Catalytic polymerization of ε-caprolactone in air†
Antti
Pärssinen
a,
Martin
Kohlmayr
a,
Markku
Leskelä
a,
Mohammed
Lahcini
b and
Timo
Repo
*a
aDepartment of Chemistry, University of Helsinki, P.O. Box 55, FIN-00014, Helsinki, Finland. E-mail: timo.repo@helsinki.fi
bLaboratoire de Chimie Bio-Organique et Macromoléculaire, Département de Chimie, Faculté des Sciences et Techniques Marrakech, Université Cadi Ayyad, BP 549, Marrakech, Morocco
First published on 27th May 2010
Abstract
A highly efficient, catalytic open-air ring opening polymerization for cyclic esters is reported. Using titanium alkoxides, Ti(OiPr)4 and Ti(OnBu)4, as catalysts reproducible ROPs even with high monomer/initiator ratios without protecting gas are feasible.
Biodegradable polymers, poly(caprolactone) (PCL), polyglycolide (PGA), polylactide (PLA) and their copolymers, besides their bulk applications in packaging and various other consumer goods are particular interesting in medicine and tissue engineering purposes due to their biocompatibility.1 Especially, interest toward polycaprolactone (PCL) homopolymer for medical devices has extensively increased ever since it was discovered that it is suitable scaffold material for cells in repairing and regenerating tissue defects1 and as a drug delivery agent.2 These polyesters are most commonly produced by ring opening polymerizations (ROPs) of the cyclic esters using cationic,3 anionic4 and coordination–insertion4 polymerization techniques. The latter has received significant interest as it offers an efficient method to control molar mass and molar mass distribution of the polyesters and thus, those properties of polyesters can be modified with great precision.5
The most studied catalyst for ROP has been tin octoate.5,6 Although various catalysts have been developed for PCL polymerization only tin octoate, to the best of our knowledge, has FDA approval for production of medical grade polyesters.7 However, with tin octoate inert-gas techniques are required in order to protect the hydrophilic monomer and the Lewis acidic catalyst as even traces of moisture appear to drop the molar mass of the polymers and hamper the polymerization process.8 Also the need of a cocatalyst, usually primary alcohols, complicates the polymerization process, and can cause variations in material properties. With all these premises, a need for new and simpler polymerization process for preparation of medical grade PCL with a catalyst based on less toxic metal is well-founded.
In this respect we report herein an exceptional and highly efficient open air procedure for ROP that is based on titanium-alkoxides Ti(OiPr)4 and Ti(OnBu)4.9 Indeed, with certain limitations ROPs can now be carried out in an open beaker. Worth of mentioning is that decomposing products of the catalysts are titanium oxide and alcohols which have already FDA approval for internal use in humans.
Although titanium-alkoxides are moderately stable towards moisture in air and they can be handled in short times without protecting gas, amount of water is still a critical issue in the open-air polymerizations. This is nicely demonstrated here with bulk polymerization experiments with ε-caprolactone. It turned out that the open-air polymerizations needed high temperatures in the initiation of the reaction. At 140 °C even high monomer–catalyst ratios (here up to 1500)10 are applicable and high conversions with all studied Mon./Cat. ratios were achieved with both titanium-alkoxides in less than ten minutes (Table 1). When lower initiation temperatures were applied, high Mon./Cat. ratios led to incomplete conversions regardless of the reaction time (Table 1). Apparently, only high temperatures facilitate efficient initiation of open-air ROP and high molar mass polymers can be produced. After initiating the polymerization at elevated temperatures, the reaction can be completed at lower temperatures slightly above the melting of PCL (∼60 °C). The corresponding bulk polymerization of ε-caprolactone at 140 °C with commercial tin octoate revealed only low conversion (Table 1, run 12).
| Run | Cat.a | Mon./Cat.b | Time/min | Temp/°C | Con.c (%) | M n | M w/Mn |
|---|---|---|---|---|---|---|---|
| a Catalyst a = (Ti(OiPr)4, b = Ti(OnBu)4 and c = tin octoate. b Monomer to catalyst metal ratio. c Conversions of the monomers were determined by 1H NMR. d Molar mass and polydispersity values of the polymers having full conversions were determined by GPC. | |||||||
| 1 | a | 70 | 3 | 70 | 73 | 1900 | 2.46 |
| a | 70 | 3 | 100 | 98 | 7100 | 2.00 | |
| a | 70 | 3 | 140 | 98 | 6100 | 2.19 | |
| 2 | a | 212 | 3 | 100 | 95 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
2.02 |
| 3 | a | 350 | 3 | 100 | 96 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.98 |
| a | 350 | 3 | 140 | 98 | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.68 | |
| 5 | a | 700 | 3 | 140 | 92 | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.80 |
| 6 | a | 920 | 5 | 140 | 85 | 61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.51 |
| 7 | b | 77 | 3 | 70 | 46 | 4900 | 2.39 |
| 8 | b | 307 | 3 | 100 | 94 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
2.00 |
| 9 | b | 384 | 3 | 100 | 76 | 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.03 |
| 10 | b | 768 | 3 | 140 | 97 | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
1.90 |
| 11 | b | 845 | 3 | 140 | 96 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
1.64 |
| 12 | c | 700 | 10 | 140 | 8 | — | — |
The reason for this peculiar polymerization behaviour is related to amount of water in the polymerization reaction. When heated in air at 70 °C and 100 °C, the water content of ε-caprolactone increased in a few minutes up to 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ppm level due to hydrophilic nature of the monomer (Fig. 1). On the contrary, heating of the monomer at 140 °C for ten minutes lowers the water content down to 200–300 ppm, which seems to be sufficient level for the efficient titanium-alkoxide catalyzed ROP.
000 ppm level due to hydrophilic nature of the monomer (Fig. 1). On the contrary, heating of the monomer at 140 °C for ten minutes lowers the water content down to 200–300 ppm, which seems to be sufficient level for the efficient titanium-alkoxide catalyzed ROP.
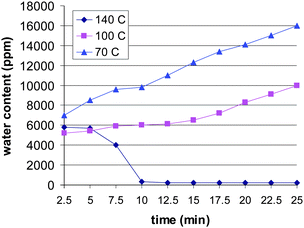 | ||
| Fig. 1 Water content of ε-caprolactone at different temperatures when exposed to air. | ||
As well as Mon./Cat. ratio also the actual amount of Ti-alkoxide has an importance in these polymerizations. Due to the residual water content the lowest amount of Ti-alkoxide to initiate the open-air ROP with any Mon./Cat. ratios was close to 300 µmol while reproducible results, regardless of the applied Mon./Cat. ratios, were obtained with catalyst loading of 470 µmol. In a typical polymerization run the addition of the catalyst over the preheated monomer impeded stirring within a minute due to increased viscosity.
Bulk polymerization of D,L-lactide with Ti(OiPr)4 can be carried out in similar conditions as described for ε-caprolactone. D,L-Lactide is melted and preheated at 140 °C and after addition of the catalyst the polymerization is rapid. Stirring is impeded within a minute and nearly quantitative conversions are obtained within 5 minutes (Table 2). However, with the polymerization system it is only possible to produce low molar mass value polylactide (PLA) with full conversions. With high monomer/catalyst ratios the bulk polymerizations solidify when conversions about 75% are reached. Apparently higher temperatures would be beneficial to improve conversions but PLA itself starts to decompose at temperatures around 200 °C.
| Mon./Cat.a | Temp/°C | Meltingb/min | Time/min | Con.c (%) | M n | PDId |
|---|---|---|---|---|---|---|
| a Monomer to titanium ratio. b Time to melt monomer in 140 °C. c Conversions of the monomers were determined by 1H NMR. d Molar mass and polydispersity of the polymers were determined by GPC. | ||||||
| 22 | 140 | 6 | 5 | 98 | 1550 | 2.04 |
| 62 | 140 | 8 | 5 | 98 | 5150 | 2.01 |
| 142 | 140 | 12 | 3 | 96 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 850 850 |
1.69 |
| 263 | 140 | 16 | 3 | 94 | 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 050 050 |
2.02 |
| 560 | 140 | 22 | 3 | 74 | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.56 |
Generally, in all polymerization runs the molar mass distribution values were between 1.5 and 2 depending on the catalyst/monomer ratios. This indicates that the titanium-alkoxide catalysts under these reaction conditions give polymers with Flory–Schulz distribution and thus possesses the single centre catalyst behaviour. As the chain end groups are detectable by 1H NMR (isopropoxide or n-butoxide depending on the applied Ti-alkoxide), the open-air polymerization proceeds with classical coordination insertion mode as described earlier for other ROP catalysts.
A clear benefit of the open-air polymerization procedure is that there is no necessity to finish the actual polymerization in a polymerization reactor. As a result of low viscosity at early stage of polymerization, and due to the stability of the polymerization catalysts, even direct pouring of the polymerization solution to a mould is feasible. Polymerization proceeds then to full conversion in the mould. In addition, the polymer can be blended effortless with any substances needed in various application, e.g. in medical devices.
The portable open-air polymerization system was applied in aseptic production of appropriate PCL for forthcoming invasive class III medical applications. In this respect the developed system is attractive as all the required devices needed for polymerization can be easily sterilized in an autoclave or purified by appropriate alcohol solution. Indeed, equipment needed now for the polymerizations includes a stirrer with heater, an aluminium block, a beaker (50–500 ml), steel needles and disposable syringes.
The reproducibility of polymerization under aseptic conditions was remarkably high and inherent viscosity values, commonly used in evaluation of properties of polymers, were in two parallel polymerizations 0.97 ± 0.02 dl g−1 when volume of monomer was 52 ml. As underlined by FDA's risk evaluation, this reproducibility is highly important for medical applications. The preliminary toxicity investigation of catalyst residues was carried out with mouse heteroploid connective tissue (L-929) revealing zero cytopathic effect. In conclusions, a highly efficient, catalytic open-air ring opening polymerization for cyclic esters is reported. Using titanium alkoxides, Ti(OiPr)4 and Ti(OnBu)4, as catalysts reproducible ROPs even with high monomer/initiator ratios without protecting gas are feasible. As a result, polymers with high molar mass and molar mass distribution values between 1.5 and 2 can be produced with high efficiency. The only limitation for the efficient ROP in an open beaker is the elevated reaction temperature that is needed in the beginning of the polymerization to reduce the amount of water in the monomer.
This novel portable polymerization system can be easily set to any clean room facility with low expenses in order to produce medical grade PCL. In general, the polymerizations for medical applications have to be carried out at laboratories following GMP regulations using clean room facility combined with laminar flow hoods. These specific regulations and toxic examinations are the main reasons for high production expenses of medical grade polyester and only tin octoate has so far FDA approval. Because of above reasoning and highly optimized processes for end products, e.g. biodegradable PLA screws, replacement of tin octoate with any new catalyst is questionable. A new catalyst must bring marked added value for a production concept and/or applications. In this respect the above described trouble-free polymerization set-up, due to excellent reproducibility, low toxicity, effortless blending of additives and a possibility for direct pouring of the polymerization solution to a mould, is highly attractive concept for aseptic preparation of medical grade PCL for various novel medical applications. Alongside with the potential commercial applications this polymerization system can be easily exploited to any lower level educational system e.g. high schools to teach production of environmentally friendly biodegradable polymers.
Financial support from Bio- and nanopolymers Centre of Excellence funded by Academy of Finland and TEKES and Academy of Finland project 123248 is gratefully acknowledged.
Notes and references
- V. Siracusa, P. Rocculi, S. Romani and M. Dalla Rosa, Trends Food Sci. Technol., 2008, 19, 634 CrossRef CAS; M. Huttunen, P. Törmälä, P. Godinho and M. Kellomäki, Biomed. Mater., 2008, 3, 034106 Search PubMed; M. Martina and D. W. Hutmacher, Polym. Int., 2007, 56, 145 CrossRef CAS.
- P. Xu, E. Van Kirk, Y. Zhan, W. Murdoch, M. Radosz and Y. J. Shen, Angew. Chem., Int. Ed., 2007, 46, 4999 CrossRef CAS.
- J. Jonté, R. Dunsing and H. R. Kricheldorf, Macromol. Chem. (Oxford), 1986, 187, 771 Search PubMed; H. R. Kricheldorf and R. Dunsing, Makromol. Chem., 1986, 187, 1611 CrossRef CAS; A. Hofman, S. Slomkowski and S. Penczek, Makromol. Chem., 1984, 185, 655 CrossRef CAS.
- A. Hofman, S. Slomkowski and S. Penczek, Makromol. Chem., 1984, 185, 91 CrossRef CAS; H. R. Kricheldorf, I. Kreiser-Saunders and N. Scharnagl, Makromol. Chem., Macromol. Symp., 1990, 32, 285 Search PubMed; Z. Jedlinski and M. Kowalczuk, Macromolecules, 1989, 22, 3242 CrossRef CAS.
- C. Jerome and P. Lecomte, Adv. Drug Delivery Rev., 2008, 60, 1056 CrossRef CAS; M. Florczak and A. Duda, Angew. Chem., Int. Ed., 2008, 47, 9088 CrossRef.
- A. P. Gupta and V. Gumar, Eur. Polym. J., 2007, 43, 4053 CrossRef CAS.
- According to ARC Surgical premarket notification (510k) they are using tin octoate synthesized polylactide as a raw material for production of biodegradable pins. Polymer is delivered by Boehringer-Ingelheim. File is dated on 19.12.2007.
- J. L. Robert and K. B. Aubrecht, J. Chem. Educ., 2008, 85, 258 CrossRef CAS.
- Both of these catalysts have been used in ROP of lactide and caprolactone by using traditional inert-gas techniques. K. Hiltunen, J. Seppälä and M. Härkönen, Macromolecules, 1997, 30, 373 Search PubMed;
F. E. Critchfield and R. D. Lundberg, Catalytic production of high-molecular-weight, solid, linear polymers from cyclic esters, patent application, FR 2
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 026
026![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 275, 1970 CrossRef CAS.
275, 1970 CrossRef CAS. - Additional polymerization of 200 ml ε-CL with 0.35 ml of Ti(OiPr)4 having monomer/titanium ratio of 1533 was carried out in a 250 ml beaker. The polymerization of ε-CL was carried out according to the method explained in experimental part. After the initiation of the polymerization stirring was impeded in 5 minutes and full conversion of monomer was achieved during 60 minutes polymerization revealing PCL having Mn value of 80
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, Mw of 140
000, Mw of 140![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and PDI value of 1.8.
000 and PDI value of 1.8.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details of the polymerization process, analyzing methods and the locations where the experiments were carried out. See DOI: 10.1039/c0py00107d |
| This journal is © The Royal Society of Chemistry 2010 |
