Highly selective bisphenol A—imprinted polymers prepared by atom transfer radical polymerization†
Shogo Sasaki
,
Tooru Ooya
and
Toshifumi Takeuchi
*
Graduate School of Engineering, Kobe University, 1-1 Rokkodai-cho, Nada-ku, Kobe 657-8501, Japan. E-mail: takeuchi@gold.kobe-u.ac.jp; Fax: +81788036158; Tel: +81788036158
First published on 7th September 2010
Abstract
Polymers molecularly imprinted toward bisphenol A (BPA-MIPs) were prepared by reverse atom transfer radical polymerization (reverse ATRP). For covalent bond-based BPA imprinting, a new template molecule, BPA di(4-vinyl benzoate), was synthesized and co-polymerized with divinylbenzene and styrene. Compared with conventional radical polymerization-based BPA-MIPs, the selectivities of the ATRP-based BPA-MIPs appear to be enhanced, suggesting that reverse ATRP could provide homogeneously cross-linked polymers and elaborate BPA recognition cavities in MIPs with size- and shape-selectivity.
1. Introduction
Bisphenol A (BPA) is suspected as an endocrine disruptor that can easily bind to estrogen receptors and exert estrogen-like effects. BPA has been used in large amounts for the production of epoxy resins and polycarbonates, and is easily eluted from many polycarbonate packages and tableware.1 Due to concern about BPA as an environmental pollutant, there has been recent interest in the development of selective adsorbents for the separation and sensing of BPA.Molecularly imprinted polymers (MIPs) have acquired a reputation as tailor-made materials capable of target recognition.2 Until now, MIPs have generally been prepared by conventional radical polymerization (RP) using a functional monomer(s) and a cross-linker(s) in the presence of a template molecule (a target molecule or a derivative). Several researchers reported BPA-imprinted polymers prepared using 4-vinylpiridine, polysulfone and methacrylic acid as functional monomers.3 Generally, when polymerization is carried out by RP, polydispersity increases as the polymerization progresses. This means that RP is often accompanied by side reactions such as termination/chain transfer reactions, potentially causing the functional monomers and cross-linkers to irregularly co-polymerize during RP and resulting in heterogeneous binding sites toward target molecules.
Recent progress in polymer chemistry has proposed some synthetic methods to create polymeric materials in a controlled manner. For example, controlled/living radical polymerization (CLRP) techniques have been extensively studied for controlling molecular weight distribution and for achieving the desired polymerization.4 When organogels were prepared by CLRP, the cross-linked network of the gel appeared to be homogeneous, i.e., no microgel formation was observed.5 CLRP-based MIPs have been also reported recently.6,7 Among them, atom transfer radical polymerization (ATRP)4,8 using halogenated Cu(I) as a catalyst holds promise as a useful method for generating MIPs, since various kinds of monomers can be used and the reaction can proceed under mild conditions such as low temperature. Indeed, there have been some recent reports about MIPs prepared by ATRP7 in which the MIPs were prepared with functional monomers non-covalently interacting with the templates. Such non-covalently interacting components may interfere with metal complex formation of the catalyst, suggesting that the ATRP reaction will not proceed smoothly. In this paper we describe the preparation of BPA-imprinted polymers by a covalent molecular imprinting approach with reverse ATRP9 using Cu(II) chloride as a catalyst and a conventional radical initiator (Fig. 1). We chose the covalent approach10 because the ATRP process can progress without inhibition by the addition of free template molecule, and is reported to give more homogeneous recognition sites than non-covalent imprinting.11 Reverse ATRP is convenient, since halogenated Cu(II) and a conventional radical initiator are used, instead of halogenated Cu(I) that is easily oxidized in air. The reverse ATRP-based BPA-imprinted polymers were characterized and the BPA binding selectivity was evaluated by comparison with MIPs prepared by reverse ATRP and RP.
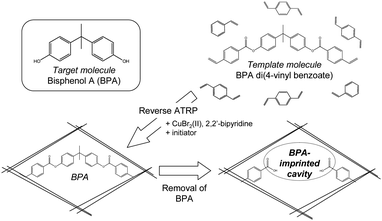 | ||
| Fig. 1 Schematic illustration for the BPA-imprinted polymers. | ||
2. Experimental
2.1 Materials
Styrene (St), dichloromethane (CH2Cl2), acetonitrile (CH3CN), Cu(II)Br2, 2,2′-bipyridine (2,2′-Bpy) and 4-dimethylaminopyridine (4-DMAP) were purchased from Nacalai Tesque Co. Ltd. (Kyoto, Japan). Divinylbenzene (DVB), resorcinol, hexestrol and 2,2′-azobis(4-methoxy-2,4-dimethylvaleronitrile) (V-70) were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Bisphenol A (BPA), bisphenol B (BPB) and 4-vinylbenzoic acid (4-VBA) were purchased from Tokyo Chemical Industry Co. Ltd. (Tokyo, Japan) and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (WSC) was purchased from Peptide Institute, Inc. (Osaka, Japan). DVB and St were purified by distillation under reduced pressure prior to use in order to remove the stabilizer. CH2Cl2 and CH3CN were purified by distillation prior to use in order to remove water. V-70 was used without further purification.2.2 Preparation of bisphenol A di(4-vinyl benzoate) (1)
BPA (0.62 g, 2.70 mmol), 4-VBA (1.00 g, 6.74 mmol), WSC (1.50 g, 7.82 mmol) and 4-DMAP (0.99 g, 7.82 mmol) were dissolved in CH2Cl2 (40 mL), and the reaction mixture was stirred at room temperature for 24 h. The reaction mixture was then washed with citric acid and NaCl aqueous solutions two times and the organic layer was dried over MgSO4. After the solvent was evaporated in vacuo, the obtained products were purified by silica gel chromatography (eluent: CH2Cl2). The collected fraction was evaporated and dried in vacuo to obtain the products as a white powder (1.25 g). The resulting products were identified by 1H-NMR measurements using a 300 Hz FT-NMR apparatus (JEOL JNM-LA300 FT NMR SYSTEM).Yield: 76%. 1H-NMR (300 MHz, CDCl3): δ = 1.72 (s, CH3, 6H), 5.45 (d, H(a)H(b)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(c)-, 2H), 5.94 (d, H(a)H(b)C
CH(c)-, 2H), 5.94 (d, H(a)H(b)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(c)-, 2H), 6.80 (q, H(a)H(b)C
CH(c)-, 2H), 6.80 (q, H(a)H(b)C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH(c)-, 2H), 7.14 (d, benzene, 4H), 7.29 (t, benzene, 2H), 7.54 (d, benzene, 4H), 8.16 (d, benzene, 2H).
CH(c)-, 2H), 7.14 (d, benzene, 4H), 7.29 (t, benzene, 2H), 7.54 (d, benzene, 4H), 8.16 (d, benzene, 2H).
2.3 Preparation of BPA-imprinted polymer
DVB, St, 1 as a template molecule, V-70, Cu(II)Br2 and 2,2′-Bpy were dissolved in porogen (CH2Cl2/CH3CN, v/v, 1/1) in a Schlenk flask. The reaction components are summarized in Table 1.| Code | Template (1)/mmol | DVB/mmol | Styrene/mmol | 4-VBA/mmol | Cu(II)Br2/mmol | 2,2'-BPY/mmol |
|---|---|---|---|---|---|---|
| a V-70 (0.16 mmol) and CH2Cl2/MeCN (v/v, 1/1) (2 mL) were used in all samples. | ||||||
| MIP-ATRP-3.2/0 | 0.16 | 3.2 | — | — | 0.16 | 0.48 |
| MIP-ATRP-2.4/0.8 | 0.16 | 2.4 | 0.8 | — | 0.16 | 0.48 |
| MIP-ATRP-1.6/1.6 | 0.16 | 1.6 | 1.6 | — | 0.16 | 0.48 |
| MIP-RP-2.4/0.8 | 0.16 | 2.4 | 0.8 | — | — | — |
| NIP-ATRP-2.4/0.8 | — | 2.4 | 0.8 | 0.64 | — | — |
| NIP-RP-2.4/0.8 | — | 2.4 | 0.8 | 0.64 | — | — |
CH3CN was mixed with CH2Cl2 to facilitate the dissolution of CuBr2. After sealing the Schlenk flask and degassing the solution under vacuum/nitrogen, the solution was incubated at 40 °C for 24 h. Then ATRP was quenched by the introduction of air into the Schlenk flask. After the obtained polymers were crushed into small particles, the remaining initiator and monomer were removed by washing with CH2Cl2 using a Soxhlet extractor for 24 h and the Cu complex was removed by washing with 1 M HCl for 12 h. The resulting polymer was dried in vacuo, and weighed, then the conversion of the obtained polymer was calculated by the following equation: conversion (%) = (weight of the resulting polymer)/(total weight of the template molecule 1, DVB and St used). Time–conversion plots were drawn by measuring the corresponding polymer weight obtained at appropriate intervals of quenching the ATRP by the introduction of air into the Schlenk flask. The template molecule 1 was removed by ester hydrolysis in 5 M NaOH in H2O/CH3OH (1/1, v/v) for 24 h. After neutralization with diluted HCl, the amount of BPA was determined using a Gilson HPLC system consisting of a 231XL auto-sampler (sample size: 10 µL), two 305 pumps (eluent: CH3OH/H2O = 65/35 and flow rate: 1.5 mL min−1) and a 117 UV/VIS detector (detection: 254 nm) to estimate the removal rate of BPA. The extracted BPA was calculated from the BPA contained in the wash solvent divided by the initial concentration of BPA.
The obtained polymer was washed with distilled water and 1 M HCl. In a similar manner, BPA-imprinted polymer was prepared by RP without using Cu(II)Br2 and 2,2′-Bpy. Moreover, non-imprinted polymers (NIPs) were likewise prepared by reverse ATRP and RP but in the absence of the template molecule 1.
2. 4 Binding of BPA
The MIPs prepared by both reverse ATRP and RP (3 mg) were incubated with various concentrations of BPA in CH2Cl2/CH3CN (1/1, v/v) (1.5 mL) at 20 °C for 24 h. The samples were then filtered to remove the polymer particles and the filtrates were evaporated. The residue was dissolved in methanol (1.5 mL), and the concentration of BPA was measured by HPLC as described previously. The amounts of bound BPA were calculated by subtracting the amount of free BPA from each initial amount added.2.5 Evaluation of selectivity for BPA-imprinted polymers
MIPs (3 mg) were incubated with 1 mM of bisphenol B (BPB), resorcinol or hexestrol in CH2Cl2/CH3CN (1 mL, 1/1, v/v) at 20 °C for 24 h. Binding experiments were carried out under the same conditions as the BPA binding experiments.2.6 BET measurement for BPA-imprinted polymer
Prior to measuring the BET surface area of the MIPs prepared by both reverse ATRP and RP, each sample (0.2 g) was degassed at 120 °C under high vacuum for 12 h, then the BET surface area of each sample was measured using a NOVA-1000 (adsorption gas: N2 and relative pressure (P/P0): 0.05–0.3).2.7 Evaluation of swelling degree for BPA-imprinted polymer
MIP-ATRP-2.4/0.8 and MIP-RP-2.4/0.8 (3 mg) were incubated with CH2Cl2/CH3CN (1 mL, 1/1, v/v) at 20 °C for 24 h. The polymer particles were then collected by centrifugation. The solvent was removed and the swelled polymer particles were weighed. The swelling degree was calculated by the following equation: swelling degree = (weight of the swelled polymer particles after incubation with CH2Cl2/CH3CN)/(the weight of polymer particles before the incubation).3. Results and discussion
In molecular imprinting, the crosslink density affects the removal of template molecules to create binding sites and the binding of samples to the binding sites. Thus, the molar ratio of styrene (St) to divinylbenzene (DVB) was investigated for the preparation of MIP-ATRP-3.2/0 (only DVB), MIP-ATRP-2.4/0.8 (DVB/St = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and MIP-ATRP-1.6/1.6 (DVB/St = 1
1) and MIP-ATRP-1.6/1.6 (DVB/St = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), by drawing the corresponding binding isotherms of BPA. As shown in Fig. 2, MIP-ATRP-2.4/0.8 bound more BPA than any of the other prepared polymers. The binding constant of MIP-ATRP-2.4/0.8 for BPA was estimated via a Langmuir plot to be 1.3 × 103 M−1 (see ESI†). Since a linear plot was observed for both MIPs, BPA might bind to the binding cavity with 1 to 1 stoichiometry.
1), by drawing the corresponding binding isotherms of BPA. As shown in Fig. 2, MIP-ATRP-2.4/0.8 bound more BPA than any of the other prepared polymers. The binding constant of MIP-ATRP-2.4/0.8 for BPA was estimated via a Langmuir plot to be 1.3 × 103 M−1 (see ESI†). Since a linear plot was observed for both MIPs, BPA might bind to the binding cavity with 1 to 1 stoichiometry.
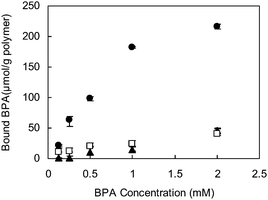 | ||
Fig. 2 Amounts of bound BPA to MIPs prepared by reverse ATRP following incubation with various concentrations of BPA in CH2Cl2/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). □: MIP-ATRP-3.2/0, ●: MIP-ATRP-2.4/0.8, and ▲: MIP-ATRP-1.6/1.6. 1, v/v). □: MIP-ATRP-3.2/0, ●: MIP-ATRP-2.4/0.8, and ▲: MIP-ATRP-1.6/1.6. | ||
MIP-ATRP-3.2/0 showed such low binding activity because only 5% of the BPA was extracted (Table 2), suggesting that the crosslink density was too high to remove BPA from the polymer under the conditions of ester hydrolysis. The small surface area of MIP-ATRP-3.2/0 (0.5 m2 g−1) also supports this presumption. As a result, insufficient binding sites were constructed in MIP-ATRP-3.2/0 for the binding of BPA.
| Code | Conversion (%)a | Extracted BPA (%) | BET surface area/m2 g−1 |
|---|---|---|---|
| a The conversion calculated by the following formula: conversion (%) = (weight of the resulting polymer/total weight of the monomers used) × 100. | |||
| MIP-ATRP-3.2/0 | 62 | 5 | 0.50 |
| MIP-ATRP-2.4/0.8 | 70 | 60 | 1.9 |
| MIP-ATRP-1.6/1.6 | 64 | 65 | 2.0 |
| MIP-RP-2.4/0.8 | 65 | 40 | 87 |
| NIP-ATRP-2.4/0.8 | 59 | — | 0.96 |
| NIP-RP-2.4/10.8 | 61 | — | 7.2 |
Regarding MIP-ATRP-2.4/0.8 and MIP-ATRP-1.6/1.6, the extracted BPA (60 and 65%) and the surface area (1.9 and 2.0 m2 g−1) were very similar, indicating that the number of binding sites in both polymers created by the hydrolysis is also similar. Nevertheless, the binding activity of MIP-ATRP-2.4/0.8 was much higher than that of MIP-ATRP-1.6/1.6. As reported by Wulff et al.,11 this may be due to the lower crosslink density of MIP-ATRP-1.6/1.6, where the pre-organized binding cavities may not be maintained during the binding events. Consequently, a molar ratio of DVB to styrene of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was chosen for the following experiments.
1 was chosen for the following experiments.
An RP-based reference MIP (MIP-RP-2.4/0.8) was prepared using the same molar ratio of DVB to styrene as that used to prepare MIP-ATRP-2.4/0.8. Forty percent of the BPA could be extracted from MIP-RP-2.4/0.8 (Table 2), suggesting that the extraction of the BPA moiety from the MIP-RP-2.4/0.8 matrix is more difficult, and that there are fewer available binding sites in MIP-RP-2.4/0.8 compared to MIP-ATRP-2.4/0.8 (Fig. 3). In contrast, less BPA was bound to the NIPs (NIP-ATRP-2.4/0.8 and NIP-RP-2.4/0.8) than to the MIPs. This indicates that the absence of the template during the polymerization is not conducive to the generation of binding sites with high binding activity for BPA, thereby confirming the importance of the imprinting effect.
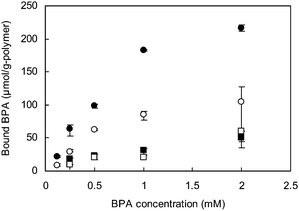 | ||
| Fig. 3 Amounts of bound BPA to MIP-ATRP-2.4/0.8 (●) and MIP-RP-2.4/0.8 (○), and bound BPA to NIP-ATRP-2.4/0.8 (■) and NIP-RP-2.4/0.8 (□). | ||
Fig. 4 shows time–conversion plots of MIP-ATRP-2.4/0.8 and MIP-RP-2.4/0.8. The solution of MIP-RP-2.4/0.8 turned into a gel after 2 h, and 60% conversion to product was attained after 8 h, suggesting that both termination and chain transfer reactions proceed very quickly in the RP system just after the initiator is decomposed, which may construct a disorderly polymer matrix. As a result, template molecule 1, DVB and styrene co-polymerized in a heterogeneous manner.5,12 The low extracted amounts of BPA from MIP-RP-2.4/0.8 (40%) also can be explained by such heterogeneous cross-linking, which made BPA extraction difficult. On the other hand, MIP-ATRP-2.4/0.8 turned into a gel after 16 h and reached 70% conversion after 20 h. It is known that initiation radicals which are produced after initiator decomposition are promptly capped by halogen atoms in the reverse ATRP system. The concentration of radicals during the polymerization decreases, and the polymerization rate of the reverse ATRP system is much slower than that of the RP system.13 Additionally, termination and chain transfer reactions would also be suppressed by the regulation of radical concentrations.
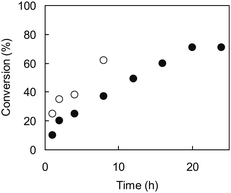 | ||
| Fig. 4 Time–conversion plots of MIP-ATRP-2.4/0.8 (●) and MIP-RP-2.4/0.8(○). | ||
The swelling degree of MIP-ATRP was approximately 2 times larger than that of MIP-RP and the NIPs also showed the same trend (Fig. 5), suggesting that the density of crosslinking of the MIP-ATRP was lower than that of the MIP-RP. Therefore, at first, liner polymers may be generated in the reverse ATRP system, and at around the gelation time, the crosslinking gradually proceeded, whereas random crosslinking was started from the initial stage and microgel formation occurred in the RP system, resulting in the early gelation was observed. These results suggest that the delayed crosslinking reaction under the ATRP conditions would proceed in a fairly homogeneous manner, resulting in the construction of highly selective binding cavity with the lower density of crosslinking.
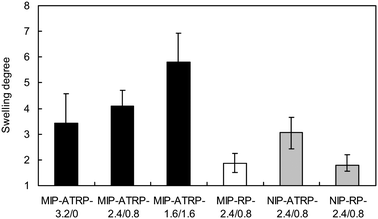 | ||
Fig. 5 Swelling degree of MIP-ATRPs (black), MIP-RP (white) and NIPs (gray) in CH2Cl2/MeCN (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). 1, v/v). | ||
The BET surface area gives further information on the BPA binding sites (Table 2). Interestingly, MIP-RP-2.4/0.8 has a much larger surface area (87 m2 g−1) than does MIP-ATRP-2.4/0.8 (1.9 m2 g−1), suggesting that RP results in a macroporous morphology. A similar phenomenon was reported by Mosbach et al.,6 where the BET surface areas of MIPs prepared by nitroxide-mediated living radical polymerization (NMP), a CLRP technique, were much smaller than those of MIPs prepared by RP. It has been reported that the more slowly the growing reaction proceeds, the thicker and more homogeneous the crosslinking.5 Therefore, RP-based MIPs would give rough and heterogeneous polymer matrices, resulting in a large BET surface area with fewer functioning binding sites. It should be noted that the apparent BET surface area of MIP-ATRPs seems to be too low. It may be due to the change of surface morphology under the dry condition of BET measurement. Thus the MIP-RPs may be more robust than MIP-ATRPs.
The amount of bound BPA, as well as reference compounds with various side chains such as bisphenol B (BPB), resorcinol (a small compound) and hexestrol (a larger compound), to MIP-ATRP-2.4/0.8 and MIP-RP-2.4/0.8 was examined (Fig. 6). The binding activity of MIP-ATRP-2.4/0.8 for these compounds increased in the order BPA, BPB, resorcinol, and hexestrol, revealing that MIP-ATRP-2.4/0.8 can recognize the side chain and the size of BPA. The distance between the oxygen atoms of the two hydroxyl groups in the most stable conformation of each compound was estimated by stochastic conformational search using the molecular force field, MMFF94x. Since the distance between the oxygen atoms of BPA was estimated to be 9.4 Å, the size of the binding cavities obtained by BPA imprinting should be smaller than that of hexestrol (12 Å), and larger than that of resorcinol (4.8 Å). The distance between the oxygen atoms in BPB (9.3 Å) is similar to that of BPA, but BPB has a larger methyl-ethyl side chain instead of two methyl groups at the central part of the ethylene linker of bisphenol. This suggests that the apparent binding behavior to MIP-ATRP-2.4/0.8 results from the highly shape-selective binding cavities generated in the ATRP-based homogeneous cross-linked polymer matrix, which can recognize mismatched structures between the binding cavities and the reference compounds. In contrast, MIP-RP-2.4/0.8 could not distinguish BPA and hexestrol. This may be due to heterogeneous cross-linking in MIP-RP-2.4/0.8 prepared by uncontrollable radical polymerization. The resulting cavities showed poor recognition of BPA, where MIP-RP-2.4/0.8 could not distinguish the difference in the distances between the two hydroxyl groups of hexestrol and BPA.
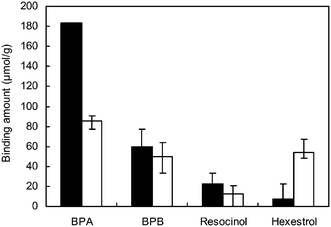 | ||
| Fig. 6 Amounts of bound BPA, BPB, resorcinol, and hexestrol to MIP-ATRP-2.4/0.8 (black) and MIP-RP-2.4/0.8 (white). The MIP was incubated with 1 mM BPA in CH2Cl2/CH3CN (v/v, 1/1) solution. Amounts of each compound were determined using HPLC. | ||
4. Conclusions
Covalent bond-based molecular imprinting was conducted using a reverse ATRP technique. The approach gave a homogeneous polymer matrix which allowed the generation of highly specific binding cavities for the target molecule compared with MIPs prepared by conventional radical polymerization. This technique would be suitable for the preparation of covalent MIPs, since the catalyst must be added to the pre-polymerization mixture containing the template monomer complexes, which should be stably maintained during the polymerization process. Careful choice of the template and functional monomer would allow this approach to be used for non-covalent and other molecular imprinting techniques. Furthermore, the application of reverse ATRP for preparing MIPs could enhance their specific binding properties, and controlling the polymerization reaction early on in the polymerization process is an effective way to achieve molecularly imprinted cavities with size- and shape-selectivity.Notes and references
- G. G. J. M. Kuiper, B. Carlsson, K. Grandien, E. Enmark, J. Höggblad, S. Nilsson and J. Å. Gustafsson, Endocrinology, 1997, 138, 863 CrossRef CAS; G. G. J. M. Kuiper, J. G. Lemmen, B. Carlsson, J. C. Corton, S. H. Safe, P. T. Saag, B. Burg and J. Å. Gustafsson, Endocrinology, 1998, 139, 4252 CrossRef CAS.
- G. Wulff, Angew. Chem., Int. Ed. Engl., 1995, 34, 1812 CrossRef CAS; T. Takeuchi and J. Haginaka, J. Chromatogr., B: Biomed. Sci. Appl., 1999, 728, 1 CrossRef CAS; K. Haupt and K. Mosbach, Chem. Rev., 2000, 100, 2495 CrossRef CAS.
- K. Hosoya, J. Haginaka, M. Morita, T. Kondo and Y. Watabe, J. Chromatogr., 2004, 1032, 45 CrossRef CAS; N. Nishi, C. S. Zhao, X. H. Zhang, M. Mao, Z. B. Liu and K. G. Yang, Anal. Chim. Acta, 2005, 546, 30 CrossRef CAS; T. Ikegami, W. Lee, H. Nariai and T. Takeuchi, Anal. Bioanal. Chem., 2004, 378, 1898 CrossRef CAS.
- K. Matyjaszewski and J. Xia, Chem. Rev., 2001, 101, 2921 CrossRef CAS.
- N. Ide and T. Fukuda, Macromolecules, 1999, 32, 95 CrossRef CAS.
- S. Boonpangrak, M. J. Whitcomebe, V. Prachayasittikul, K. Mosbach and L. Ye, Biosens. Bioelectron., 2006, 22, 349 CrossRef CAS; Y. Li, W-H. Zhou, H-H. Yang and X-R. Wang, Talanta, 2009, 79, 141 CrossRef CAS.
- X. L. Wei, X. Li and S. M. Husson, Biomacromolecules, 2005, 6, 1113 CrossRef CAS; B. Y. Zu, C. Q. Pan, X. Z. Guo, Y. Zhang and H. Q. Zhang, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 3257 CrossRef CAS.
- M. Kamigaito, T. Ando and M. Sawamoto, Chem. Rev., 2001, 101, 3689 CrossRef CAS.
- J. H. Xia and K. Matyjaszewski, Macromolecules, 1999, 32, 5199 CrossRef CAS; J. Gromada and K. Matyjaszewski, Macromolecules, 2001, 34, 7664 CrossRef CAS.
- G. Wulff, B. Heide and G. Helfmeier, J. Am. Chem. Soc., 1986, 108, 1089 CrossRef CAS; T. Ikegami, W. Lee, H. Nariai and T. Takeuchi, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2004, 804, 197 CrossRef CAS.
- G. Wulff, R. Kemmerer, J. Vietmeier and H. G. Poll, Nouv. J. Chim, 1982, 6, 681 Search PubMed.
- A. D. Vaughan, S. P. Sizemore and M. E. Byrne, Polymer, 2007, 48, 74 CrossRef CAS.
- Q. Yu, F. Zeng and S. Zhu, Macromolecules, 2001, 34, 1612 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Langmuir plot. See DOI: 10.1039/c0py00140f |
| This journal is © The Royal Society of Chemistry 2010 |