Preparation of shell cross-linked nanoparticles via miniemulsion RAFT polymerization†
Yin
Wang
ab,
Guohua
Jiang
*ab,
Xinke
Sun
ab,
Miaojun
Ding
b,
Hongyuan
Hu
b and
Wenxing
Chen
ab
aKey Laboratory of Advanced Textile Materials and Manufacturing Technology, Zhejiang Sci-Tech University, Ministry of Education, Hangzhou, 310018, P. R. China. E-mail: polymer_jiang@hotmail.com; Tel: +86-571-86843527
bDepartment of Materials Engineering, College of Materials and Textile, Zhejiang Sci-Tech University, Hangzhou, 310018, P. R. China
First published on 14th September 2010
Abstract
Shell cross-linked polymeric nanoparticles were prepared through an interfacially confined copolymerization of styrene and disulfide cross-linker in a reversible addition-fragmentation chain transfer (RAFT) miniemulsion system using poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) as stabilizer and macroinitiator. 1H NMR, FT-IR, and FE-SEM have been applied to investigate the structures and morphologies of the resultant nanoparticles. Fluorescent spectra results show that the as-prepared samples have fluorescent properties, which may be used as a fluorescent carrier for control release of guest molecules.
1. Introduction
Micelles obtained from self-assembly of amphiphilic block copolymers in aqueous solution have been well researched in the past decades.1–3 Most attention has been paid to the potential application of nanomedicine carriers for therapeutic and diagnostic agents since they were first reported by Ringsdorf in 1984.4 However, in the practical applications of conventional micelles, the stability of the micelles is of great challenge since they would disassemble at concentrations below the critical micelle concentration (CMC) by large volume dilution or other stimulations.5To overcome this problem, a great amount of effort has been devoted to developing cross-linked micelles, which can maintain the micellar structural integrity. Two representative micelles are core cross-linked (CCL) and shell cross-linked (SCL) ones, which can be divided into three types according to the cross-linking regents, such as organic small molecules, inorganic molecules and macromolecules. Xu and his partners6 found water-soluble temperature responsive triblock copolymers, poly(ethylene oxide)-b-poly(acrylic acid)-b-poly(N-isopropyl acrylamide) (PEO-b-PAA-b-PNIPAM) could form vesicles when heating the solution above 32 °C, which could be further cross-linked at the interface using cystamine via carbodiimide chemistry. After cross-linking, the thus-prepared polymersomes showed remarkable stability against dilution, organic solvent, high salt conditions and changes of temperature in water. Recently, Chang et al.7 used an inorganic silica-based cross-linking strategy to prepare CCL and SCL from poly(N-isopropylacrylamide-co-3-(trimethoxysilyl) propyl methacrylate)-b-poly(2-(diethyl-amino) ethyl methacrylate) ((PNIPAAm-co-PMPMA)-b-PDEA) copolymer upon changing the pH and temperature of the solution. Xu and co-workers8 prepared shell cross-linked micelles by directly adding amine-reactive polymeric cross-linking agent, NHS-PNIPAM-NHS, into α-methoxypoly(ethylene oxide)-b-poly[N-(3-aminopropyl)methacryl amide]-b-poly[2-(diisopropy amino) ethyl methacrylate] (mPEO-b-PAPMA-b-PDPAEMA) solution at pH = 10. Although the stable micelles with relatively uniform size can be prepared with this methodology, the multistep cross-linking procedure is always laborious and the preparation efficiency is relatively low. Thus it cannot be scaled up when the concentration of the micelles solution is high.
At present, several research groups have explored the application of one-pot method in synthesizing cross-linked materials because of its outstanding properties, which can overcome the disadvantages mentioned above. Wan and Pan9 directly cross-linked one of the blocks during the polymerization of 4-vinylpyridine (4VP) and N,N′-methylene bisacrylamide (MBA) in an ethanol–H2O solution to prepare core-stabilized nanoparticles. Chen et al.10 reported another way to obtain CCL micelles by locking one of the blocks in its nonselective solvent. In order to lower the toxicity of the resultant production and avoid the usage of organic solvents, the researchers favoured conducting the reactions in water. Liu and his partners further extended Chen's principle to fabricate cross-linked micelles in aqueous solution by the direct addition of cross-linker into the polymerization solution.11,12
To control the resultant morphology, which is related to the composition of the copolymers firstly reported by Discher and Eisenberg,13 chemists tended to select controlled radical polymerization (CRP) techniques for synthesizing copolymers. Reversible addition-fragmentation chain transfer (RAFT), in particular, can enable the incorporation of a wide variety of functional monomers under conditions that are similar to conventional free radical polymerization.14 Great efforts have been directed toward investigating the synthesis and assembly of block copolymers in aqueous media. Moreover, miniemulsion polymerization combined with RAFT, has already been explored to prepare polymeric nanoparticles. Because it can reduce the mass transport through aqueous dispersed media and get unformed particles with well defined structures. Recently, Li and his partners synthesized shell cross-linked hollow polymeric nanocapsules using a reactive surfactant, poly(ethylene oxide)-b-poly(n-butyl methacrylate) (PEO-b-PBMA-Cl), via interfacially confined miniemulsion atom transfer radical polymerization (ATRP).15 The reports on utilizing miniemulsion RAFT polymerization to prepare shell cross-linked nanoparticles are limited, to the best of our knowledge. Herein, we report the preparation of poly(2-(dimethylamino) ethyl methacrylate)-b-polystyrene (PDMAEMA-b-PS) nanoparticles with a cross-linked shell by this methodology using poly(2-(dimethylamino) ethyl methacrylate) (PDMAEMA) as the macroinitiator and stabilizing agent.
2. Experimental section
2.1 Materials
Styrene (St) was firstly washed by 5 wt% NaOH solution and deionized water to remove the inhibitors and then vacuum distilled prior to use. Azobisisobutyronitrile (AIBN, 98%) was recrystallized twice from ethanol and dried in vacuum prior to use. St and AIBN were purchased from East China Chemical Co. (Shanghai, China). S-1-Dodecyl-S′-(α, α′-dimethyl-α′′-acetic acid) trithiocarbonate (DMP) was synthesized according to the previously published procedure.16 Bis(2-hydroxyethyl) disulfide(BHEDS, 90%) and 2-(dimethylamino)ethyl methacrylate (DMAEMA, 99%) were used as received, which were purchased from Alfa and Acros Organics respectively. Acryloyl chloride (96%) was purchased from Aladdin Reagent Co. (Shanghai, China). All other reagents and solvents were of analytical grade and used as received without further purification. MilliQ Water (18.2 MΩcm−1) was generated using a Millipore MilliQ Academic Water Purification System.2.2 Synthesis of the disulfide-based diacrylate branching agent
Bis(acryloyxyethyl) disulfide (BAEDS) was synthesized according to the previously published procedure.17,18 A 250 mL three necked flask equipped with a magnetic stirrer and a 50 mL dropping funnel was charged with 75 mL dry tetrahydrofuran. This flask was immersed in an ice bath for 15 min. Bis(2-hydroxyethyl) disulfide (BHEDS, 3.85 g, 25 mmol, 0.5 equiv) and triethylamine (25 mL, 20 mmol, 4.0 equiv) were added to the flask under N2 atmosphere. After stirring for 10 min, acryloyl chloride (10.5 g, 100 mmol, 2.0 equiv) was added dropwise to the stirred THF solution. The resulting heterogeneous mixture was allowed to stir at 20 °C for 24 h and then filtered to remove the byproduct. The solvent was removed by evaporation, and the crude product was dissolved in chloroform. This solution was washed three times with an aqueous solution of 0.1 M Na2CO3 and deionized water respectively. The purified organic solution was dried using anhydrous MgSO4 over night and the chloroform was removed under reduced pressure. The final disulfide-based diacrylate product was obtained as a slightly yellow liquid and was stored in a freezer under nitrogen in the absence of light prior to use. 1H NMR (CDCl3, ppm): δ 6.4 and 5.9 (4 H, doublet, CH2CHCOO); 6.1 (2 H, quartet, CH2CHCOO); 4.43 (4 H, triplet, COOCH2CH2SS); 3.0 (4 H, triplet, COOCH2CH2SS) (see ESI†).2.3 Synthesis of the PDMAEMA macroinitiators via RAFT polymerization
PDMAEMA macroinitiators were fabricated in a one-pot synthesis via RAFT using DMP as the initiator. A typical polymerization procedure is described as follows: DMP (0.182 g, 0.5 mmol), AIBN (0.016 g, 0.1 mmol) and DMAEMA (5.39 mL, 0.03 mol) were added to a dry flask, which was then immersed in liquid nitrogen for a while. Then, the reaction mixture was degassed three times using the freeze-pump-thaw procedure. Finally, the flask was flame-sealed under vacuum and placed in a pre-heated oil-bath at 80 °C for 12 h. After cooled to the room temperature, an amount of THF was added to the flask to dissolve the polymer completely. The excess THF was evaporated under reduced pressure and the product was precipitated from excess petroleum ether, filtered, and dried under vacuum to constant weight. The resultant product with Mn = 1.10 × 104 g mol−1 (GPC) was obtained at >95% yield after freeze-drying. 1H NMR (CDCl3, ppm): δ 0.8–0.9 (3 H, –CH3); 1.8–1.9 (2 H, –CH2–); 2.20–2.30 (6 H,–N(CH3)2); 2.50–2.60 (2 H, –CH2–N); 4.00–4.20 (2 H, –CH2–CH2–).2.4 Synthesis of shell cross-linked nanoparticles via miniemulsion RAFT polymerization
Shell cross-linked nanoparticles were fabricated in a one-pot method via miniemulsion RAFT polymerization using PDMAEMA as the macroinitiator and stabilizing agent. A typical polymerization procedure is described as follows: a flask with a magnetic stirrer was charged with ethyltrimethylammonium bromide (CTAB, 0.0033 g, 0.009 mmol) and 5 mL water. After complete dissolution, an equal volume of PDMAEMA (0.1041 g, 0.01 mmol) solution was added into the flask and stirred for 5 min. Meanwhile, an aliquot of organic mixture containing AIBN (0.0003 g, 0.0018 mmol), St (0.25 mL, 2.2 mmol), BAEDS (0.0524 g, 0.2 mmol) and cyclohexane (0.055 mL, 0.5 mmol) was then transferred to the flask. After stirring for another 10 min, the reaction mixture turned white and the flask was immersed in liquid nitrogen followed by three cycles of freeze-pump-thaw procedure. Finally, the flask was flame-sealed under vacuum and placed in a pre-heated oil-bath at 70 °C for 24 h. After cooled to the room temperature, an amount of resultant solution was taken to lyophilize for measurement. The nanoparticles with non cross-linked shells have also been synthesized in the same condition except the cross-linker, BAEDS, which is not added into the reaction mixture.2.5 Characterization
The 1H NMR spectra were recorded on an AVANCE AV 400 MHz Digital FT-NMR spectrometer operating at 400 MHz using deuterated chloroform (CDCl3) as a solvent. Gel permeation chromatographic (GPC) analysis was carried out using a Waters 1525 pumping system (USA) at the flow rate of 0.5 mL min−1 with an Ultrahydrogel 500 column (Waters). The eluent was THF. UV-visible spectra were obtained using a Hitachi U-3010 spectrophotometer. Fluorescence spectra at room temperature were measured with a LS-45 fluorescence spectrophotometer. The sizes and morphologies of the resultant samples were characterized by JSM-5610LV field-emission scanning electron microscopy (FE-SEM). Thermogravimetric analysis (TGA) was performed on a Pyris Diamond 1 instrument (America) at a heating rate of 20 °C min−1 from 25 to 500 °C in a flow of nitrogen. Fourier transform infrared (FT-IR) spectra were recorded on a Nicolet 5700 spectrophotometer using an ATR cell or KBr pellets for samples.3. Results and discussion
3.1 Synthesis of the PDMAEMA-b-PS nanoparticles via RAFT polymerization conducted in water
RAFT is a facile technique for the controlled polymerization of many functional hydrophilic and amphiphilic monomers and can be conveniently employed for the preparation of block copolymers containing DMAMEA and St. The PDMAEMA macroinitiators and PDMAEMA-b-PS nanoparticles were synthesized by RAFT polymerization as shown in Scheme 1.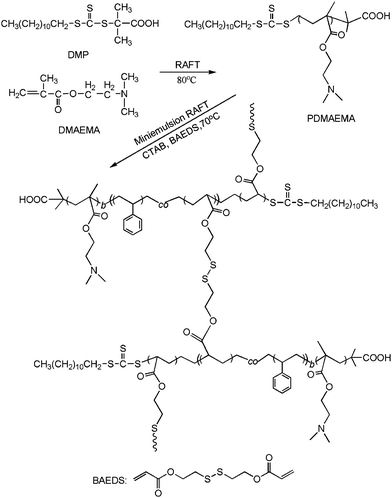 | ||
| Scheme 1 The synthetic pathway for the fabrication of the shell cross-linked (SCL) nanoparticles via miniemulsion RAFT polymerization. | ||
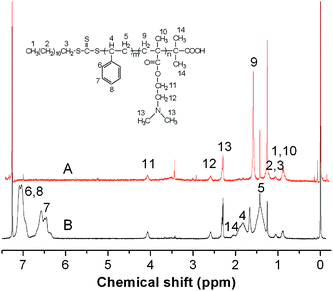
Fig. 1 shows the 1H NMR spectra of PDMAEMA (A) and PDMAEMA-b-PS (B) in CDCl3. The peaks at δ = 1.82 and 0.90 ppm are attributed to methylene and methyl groups of (S–CH2–C–CH3). Meanwhile, the proton peaks at δ = 4.06 and 2.56 ppm are assigned to methylene groups (–CH2–CH2–N). The signal at δ = 2.28 ppm is the characteristic peaks of methyl group connecting the nitrogen (N–CH3), which indicates that PDMAEMA macroinitiators have been successfully synthesized. PDMAEMA-b-PS nanoparticles were then fabricated via miniemulsion RAFT polymerization in the presence of PDMAEMA macroinitiators. PDMAEMA-b-PS can be dissolved in CDCl3, so all the signals characteristic of PDMAEMA block and the PS block are observed as shown in Fig. 1B. Compared to Fig. 1A, the peaks intensity attributed to PDMAEMA decreases significantly. Furthermore, it reveals the presence of new peaks at δ = 6.29–6.74 and δ = 6.87–7.21 ppm, which are ascribed to the PS blocks indicating a successful chain extension of PDMAEMA.
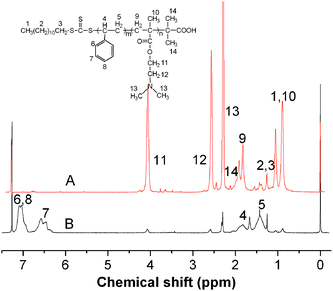 | ||
| Fig. 1 1H NMR spectra of PDMAEMA (A), PDMAEMA-b-PS (B) in CDCl3. | ||
FT-IR spectroscopy was then employed to characterize the compositions of PDMAEMA and PDMAEMA-b-PS. Fig. 2A is the FT-IR spectrum of PDMAEMA, whose characteristic absorption bands of C–N stretching vibration at 1148 cm−1 and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1726 cm−1. Comparing to the PDMAEMA sample, the phenyl ring stretching vibration at 1492 and 1600.8 cm−1, the ring in phase C–H stretching vibration at 1028 cm−1 and the ring out-of-plane bend at 697 cm−1 can be clearly observed in Fig. 2B which are the typical absorption bands for polystyrene. The absorption bands of the C–N stretching vibration at 1148 cm−1 and the C
O stretching vibration at 1726 cm−1. Comparing to the PDMAEMA sample, the phenyl ring stretching vibration at 1492 and 1600.8 cm−1, the ring in phase C–H stretching vibration at 1028 cm−1 and the ring out-of-plane bend at 697 cm−1 can be clearly observed in Fig. 2B which are the typical absorption bands for polystyrene. The absorption bands of the C–N stretching vibration at 1148 cm−1 and the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration at 1726 cm−1 appear in both samples, indicating a successful chain extension of PDMAEMA, which is in accordance with 1H NMR results.
O stretching vibration at 1726 cm−1 appear in both samples, indicating a successful chain extension of PDMAEMA, which is in accordance with 1H NMR results.
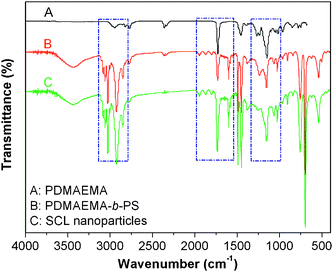 | ||
| Fig. 2 FT-IR spectra of PDMAEMA (A), PDMAEMA-b-PS (B) and the synthesized shell cross-linked nanoparticles (C). | ||
The curves of the loss weight rate of the macroinitiators and the resultant nanoparticles in a nitrogen stream from room temperature to 500 °C were gained by way of TGA. Fig. 3 shows the weight lose curves of PDMAEMA, PDMAEMA-b-PS and the synthesized SCL nanoparticles. As it can be seen, the thermal stability of the three polymers is different. For PDMAEMA, it begins to decompose when the temperature increases to 178 °C. The sample weight lose is about 50% when the temperature is reached at 370 °C, and decomposition almost completely at 450 °C. In the case of PDMAEMA-b-PS, three decomposition stages can be observed. Below 100 °C, the water begins to evaporate from the sample. The second decomposition temperature is 138 °C, and weight lose reaches about 20% up to 354 °C, which is attributed to the decomposition of PDMAEMA. The third decomposition temperature is 370 °C which is near the initial decomposition temperature of homo-polystyrene. It also confirms that PDMAEMA-b-PS nanoparticles have been successfully prepared via aqueous RAFT polymerization.
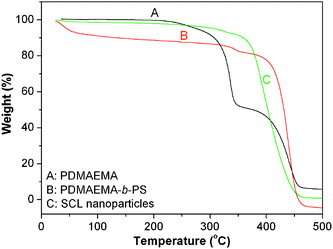 | ||
| Fig. 3 TGA curves of PDMAEMA (A), PDMAEMA-b-PS (B) and the synthesized shell cross-linked nanoparticles (C). | ||
3.2 Synthesis of shell cross-linked nanoparticles via miniemulsion RAFT polymerization
Miniemulsion polymerization is considered to be one of the most commonly used methods for preparing polymeric nanoparticles,19,20 which starts with stabilized oil droplets with average diameter between 50 and 500 nm and narrow size distribution dispersed in water.21 Compared to the traditional emulsion polymerization, it has well overcome the limitation of using a large amount of surfactant to stabilize the emulsions or form vesicles, which remains part of the system or has to be removed in a laborious manner. Furthermore, RAFT polymerization conducted in aqueous dispersed media has already been well applied by McCormick to copolymerize different kinds of monomers.8,22 And, all the components necessary for a RAFT process, including the initiators, emulsifiers, and monomers, are initially located within the stabilized monomer droplets, or the “mini-reactor”.23 Particle nucleation occurs in the monomer droplets and ideally each droplet turns into a polymeric particle after polymerization, since mass transport through aqueous dispersed media is limited.21Surfactant/stabilizer plays an important role in miniemulsion polymerization, which has a great impact on the stability of the latexes and the particle size. In recent years, reactive amphiphilic block copolymer has been widely used in miniemulsion polymerization because it can serve as both stabilizer and initiator for the polymerization that the hydrophobic block initially emulsifies the monomer phase, and the hydrophilic one extends into the water phase to stabilize the latexes.24,25 In the present approach, PDMAEMA macroinitiators, were used as a reactive surfactant for the preparation of polymeric nanoparticles in miniemulsion polymerization. It firstly co-stabilized St and BAEDS with CTAB to form the oil drops and then initiated the polymerization to progress in the water/monomer droplet interface, resulting in the formation of nanoparticles with cross-linked shells. A schematic representation of the fabrication of the shell cross-linked (SCL) nanoparticles is displayed in Scheme 2.
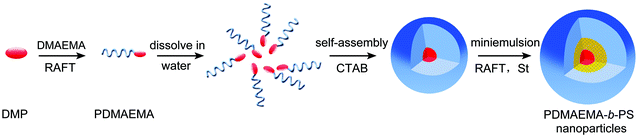 | ||
| Scheme 2 A schematic representation of the fabrication of the shell cross-linked (SCL) nanoparticles via miniemulsion RAFT polymerization. | ||
The shell cross-linked nanoparticles were also identified by 1H NMR and FT-IR spectra. If the shell cross-linking was unsuccessful, dissociation into individual copolymer chains would be expected since CDCl3 was a good solvent for both the PDMAEMA and PS blocks, whose characteristic peaks could be both observed. Fig. 4 shows the 1H NMR spectra of the synthesized shell cross-linked nanoparticles and PDMAEMA-b-PS in CDCl3. Comparing the two samples, the peaks pertinent to the PS blocks are invisible after the shell cross-linking reaction because of the molecular mobility trapped by the cross-linked shell, while the PDMAEMA blocks are still well-solvated and can be detected. Moreover, in Fig. 2C, all the characteristic absorption bands for polystyrene and PDMAEMA can still be well detected, but the relatively intensity of two absorption bands at 1732 cm−1 and 1601 cm−1 have changed significantly, which is attributed to the relative amount of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, which increases with the introduction of the cross-linker. All the above confirms that the PDMAEMA-b-PS has been successfully fixed by BAEDS to form shell cross-linked nanoparticles.
O, which increases with the introduction of the cross-linker. All the above confirms that the PDMAEMA-b-PS has been successfully fixed by BAEDS to form shell cross-linked nanoparticles.
 | ||
| Fig. 4 1H NMR spectra of the synthesized shell cross-linked nanoparticles (A), PDMAEMA-b-PS (B) in CDCl3. | ||
In addition, the nanopaticles with a cross-linked shell can exhibit an excellent thermal stability. Fig. 3C reveals that the two decomposition temperatures due to decomposition of PDMAEMA and polystyrene are not so obvious, indicating that the cross-linker can enhance the thermal stability of the resultant shell cross-linked nanoparticles. It further confirms that the shell cross-linked nanoparticles have been successfully fabricated by simply introducing the cross-linker to the miniemulsion RAFT polymerization system.
The morphologies of the PDMAEMA-b-PS nanoparticles with a cross-linked shell or not were investigated by field-emission scanning electron microscopy (Fig. 5). As expected, the as-prepared samples consist of spherical particles. In case of PDMAEMA-b-PS nanoparticles, the size of these spheres is around 150 nm and the surface is very smooth as shown in Fig. 5A and 5B. Compared to PDMAEMA-b-PS nanoparticles, the shell cross-linked nanoparticles have a slight difference in morphology. Shrivelled spheres can be found from Fig. 5C and 5D, and the diameter of as-prepared nanoparticles is around 300 nm. This interesting phenomenon is also reported by another research group.26 We speculated that this phenomenon was attributed to the shrink difference between the shell and core. The fold surface was formed for the shrink rate of cross-linked shell was relatively lower than that of the core during the sample preparation process.26
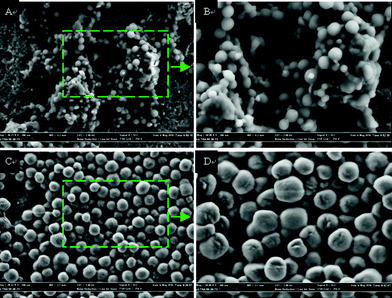 | ||
| Fig. 5 FE-SEM images of PDMAEMA-b-PS (A, B) and the synthesized shell cross-linked nanoparticles (C, D). All scale bars are 200 nm. | ||
PDMAEMA-b-PS nanoparticles with a cross-linked shell or not were used to study the UV-visible properties. As shown in Fig. 6, both the samples in aqueous solution (0.02 wt%) show a strong absorption band at 225 nm and weaker broad band at 280 nm. However, the absorption intensity for the uncross-linked nanoparticles is stronger than that of the cross-linked sample.
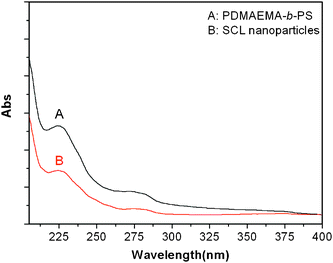 | ||
| Fig. 6 UV-vis spectra of PDMAEMA-b-PS (A) and the synthesized shell cross-linked nanoparticles (B) aqueous solution samples with a concentration of 0.02 wt%. | ||
Since the as-prepared nanoparticles with a cross-linked shell or not do not contain fluorescent functional groups from the classical viewpoint, exploring the source of the fluorescence should be interesting. Fig. 7 shows the fluorescence excitation and emission spectra of the samples. The nanoparticles without a cross-linked shell have an excited band at 255 nm. Under light irradiation at 255 nm, three emission bands at 284, 322 and 358 nm can be observed. The intensity of the emission band at 358 nm is stronger than that at 284, 322 nm. For the sample with a cross-linked shell, a similar phenomenon is observed. For more intuitive observation the fluorescence properties of as-prepared nanoparticles, the illumination photographs of dispersion aqueous solution of shell uncross-linked and shell cross-linked nanoparticles irradiated under 365 nm are recorded with a digital camera. The blue fluorescence phenomenon can be observed compared the solvent in the cell (Fig. 7A-a and 7B-a) when the concentration is controlled at 0.5 wt% as shown the inset photographs in Fig. 7A-b and 7B-b. The intensity of emission spectrum for cross-linked nanoparticles is weaker than that of uncross-linked ones. We speculated that the fluorescence properties of the resultant polymers had a close relationship with their architecture. The tertiary nitrogen with lone pair electrons inside the nanoparticles played an important role in the photoluminescence, which is similar to other results reported.27–32 The PDMAEMA segment in the shell uncross-linked nanoparticles is non-rigid and the transition of lone pair electrons on the tertiary nitrogen is easier and thus the stronger photoluminescence can be observed. However, in the shell cross-linked nanoparticles, the relaxation of some PDMAEMA is blocked for the cross-linked shell. Therefore, only the weaker photoluminescence can be obtained. The fluorescence intensity is also dependent on the concentration of nanoparticles (see ESI†).
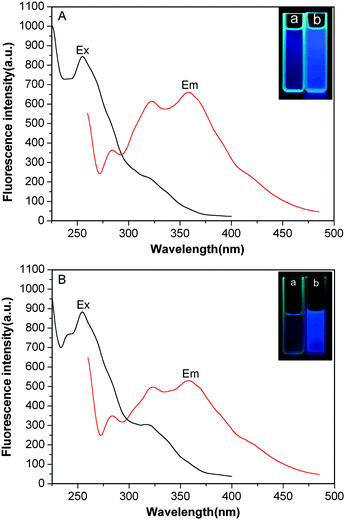 | ||
| Fig. 7 Excitation and emission spectra for PDMAEMA-b-PS (A) and the synthesized shell cross-linked nanoparticles (B) samples. | ||
As known to all, nanoparticles or micelles have been extensively used as a candidate for delivery of guest molecules, however, the controlled release of the molecules to targeted area is the ultimate goal. By introducing the specific cleavable linkers to the shell of the nanoparticles should help control the delivery of the encapsulated substances. For this purpose, BAEDS was chosen as the cross-linker, since the disulfide group can be cleaved to the corresponding thiols in the presence of reducing agents, such as tri(n-butyl) phosphine (Bu3P),33 dithiothreitol (DTT),34 and glutathione.35 As aforementioned, the resultant nanoparticles have fluorescent properties, and can be further used as a fluorescent probe without any treatment, so it will lower the toxicity of the nanoparticles to cells. Investigation of their potential applications in fluorescence imaging technology and biological science is in progress.
4. Conclusion
In summary, we have established a facile way to prepare shell cross-linked nanoparticles via miniemulsion RAFT polymerization of St and BAEDS in the presence of reactive amphiphilic block copolymer, PDMAEMA, which used as both stabilizer and macroinitiator. The shell cross-linked nanoparticles have a slight shrivelled morphology with a size of 300 nm. Furthermore, the resultant samples having fluorescent and redox-responsive properties can be potentially used as a carrier for control release of guest molecules.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (20604024), Preferentially Financing projects of scientific and technological activities of overseas students in Zhejiang Province (0903677-M), Scientific Research Foundation of Zhejiang Sci-Tech University (0901808-Y), Training Foundation for the Excellent Young Talents by the Key Laboratory of Advanced Textile Materials and Manufacturing Technology (ATMT), Ministry of Education (2008QN10), Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT: 0654) and Natural Science Foundation of Zhejiang Province (Y4100045).References
- G. Riess, Prog. Polym. Sci., 2003, 28, 1107–1170 CrossRef CAS.
- J. F. Gohy, Adv. Polym. Sci., 2005, 190, 65–136 CAS.
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173–1222 CrossRef CAS.
- H. Bader, H. Ringsdorf and B. Schmidt, Angew. Makromol. Chem., 1984, 123, 457–485 CrossRef.
- X. Z. Jiang, Z. S. Ge, J. Xu, H. Liu and S. Y. Liu, Biomacromolecules, 2007, 8, 3184–3192 CrossRef CAS.
- H. F. Xu, F. H. Meng and Z. Y. Zhong, J. Mater. Chem., 2009, 19, 4183–4190 RSC.
- C. Chang, H. Wei, J. Feng, Z. C. Wang, X. J. Wu, D. Q. Wu, S. X. Cheng, X. Z. Zhang and R. X. Zhuo, Macromolecules, 2009, 42, 4838–4844 CrossRef CAS.
- X. W. Xu, A. E. Smith, S. E. Kirkland and C. L. McCormick, Macromolecules, 2008, 41, 8429–8435 CrossRef CAS.
- W. M. Wan and C. Y. Pan, Macromolecules, 2007, 40, 8897–8905 CrossRef CAS.
- D. Y. Chen, H. S. Peng and M. Jiang, Macromolecules, 2003, 36, 2576–2578 CrossRef CAS.
- Y. M. Zhou, K. Q. Jiang, Y. Q. Chen and S. Y. Liu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6518–6531 CrossRef CAS.
- X. Z. Jiang, J. Y. Zhang, Y. M. Zhou, J. Xu and S. Y. Liu, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 860–871 CrossRef CAS.
- D. E. Discher and A. Eisenberg, Science, 2002, 297, 967–973 CrossRef CAS.
- G. Moad, J. Chiefari, Y. K. Chong, J. Krstina, R. T. A. Mayadunne, A. Postma, E. Rizzardo and S. H. Thang, Polym. Int., 2000, 49, 993–1001 CrossRef CAS.
- W. W. Li, K. Matyjaszewski, K. Albrecht and M. Möller, Macromolecules, 2009, 42, 8228–8233 CrossRef CAS.
- J. T. Lai, D. Filla and R. Shea, Macromolecules, 2002, 35, 6754–6756 CrossRef CAS.
- Y. T. Li and S. P. Armes, Macromolecules, 2005, 38, 8155–8162 CrossRef CAS.
- L. Zhang, W. G. Liu, L. Lin, D. Y. Chen and M. H. Stenzel, Biomacromolecules, 2008, 9, 3321–3331 CrossRef CAS.
- F. Tiarks, K. Landfester and M. Antonietti, Langmuir, 2001, 17, 908–918 CrossRef CAS.
- A. V. Zyl, R. F. Bosch, J. B. McLeary, R. D. Sanderson and B. Klumperman, Polymer, 2005, 46, 3607–3615 CrossRef.
- J. M. Asua, Prog. Polym. Sci., 2002, 27, 1283–1346 CrossRef CAS.
- Y. T. Li, B. S. Lokitz and C. L. McCormick, Macromolecules, 2006, 39, 81–89 CrossRef CAS.
- C. N. Urbani and M. J. Monteiro, Macromolecules, 2009, 42, 3884–3886 CrossRef CAS.
- F. Stoffelbach, B. Belardi, J. Santos, L. Tessier, K. Matyjaszewski and B. Charleux, Macromolecules, 2007, 40, 8813–8816 CrossRef CAS.
- W. W. Li, K. Min, K. Matyjaszewski, F. Stoffelbach and B. Charleux, Macromolecules, 2008, 41, 6387–6392 CrossRef CAS.
- D. A. Xiong, Z. Li, R. J. Ma, Y. L. An and L. Q. Shi, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1651–1660 CrossRef CAS.
- G. H. Jiang, Y. Wang, X. K. Sun and J. J. Shen, Polym. Chem., 2010, 1, 618–620 RSC.
- W. I. Lee, Y. J. Bae and A. J. Bard, J. Am. Chem. Soc., 2004, 126, 8358–8359 CrossRef CAS.
- D. Wang and T. Imae, J. Am. Chem. Soc., 2004, 126, 13204–13205 CrossRef CAS.
- Y. Lin, J. W. Gao, H. W. Liu and Y. S. Li, Macromolecules, 2009, 42, 3237–3246 CrossRef CAS.
- D. J. Wang, D. D. Jia, Y. G. Zhao, X. H. Shen and Y. D. Yang, Chin. Chem. Lett., 2006, 17, 502–504 CAS.
- W. Wang and C. Y. Pan, Macromol. Rapid Commun., 2009, 30, 2096–2101 CrossRef.
- J. K. Oh, C. B. Tang, H. F. Gao, N. V. Tsarevsky and K. Matyjaszewski, J. Am. Chem. Soc., 2006, 128, 5578–5584 CrossRef CAS.
- H. L. Sun, B. N. Guo, R. Cheng, F. H. Meng, H. Y. Liu and Z. Y. Zhong, Biomaterials, 2009, 30, 6358–6366 CrossRef CAS.
- J. K. Oh, D. J. Siegwart, H. Lee, G. Sherwood, L. Peteanu, J. O. Hollinger, K. Kataoka and K. Matyjaszewski, J. Am. Chem. Soc., 2007, 129, 5939–5945 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR of disulfide-based monomer and photoluminescence photographs of shell cross-linked and uncross-linked nanoparticles in aqueous solution. See DOI: 10.1039/c0py00170h |
| This journal is © The Royal Society of Chemistry 2010 |
