a and 8b), no such bright injection region can be found in the CT image even at 5 min post injection. This is due to the high diffusion rate of the small molecular contrast agent in the subcutaneous tissues. Besides the ability of Ag DSNPs in prolonging the CT imaging time at the localized region, the injected Ag DSNPs did not show any toxicity to the mice. The mice injected with the Ag DSNPs behaved healthy and normal as compared with those before injection.
![CT image of a mouse with 50 μL of [(Ag0)75-G5.NHAc] DSNPs ([Ag] = 0.1 mol L−1) subcutaneously injected into its back on the left at 5 min (a) and 25 min (b) post injection. The white arrow points to the Ag DSNP injection region. For a control experiment, Omnipaque (50 μL, iodine concentration = 0.1 mol L−1) was injected into the mouse's back on the right. The white arrow head points to the inject area of Omnipaque with iodine concentration similar to Ag.](/image/article/2010/PY/c0py00218f/c0py00218f-f8.gif) | ||
| Fig. 8 CT image of a mouse with 50 μL of [(Ag0)75-G5.NHAc] DSNPs ([Ag] = 0.1 mol L−1) subcutaneously injected into its back on the left at 5 min (a) and 25 min (b) post injection. The white arrow points to the Ag DSNP injection region. For a control experiment, Omnipaque (50 μL, iodine concentration = 0.1 mol L−1) was injected into the mouse's back on the right. The white arrow head points to the inject area of Omnipaque with iodine concentration similar to Ag. | ||
Conclusions
In summary, Ag DSNPs were formed by acetylation-mediated conversion of amine-surfaced Ag DENPs. By simply varying the molar ratio of G5 PAMAM dendrimer/Ag salt, the size of crystalline Ag DSNPs with fcc crystal structures can be controlled at the range of 8.8–23.2 nm. The formed Ag DSNPs are stable not only in water, PBS buffer, and fetal bovine serum but also at different pH and temperature conditions. X-Ray absorption results revealed that the attenuation intensity of Ag DSNPs was both size- and concentration-dependent. The Ag DSNPs with a diameter of 16.1 nm were found to have approximately similar X-ray attenuation intensity to that of iodine-based contrast agents at the same molar concentration. Furthermore, we show that subcutaneously injected Ag DSNPs can significantly increase the contrast of the injection region of a mouse in the CT image, suggesting the use of Ag DSNPs as an in vivo contrast agent for CT imaging applications. Our future efforts will be focused on the application of Ag DSNPs for blood pool imaging in vivo through intravenous injection of the particles. Moreover, taking into consideration the unique structural features of PAMAM dendrimers and the capability for synthesis of multifunctional metal DSNP,33,35 Ag DSNPs are expected to be a useful platform for the construction of multifunctional theranostic nanoagents for targeted CT imaging of various diseases with prolonged imaging times.Acknowledgements
This research is financially supported by the National Natural Science Foundation of China (20974019 and 30901730), the National Basic Research Program of China (973 Program, 2007CB936000), the Shanghai Pujiang Program (09PJ1400600), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, and the Fundamental Research Funds for the Central Universities (For R. G., X. C., M. S., and X. S.). R. G. thanks the supports from Young Teacher Foundation of Donghua University (200802) and Shanghai Natural Science Foundation (10ZR1400800). G. Z. thanks the Ph.D. Programs Foundation of Ministry of Education of China (No. 20090073110072).Notes and references
- J. Zheng, J. T. Petty and R. M. Dickson, J. Am. Chem. Soc., 2003, 125, 7780–7781 CrossRef CAS.
- G. Mattei, P. Mazzoldi and H. Bernas, Top. Appl. Phys., 2010, 116, 287–316 CAS.
- D. Graham, K. Faulds and W. E. Smith, Chem. Commun., 2006, 4363–4371 RSC.
- M.-C. Daniel and D. Astruc, Chem. Rev., 2004, 104, 293–346 CrossRef CAS.
- X. Shi, S. H. Wang, S. Meshinchi, M. E. Van Antwerp, X. Bi, I. Lee and J. R. Baker Jr., Small, 2007, 3, 1245–1252 CrossRef CAS.
- N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547–1562 CrossRef CAS.
- W. Lesniak, A. U. Bielinska, K. Sun, K. W. Janczak, X. Shi, J. R. Baker, Jr. and L. P. Balogh, Nano Lett., 2005, 5, 2123–2130 CrossRef CAS.
- P. Cherukuri, E. S. Glazer and S. A. Curley, Adv. Drug Delivery Rev., 2010, 62, 339–345 CrossRef CAS.
- X. Huang, S. Neretina and M. A. El-Sayed, Adv. Mater., 2009, 21, 4880–4910 CrossRef CAS.
- M. Shen and X. Shi, Nanoscale, 2010, 2, 1596–1610 RSC.
- R. M. Crooks, M. Q. Zhao, L. Sun, V. Chechik and L. K. Yeung, Acc. Chem. Res., 2001, 34, 181–190 CrossRef CAS.
- K. Esumi, R. Isono and T. Yoshimura, Langmuir, 2004, 20, 237–243 CrossRef CAS.
- X. H. Peng, Q. M. Pan and G. L. Rempel, Chem. Soc. Rev., 2008, 37, 1619–1628 RSC.
- A. Sutton, G. Franc and A. Kakkar, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 4482–4493 CrossRef CAS.
- I. Sondi and B. Salopek-Sondi, J. Colloid Interface Sci., 2004, 275, 177–182 CrossRef CAS.
- L. Balogh, D. R. Swanson, D. A. Tomalia, G. L. Hagnauer and A. T. McManus, Nano Lett., 2001, 1, 18–21 CrossRef CAS.
- Y.-C. Chung, I.-H. Chen and C.-J. Chen, Biomaterials, 2008, 29, 1807–1816 CrossRef CAS.
- W. Lu, D. Senapati, S. Wang, O. Tovmachenko, A. K. Singh, H. Yu and P. C. Ray, Chem. Phys. Lett., 2010, 487, 92–96 CrossRef CAS.
- C. M. Cobley, S. E. Skrabalak, D. J. Campbell and Y. Xia, Plasmonics, 2009, 4, 171–179 CrossRef CAS.
- L. S. Nair and C. T. Laurencin, J. Biomed. Nanotechnol., 2007, 3, 301–316 Search PubMed.
- S. E. Skrabalak and Y. Xia, ACS Nano, 2009, 3, 10–15 CrossRef CAS.
- Dendrimers and Other Dendritic Polymers, ed. D. A. Tomalia and J. M. J. Frechet, John Wiley & Sons Ltd, New York, 2001 Search PubMed.
- D. A. Tomalia, A. M. Naylor and W. A. Goddard III, Angew. Chem., Int. Ed. Engl., 1990, 29, 138 CrossRef.
- J. Maly, H. Lampova, A. Semeradtova, M. Stofik and L. Kovacik, Nanotechnology, 2009, 20, 385101 CrossRef CAS.
- K. Esumi, A. Suzuki, A. Yamahira and K. Torigoe, Langmuir, 2000, 16, 2604–2608 CrossRef CAS.
- R. W. J. Scott, O. M. Wilson and R. M. Crooks, J. Phys. Chem. B, 2005, 109, 692–704 CrossRef CAS.
- X. Shi, I. Lee and J. R. Baker, Jr., J. Mater. Chem., 2008, 18, 586–593 RSC.
- M. Shen, K. Sun and X. Shi, Curr. Nanosci., 2010, 6, 307–314 Search PubMed.
- O. M. Wilson, R. W. J. Scott, J. C. Garcia-Martinez and R. M. Crooks, J. Am. Chem. Soc., 2005, 127, 1015–1024 CrossRef CAS.
- K. Esumi, Colloid Chemistry II, Springer-Verlag Berlin, Berlin, 2003, pp. 31–52 Search PubMed.
- A. Manna, T. Imae, K. Aoi, M. Okada and T. Yogo, Chem. Mater., 2001, 13, 1674–1681 CrossRef CAS.
- X. P. Sun, S. J. Dong and E. K. Wang, Macromolecules, 2004, 37, 7105–7108 CrossRef CAS.
- X. Shi, K. Sun and J. R. Baker, Jr., J. Phys. Chem. C, 2008, 112, 8251–8258 CrossRef CAS.
- X. Shi, S. H. Wang, H. P. Sun and J. R. Baker, Jr., Soft Matter, 2007, 3, 71–74 RSC.
- X. Shi, S. H. Wang, M. E. Van Antwerp, X. Chen and J. R. Baker, Jr., Analyst, 2009, 134, 1373–1379 RSC.
- R. Popovtzer, A. Agrawal, N. A. Kotov, A. Popovtzer, J. Balter, T. E. Carey and R. Kopelman, Nano Lett., 2008, 8, 4593–4596 CrossRef CAS.
- C. Alric, J. Taleb, G. Le Duc, C. Mandon, C. Billotey, A. Le Meur-Herland, T. Brochard, F. Vocanson, M. Janier, P. Perriat, S. Roux and O. Tillement, J. Am. Chem. Soc., 2008, 130, 5908–5915 CrossRef CAS.
- R. Guo, H. Wang, C. Peng, M. Shen, M. Pan, X. Cao, G. Zhang and X. Shi, J. Phys. Chem. C, 2010, 114, 50–56 CrossRef CAS.
- C. J. Xu, G. A. Tung and S. H. Sun, Chem. Mater., 2008, 20, 4167–4169 CrossRef CAS.
- D. Kim, S. Park, J. H. Lee, Y. Y. Jeong and S. Jon, J. Am. Chem. Soc., 2007, 129, 7661–7665 CrossRef CAS.
- X. Shi, T. R. Ganser, K. Sun, L. P. Balogh and J. R. Baker, Jr., Nanotechnology, 2006, 17, 1072–1078 CrossRef CAS.
- X. P. Sun, X. Jiang, S. J. Dong and E. K. Wang, Macromol. Rapid Commun., 2003, 24, 1024–1028 CrossRef CAS.
- I. J. Majoros, B. Keszler, S. Woehler, T. Bull and J. R. Baker, Jr., Macromolecules, 2003, 36, 5526–5529 CrossRef CAS.
- Y. Takeuchi, T. Ida and K. Kimura, J. Phys. Chem. B, 1997, 101, 1322–1327 CrossRef CAS.
- C. Kojima, Y. Umeda, M. Ogawa, A. Harada, Y. Magata and K. Kono, Nanotechnology, 2010, 21, 245104 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Additional 1H NMR spectra and ζ-potential values of all Ag DSNPs and the photographs of the Ag DSNP solutions dispersed in water, PBS buffer, and fetal bovine serum. See DOI: 10.1039/c0py00218f |
| This journal is © The Royal Society of Chemistry 2010 |
