PMMA based soluble polymeric temperature sensors based on UCST transition and solvatochromic dyes†
Christian
Pietsch
ab,
Richard
Hoogenboom
*cd and
Ulrich S.
Schubert
abc
aLaboratory of Organic and Macromolecular Chemistry, Friedrich-Schiller-University Jena, Humboldtstrasse 10, 07743, Jena, Germany
bDutch Polymer Institute, John F. Kennedylaan 2, 5612 AB, Eindhoven, The Netherlands
cLaboratory of Macromolecular Chemistry and Nanoscience, Eindhoven University of Technology, P.O. Box 513, 5600 MB, Eindhoven, The Netherlands. E-mail: r.hoogenboom@tue.nl; Fax: +31 (0)40 247 4186
dInstitute for Molecules and Materials, Radboud University Nijmegen, Heyendaalseweg 135, 6525 AJ, Nijmegen, The Netherlands
First published on 12th June 2010
Abstract
Poly(methyl methacrylate) copolymers labeled with a UV/vis or fluorescent dye were synthesized by RAFT polymerization and subsequently investigated as optical or fluorescent temperature sensors in ethanol. The UCST transition of PMMA in combination with solvatochromic dyes was found to result in a wide linear temperature response and to provide insights into the structural conformation of the PMMA chains during the UCST phase transition.
Water-soluble thermoresponsive polymers functionalized with dyes have received significant attention as sensing materials by simple detection of the absorbance or the fluorescence signal. The high sensitivities arise from the incorporated solvatochromic dye molecules,1 which respond to minor local environmental changes that occur upon the temperature induced polymer phase transition.2 A variety of optical polymeric sensors have been reported as sensing materials for a wide range of applications, such as biosensors,3 drug delivery,4 logic gates,5 and optical sensing.6–9 The majority of these ‘smart’ materials are based on stimuli-responsive polymers exhibiting a lower critical solution temperature (LCST). Such LCST polymers are water-soluble at low temperatures and undergo a sharp entropy-driven collapse with increasing temperature.10 As a result the temperature sensing regime of LCST-based sensors is often limited to 10 °C.3–7 The reverse solubility transition is called upper critical solution temperature (UCST), i.e. the polymer is insoluble at low temperatures and dissolves upon heating. In contrast to the LCST transition, UCST behavior is mostly enthalpy driven causing a broader transition compared to the LCST transition. The LCST and UCST behaviors of polymers in solution are described by the Flory–Huggins theory.11 For our current research, we anticipated that the broader UCST transition would allow expanding the temperature sensing regime compared to LCST polymers.
In contrast to the large number of reports on LCST polymers, only a limited number of UCST polymers are discussed in recent literature. The majority of these studies involved toxic zwitterionic poly(betaine)s.12 In addition, UCST behavior has been reported for poly(methyl methacrylate) (PMMA) based polymer blends13 as well as for PMMA solutions in cyclohexanol14 and ethanol/water mixtures.15 Furthermore, UCST behavior of copolymers of hydroxyethyl methacrylate and acetoacetoxyethyl methacrylate16 as well as various poly(2-oxazoline)s17 was reported in ethanol/water mixtures.
In the current work, we developed optical and fluorescent temperature sensors with a broad temperature sensing regime based on the UCST transition of PMMA in ethanol/water mixtures.
For this purpose, UV/vis and fluorescent solvatochromic dyes, namely disperse red 1 (1a, DR1)18 and pyrene-1-methanol (1b),19 were incorporated into the side chain of PMMA (Scheme 1). It should be noted that for these copolymers only a small solvatochromic response is expected due to the limited polarity difference between ethanol/water and the polymer.
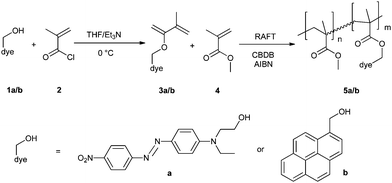 | ||
| Scheme 1 Schematic representation of the synthesis of DR1- and pyrene-functionalized monomers and subsequent reversible addition fragmentation chain transfer (RAFT) copolymerization with MMA (MMA = methyl methacrylate, CBDB = 2-cyano-2-butyl-dithiobenzoate chain-transfer agent; AIBN = azoisobutyronitrile initiator). | ||
The copolymer synthesis was performed using the RAFT polymerization process20 (Scheme 1) to ensure the formation of well-defined polymers allowing a straightforward interpretation of the sensing results. The sensing ability of these dye functionalized UCST polymers was investigated by temperature-controlled UV/vis and fluorescence spectroscopy.
The polymerizable dyes 3a and 3b were prepared by esterification of the hydroxyl group of 1a/b with methacryloyl chloride 2.21 In a next step, these dye-functionalized monomers 3a/b were statistically copolymerized with methyl methacrylate (4, MMA) by RAFT polymerization. In both cases the monomer to RAFT agent ratio was 100 using 3–5% of dye-functionalized monomer, aiming for a theoretical degree of polymerization of 100. Size exclusion chromatography (SEC) revealed the formation of well-defined copolymers as indicated by the low polydispersity indices (PDI < 1.20; Table 1).
The DR1 and pyrene dyes were successfully incorporated into the copolymers as determined by 1H NMR spectroscopy (Table 1). To quantify the temperature sensing ability of the DR1-functionalized PMMA 5a in ethanol, UV/vis absorption spectra were recorded at different temperatures. A 3D representation of the resulting spectra (Fig. 1) clearly shows the intensity change of the two absorption maxima of the DR1 dye at 291 nm (π*(n) ← π) and 480 nm (π* ← π). The importance of the polymer is evidenced by the minor absorption change of a DR1 1a solution in ethanol with varying temperature (see Fig. S1†).
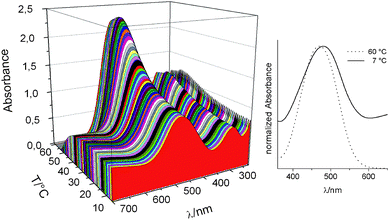 | ||
| Fig. 1 Temperature dependence of the UV/vis absorption of a solution of DR1-functionalized PMMA 5a in ethanol (0.59 mg mL−1). | ||
At 60 °C, when the polymer is completely dissolved in ethanol, the polymer revealed the highest absorbance at both absorption maxima and no scattering was observed above 600 nm as expected for molecularly dissolved polymer chains. The precipitated polymer at lower temperatures (0 to 20 °C) showed significantly lower UV/vis absorption values, which can be ascribed to shielding of the dye by the precipitated polymer, i.e. light is scattered away by the particles before it can be absorbed by the dye as was also observed by Hirai for a LCST based polymeric thermometer.22 Since scattering is more pronounced at higher wavelengths, the decrease of the π* ← π absorption with decreasing temperature is more pronounced compared to the π*(n) ← π absorption.
The observed solvatochromic shift of 5a from 480 nm at 7 °C to 470 nm at 60 °C is rather small (Fig. 1 right), but unexpectedly indicates a more polar environment in the precipitated state. The changes in the λmax absorption values are indicators for the solvent polarity and allow the interpretation of the environment polarity (ET(30) values are recorded for most solvents). Hirai and co-workers showed that a small polarity change occurred for the heat-induced phase transition (LCST) of polyNIPAM, which could not explain the change in optical properties of the incorporated dye,7b,8 as is also the case in the current work. This unanticipated absorption shift is presumably caused by interactions of DR1 with the polymeric ester groups in the precipitated state influencing the tautomeric equilibrium of the dye resulting in a shift of the absorption wavelength.6
The sensing ability of copolymer 5a was quantified by plotting the ratio of the λmax absorption values at 291 nm and 480 nm against temperature (Fig. 2). A linear dependence of the absorbance ratio versus temperature was found in between 25 °C and 55 °C demonstrating the sensing capability of copolymer 5a. Plotting the absorbance of the individual absorption maxima also resulted in a linear response. However, the absorbance ratio is a more robust method since it excludes fluctuations in absorbance due to variations in concentration.
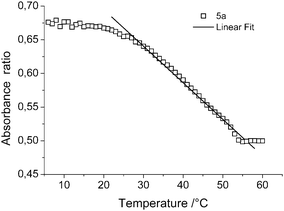 | ||
| Fig. 2 Temperature dependence of the absorbance ratio (π*(n) ← π/π* ← π) of copolymer 5a with a linear fit between 25 and 55 °C ethanol (0.59 mg mL−1). | ||
At temperatures below 25 °C and above 55 °C the absorption ratio is independent of the temperature indicating that the UCST transition from insoluble to soluble gradually occurs in between 25 °C and 55 °C. In fact, these results clearly demonstrate that indeed the UCST transition can be exploited to create a broader temperature sensing regime compared to sensors based on LCST polymers.
The pyrene functionalized PMMA 5b exhibits a clear UCST transition in ethanol/water mixtures (vol%) of 100/0 at 46.9 °C, 90/10 at 27.8 °C and 80/20 at 37.1 °C, respectively, as indicated by the reversible cloud points. Further increasing the water content renders the polymer (partially) insoluble. The optimal solubility, i.e. lowest cloud point, was slightly shifted from 80/20 to 90/10 compared to PMMA15 due to the more hydrophobic character of the pyrene containing copolymer 5b.The sensoric behavior of copolymer 5b was evaluated in ethanol/water 80/20 vol% by recording fluorescence spectra at different temperatures (Fig. 3). This specific solvent mixture was chosen since it is the most polar solvent mixture showing UCST behavior23 and, thus, the strongest solvatochromic shift is expected. The 3D representation of the fluorescence spectra reveals that the fluorescence intensity is higher below the UCST, which is in clear contrast to the absorption behavior of DR1–PMMA 5a.
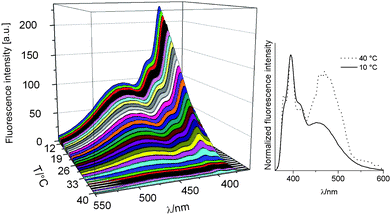 | ||
| Fig. 3 Temperature dependence of the fluorescence spectra of copolymer 5b in ethanol/water 80/20 vol% (excitation at 342 nm; 1.0 mg mL−1). | ||
The decrease in fluorescence intensity of polymer 5b above the cloud point indicates pyrene excimer formation (i.e. pyrene dimers or higher aggregates between the same and/or different chains) driven by solvophobic interactions resulting in fluorescence quenching.24 Below the UCST transition, the pyrene molecules are randomly distributed in the collapsed hydrophobic polymer globules due to effective solvation that suppresses excimer formation. This coil-to-globule transition only induces a very low polarity change (as discussed before for copolymer 5a). The minor polarity change of ethanol/water mixture versus unpolar polymer backbone does not explain the observed fluorescence enhancement, which shows a characteristic ON–OFF behavior. Similar ON–OFF behavior was also reported for different LCST based thermoresponsive copolymer solutions and pH changes.5
A closer look at the fluorescence spectra reveals two maxima, one for individual pyrene molecules at 395 nm and a broad maximum for pyrene excimers at 454 nm (Fig. 3 right).9,19 The proposed increase in pyrene excimer formation above the cloud point is confirmed by the strong increase in excimer emission at 40 °C compared to 10 °C accompanied by a shift of the excimer emission to 462 nm.
The temperature sensing regime of copolymer 5b was evaluated by plotting the fluorescence intensity at 395 nm as a function of temperature (Fig. 4). Above 35 °C the polymer is molecularly dissolved and the intensity remains constant. However, a linear decrease is observed in fluorescence intensity in between 10 °C and 35 °C representing the broad UCST transition, which can be considered as the temperature sensing regime. The ratio of excimer to monomer emission intensities (IE/IM) versus temperature provides additional information on the enthalpy driven UCST phase transition of PMMA revealing three distinct regimes (Fig. 4). From 10 to 30 °C IE/IM gradually increases indicating swelling of the polymer particles resulting in a small increase in excimer formation due to increased hydrophilicity of the swollen particles in combination with still rather limited chain motion. In between 30 and 37 °C a strong increase in IE/IM, i.e. excimer formation, is observed driven by the increased chain mobility of the macroscopically dissolved polymer chain as well as the further increase in hydrophilicity of the pyrene microenvironment. Above the phase transition at 35 °C the IE/IM ratio remains constant since no change in polymer solubility occurs upon further heating.
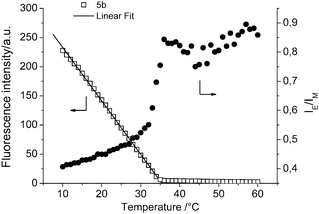 | ||
| Fig. 4 Fluorescence intensity as a function of temperature of copolymer 5b with a linear fit between 10 and 35 °C. In addition, the ratio of excimer (462 nm) to monomer (395 nm) emission intensities (IE/IM) of copolymer 5b is plotted as function of temperature (excitation wavelength 342 nm). | ||
In conclusion, thermoresponsive copolymers based on PMMA side-chain functionalized with DR1 or pyrene are developed that act as soluble temperature sensors in ethanol or ethanol/water solvent mixtures. These UCST polymers have a much broader temperature sensing regime compared to sensors based on LCST polymers. The observed sensing capability of the copolymers was not based on the anticipated solvatochromic shift of DR1 and pyrene, but could be related to scattering effects of the DR1 copolymer and excimer formation of the pyrene copolymer. In addition, the results clearly demonstrate the potential of including solvatochromic dyes into thermoresponsive polymers to gain a better fundamental understanding of polymer solubility transitions.
The Dutch Polymer Institute (DPI) and the Netherlands Scientific Organisation (NWO; Veni-grant for RH and VICI award for USS) are acknowledged for financial support.
Notes and references
- (a) P. Suppan, J. Photochem. Photobiol., A, 1990, 50, 293–330 CrossRef CAS; (b) C. Reichardt, Chem. Rev., 1994, 94, 2319–2358 CrossRef CAS.
- Z. Q. Guo, W. H. Zhu, Y. Y. Xiong and H. Tian, Macromolecules, 2009, 42, 1448–1453 CrossRef CAS.
- C. Gota, K. Okabe, T. Funatsu, Y. Harada and S. Uchiyama, J. Am. Chem. Soc., 2009, 131, 2766–2767 CrossRef CAS.
- S. Aoshima and S. Kanaoka, Adv. Polym. Sci., 2008, 210, 169–208 CAS.
- (a) S. Uchiyama, N. Kawai, A. P. de Silva and K. Iwai, J. Am. Chem. Soc., 2004, 126, 3032–3033 CrossRef CAS; (b) S. Uchiyama and Y. Makino, Chem. Commun., 2009, 2646–2648 RSC.
- C. Pietsch, R. Hoogenboom and U. S. Schubert, Angew. Chem., Int. Ed., 2009, 48, 5653–5656 CrossRef CAS.
- (a) C. Koopmans and H. Ritter, J. Am. Chem. Soc., 2007, 129, 3502–3503 CrossRef CAS; (b) Y. Shiraishi, R. Miyamoto and T. Hirai, Langmuir, 2008, 24, 4273–4279 CrossRef CAS.
- D. Wang, R. Miyamoto, Y. Shiraishi and T. Hirai, Langmuir, 2009, 25, 13176–13182 CrossRef CAS.
- Y. Shiraishi, Y. Tokitoh and T. Hirai, Org. Lett., 2006, 8, 3841–3844 CrossRef CAS.
- E. Dormidontova, Macromolecules, 2002, 35, 987–1001 CrossRef CAS.
- U. W. Gedde, Polymer Physics, Kluwer Academic Publishers, Dordrecht, 1st edn, 1995, pp. 55–69 Search PubMed.
- S. Kudaibergenov, W. Jaeger and A. Laschewsky, Adv. Polym. Sci., 2006, 201, 157–224 CAS.
- (a) S. Li and E. Woo, Colloid Polym. Sci., 2008, 286, 253–265 CrossRef CAS; (b) J. Kressler, H. Kammer and K. Klostermann, Polym. Bull., 1986, 15, 113–119 CrossRef CAS.
- V. Sakai, J. Higgins and J. Trusler, J. Chem. Eng. Data, 2006, 51, 743–748 CrossRef CAS.
- (a) S. Piccarolo and G. Titomanlio, Makromol. Chem. Rapid Commun., 1982, 3, 383–387 CrossRef CAS; (b) R. Hoogenboom, S. Rogers, A. Can, C. R. Becer, C. Guerrero-Sanchez, D. Wouters, S. Hoeppener and U. S. Schubert, Chem. Commun., 2009, 5582–5584 RSC.
- V. Boyko, Y. Lu, A. Richter and A. Pich, Macromol. Chem. Phys., 2003, 204, 2031–2039 CrossRef CAS.
- (a) R. Hoogenboom, H. M. L. Thijs, D. Wouters, S. Hoeppener and U. S. Schubert, Soft Matter, 2008, 4, 103–107 RSC; (b) R. Hoogenboom, H. M. L. Lambermont-Thijs, M. Jochems, S. Hoeppener, C. Guerlain, C.-A. Fustin, J.-F. Gohy and U. S. Schubert, Soft Matter, 2009, 5, 3590–3592 RSC.
- (a) P. Lacroix, I. Malfant, G. Iftime, A. Razus, K. Nakatani and J. Delaire, Chem.–Eur. J., 2000, 6, 2599–2608 CrossRef CAS; (b) L. De Boni, C. Toro, A. E. Masunov and F. E. Hernandez, J. Phys. Chem. A, 2008, 112, 3886–3890 CrossRef CAS.
- T. Förster, Angew. Chem., Int. Ed. Engl., 1969, 8, 333–343 CrossRef.
- (a) G. Moad, E. Rizzardo and S. H. Thang, Aust. J. Chem., 2009, 62, 1402–1472 CrossRef CAS; (b) C. Pietsch, M. W. M. Fijten, H. M. L. Thijs, R. Hoogenboom and U. S. Schubert, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 2811–2820 CrossRef CAS.
- (a) R. R. Barto, C. W. Frank, P. V. Bedworth, R. E. Taylor, W. W. Anderson, S. Ermer, A. K. Jen, J. D. Luo, H. Ma, H. Tang, M. Lee and A. S. Ren, Macromolecules, 2006, 39, 7566–7577 CrossRef CAS; (b) J. Jiang, X. Tong and Y. Zhao, J. Am. Chem. Soc., 2005, 127, 8290–8291 CrossRef CAS; (c) X. Lou, R. Daussin, S. Cuenot, A. Duwez, C. Pagnoulle, C. Detrembleur, C. Bailly and R. Jerome, Chem. Mater., 2004, 16, 4005–4011 CrossRef CAS.
- Y. Shiraishi, R. Miyamoto, X. Zhang and T. Hirai, Org. Lett., 2007, 9, 3921–3924 CrossRef CAS.
- R. Hoogenboom, C. R. Becer, C. Guerrero-Sanchez, S. Hoeppener and U. S. Schubert, Aust. J. Chem., 2010, 63 DOI:10.1071/CH10083.
- (a) F. M. Winnik, Macromolecules, 1990, 23, 233–242 CrossRef CAS; (b) H. Ringsdorf, J. Venzmer and F. M. Winnik, Macromolecules, 1991, 24, 1678–1686 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details. See DOI: 10.1039/c0py00162g |
| This journal is © The Royal Society of Chemistry 2010 |

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500
500