Dispersion nitroxide mediated polymerization of methyl methacrylate in supercritical carbon dioxide using in situ formed stabilizers†
Bruno
Grignard
a,
Trang
Phan
b,
Denis
Bertin
b,
Didier
Gigmes
b,
Christine
Jérôme
a and
Christophe
Detrembleur
*a
aCenter for Education and Research on Macromolecules (CERM), University of Liège, Sart-Tilman, B6a, B-4000, Liège, Belgium. E-mail: christophe.detrembleur@ulg.ac.be; Fax: (+32) 4-3663497; Fax: (+32) 4-3663565; Tel: (+32) 4-3663565
bLaboratoire Chimie Provence, Université de Provence UMR 6264, Avenue Escadrille Normandie-Niemen, Case 542, Marseille, 13397, Cedex 20, France
First published on 13th April 2010
Abstract
Controlled dispersion nitroxide mediated polymerization (NMP) of methyl methacrylate (MMA) was successfully carried out in supercritical carbon dioxide (scCO2) in the presence of a CO2-philic perfluorinated stabilizer generated “in situ”. This is an efficient green approach for preparing free flowing PMMA powder consisting of small sized microspheres.
Introduction
Nitroxide mediated polymerization (NMP) of methacrylates has been challenging for a long time due to cross disproportionation and large activation–deactivation equilibrium constants.1 In 2002, Detrembleur and co-workers reported on the successful polymerization of methacrylate derivatives in water by in situ NMP2 using sodium nitrite,3 a nitroso compound4 or nitric oxide5 as nitroxide precursors. Although an efficient control over the molecular weight was observed for methyl methacrylate (MMA) and tert-butyl methacrylate (tBMA), the polydispersity was increased with the monomer conversion from 1.1 to 1.8. In 2007, Bertin and co-workers reported on the successful MMA polymerization in bulk using new alkoxyamines based on 2,2-diphenyl-3-phenylimino-2,3-dihydroindol-1-yloxyl nitroxide (DPAIO). NMP of MMA remained controlled until the monomer conversion became higher than 60% and the molecular weight distribution was relatively low (Mw/Mn ≈ 1.4).6 Very recently, Greene and Grubbs7 prepared a novel N-phenylalkoxyamine that controlled the NMP of MMA until about 40% monomer conversion. In 2006, Charleux and co-workers demonstrated that MMA could be polymerized in a controlled way with a very narrow polydispersity using a SG1 (N-tert-butyl-N-(1-diethylphosphono-2,2-dimethylpropyl) nitroxide) based alkoxyamine provided that a small amount of styrene (∼8.8 mol%) was added.8 Using this powerful approach, the authors also reported on the emulsion polymerization of MMA using poly(methacrylic acid-co-styrene) macroalkoxyamines.9 Based on this Charleux's work, tert-butyl methacrylate could also be controlled and various block copolymers were successfully prepared.10In the past 5 years, the use of supercritical carbon dioxide (scCO2) as a green polymerization medium has gained interest for the preparation of well-defined polymers free of any organic residues (monomer, solvent) by controlled radical polymerizations, i.e. ATRP,11–16 RAFT17–21 and NMP.22–30 However, examples of polymers prepared by NMP in supercritical carbon dioxide (scCO2) are limited and only focus on the synthesis of polystyrene (PS) in precipitation22,23,25,29,30 or in dispersion.24,26,27 In the latter case, SG1 was the controlling agent for the dispersion polymerization of styrene initiated by a conventional free radical initiator in the presence of an external preformed stabilizer such as a diblock copolymer with a CO2 philic and CO2 phobic block.24,26 To the best of our knowledge, the polymerization of MMA initiated by a SG1 based alkoxyamine in scCO2 and stabilized by an in situ formed stabilizer has never been reported.
In this communication, we report on the first self-stabilized dispersion nitroxide mediated polymerization of MMA in the presence of small amount of styrene in scCO2 using a binary system, (i) 2-methyl-2-[N-tert-butyl-N-(diethoxyphosphoryl-2,2-(dimethylpropyl)aminooxy]propionic acid (BlocBuilder, Arkema) as the alkoxyamine to initiate and control the MMA polymerization, and (ii) a SG1-terminated perfluorinated poly(acrylate), prepared in scCO2, that generates “in situ” the dispersion stabilizer during the MMA polymerization and consequently ensures the formation of stabilized PMMA particles (Scheme 1).
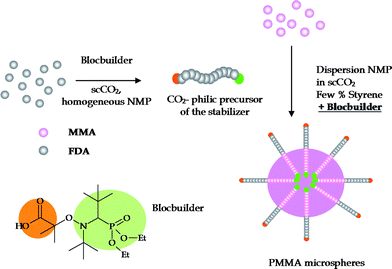 | ||
| Scheme 1 Dispersion NMP of MMA using BlocBuilder as polymerization initiator and SG1-terminated poly(heptadecafluorodecyl acrylate) (PFDA-SG1) as precursor of the stabilizer. | ||
For the dispersion polymerization of MMA in scCO2, a diblock copolymer composed of a CO2-philic block (poly(heptadecafluorodecyl acrylate) (PFDA)) and a CO2-phobic block (PMMA) was chosen as the stabilizer. Importantly, to simplify the system as much as possible, this PFDA-b-PMMA diblock copolymer is generated “in situ” from SG1-terminated PFDA macroinitiators (PFDA-SG1) during the dispersion NMP of MMA initiated by BlocBuilder. PFDA-SG1 is first prepared by homogeneous NMP in scCO2 using BlocBuilder as the alkoxyamine, at 100 °C and 300 bar for 24 h (Table 1 entries 1–4).
| Entry | Monomer | [Monomer]/[BlocBuilder] | Conv. (%) | Mn theo/g mol−1 | Mn/g mol−1 | PDI | f e |
|---|---|---|---|---|---|---|---|
| a Conditions: entries 1–4: 100 °C, 300 bar, 24 h; entries 5–7: 70 °C, 300 bar 114 h, 5 wt% PFDA-SG1 (wt% compare to MMA + styrene; Mn = 70000 g mol−1), 8.8 mol% styrene. b Estimated from Mn = ((weight FDA)/n BlocBuilder) × conv. c Mn determined by 1H NMR in a 50/50 v/v CFC113/CDCl3 mixture. d Low molecular weight PFDA (Mn < 5000 g mol−1) exhibits solubility in THF. e f = initiator efficiency = Mn theo./Mn exp. f MMA + 8.8 mol% styrene. g Estimated from Mn = ((weight MMA + weight styrene)/n alkoxyamines) × conv. h Estimated by SEC using a PMMA standard calibration. | |||||||
| 1 | FDA | 6 | 88 | 2700 b | 3800 c | 1.09 d | 0.70 |
| 2 | FDA | 11 | 80 | 4500 b | 7000 c | — | 0.65 |
| 3 | FDA | 19 | 78 | 8000 b | 12000 c | — | 0.67 |
| 4 | FDA | 96 | 94 | 48000 b | 70000 c | — | 0.68 |
| 5 | MMA f | 200 | 89 | 17800 g | 18500 h | 1.29 | 0.96 |
| 6 | MMA f | 550 | 94 | 52000 g | 57000 h | 1.26 | 0.91 |
| 7 | MMA f | 1000 | 90 | 90000 g | 94000 h | 1.31 | 0.96 |
After depressurisation of the cell, the polymer was collected as a white powder, the monomer conversion, gravimetrically determined, was high and number average molecular weight (Mn) was estimated by 1H NMR spectroscopy by comparison of the relative intensities of the CH group of the alkoxyamine (δ = 3.4 ppm, d) and the methylene protons of the repetitive FDA units (CH2, δ = 4.4 ppm) (Fig. S1, ESI†). It has to be noted that whatever the targeted molecular weight, the BlocBuilder efficiency is close to 0.7 (Table 1). This result could be explained by the particularly fast dissociation of the BlocBuilder when the reaction mixture is directly immersed at the desired temperature. A high concentration of 1-carboxy-1-methylethyl initiating radical is rapidly generated and therefore, a non-negligible proportion of self-termination reaction of this species was probably occurring before the addition onto FDA.31
In order to demonstrate the livingness of the PDFA chains, the synthesis of a PFDA-b-PnBuA diblock copolymer in scCO2 starting from a PFDA-SG1 macroinitiator (Mn = 3800 g mol−1, Mw/Mn = 1.09, Table 1, entry 1) was considered. After 24 h at 300 bars and 100 °C, the high pressure cell was cooled to room temperature, the pressure was slowly released and the diblock copolymer was collected as a viscous material (90% conversion). SEC analysis clearly evidences the successful block copolymerization with the complete shift of the SEC chromatogram of PFDA-SG1 macroinitiator toward the higher molecular weight side, although some polydispersity increase (Mw/Mn = 1.45) is observed because no free SG1 was added to the polymerization (Fig. S2†). The presence of the SG1 at the chain-end and the yield of functionalization were determined by electron spin resonance (ESR). Typically a trifluorotoluene (TFT) solution (10−4 M) of PFDA-SG1 (Mn = 3800 g mol−1, Mw/Mn = 1.09) was heated at 100 °C in presence of oxygen (to suppress the recombination reaction) for 2 h. The characteristic ESR spectrum of SG1 was observed assessing the presence of SG1 living chains. By comparing the area of the ESR SG1 spectrum released by the polymer with the one obtained with a free sample of SG1 in TFT solution (10−4M) chosen as reference, the yield of functionalization was estimated to be around 90 ± 10%.
NMP of MMA was then carried out at 70 °C and 300 bar using BlocBuilder as the initiator in the presence of 8.8 mol% styrene, and 5 wt% PFDA-SG1 (Mn = 70000 g mol−1; entry 4 Table 1) as the stabilizer precursor. First, different monomer to initiator molar ratios were investigated in order to target different molecular weights (Table 1, entries 5–7). After 114 h, the monomer conversion was high (≥ 90%), and the experimental molecular weights determined by SEC using a PMMA calibration are in good agreement with the theoretical values. Moreover, the molecular weight distributions were narrow even for high molecular weight PMMA (Table 1). The control of the polymerization is maintained when using only 4.4 mol% styrene, but is negatively affected when the styrene content is further decreased to 2.2 mol% as assessed by the broadening of the polydispersity and the important discrepancy between theoretical and experimental molecular weights (Table S1, ESI†). More interestingly, after depressurisation of the cell, PMMA was collected as a white free flowing powder. Depending on the targeted molecular weight, the polymer was received as small size microparticles of undefined morphology (Fig. 1A, Mn = 18500 g mol−1), or microspheres of ∼100 μm diameter that coexisted with larger elongated particles (Fig. 1B, Mn = 57000 g mol−1), or well defined microspheres with an average diameter of 91 ± 7 μm (Fig. 1C, Mn = 94000g mol−1).
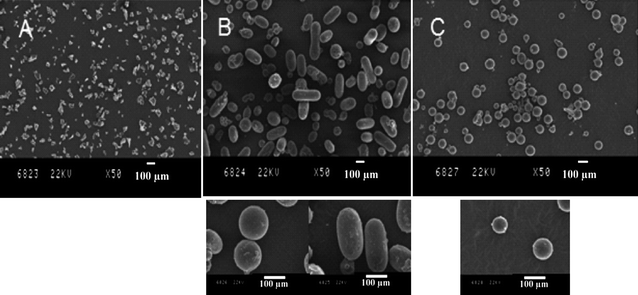 | ||
| Fig. 1 SEM characterizations of PMMA particles obtained by dispersion NMP in scCO2 at 70 °C in the presence of 5 wt% of PDFA-SG1 surfactant. A: PMMA, Mn = 18500 g mol−1, PDI = 1.29, Table 1, entry 5; B: PMMA, Mn = 57000 g mol−1, PDI = 1.26, Table 1, entry 6; C: PMMA, Mn = 94000 g mol−1, PDI = 1.31, Table 1, entry 7. | ||
In order to determine the effect of the PFDA-SG1 loading on the polymerization control and on the particles stabilization, dispersion NMP of MMA was repeated in the same conditions than those described previously in the presence of 10 wt%, 5 wt% and without PFDA-SG1 of Mn = 70000 g mol−1 (Table 2). Whatever the surfactant loading, in each case, the monomer conversion was high after 114 h (> 90%), the polydispersity narrow (1.2 ≤ Mw/Mn ≤ 1.3) and the experiment molecular weight was in good agreement with the theoretical value.
| Entry | PFDA-SG1 loading (%) | Conv (%) a | Mn theo/g mol−1b | Mn exp/g mol−1c | PDI |
|---|---|---|---|---|---|
| a Gravimetrically determined. b Estimated from the relation Mn = ((w MMA + w styrene)/n alkoxyamines) × conv. c Estimated using a PMMA standard calibration. | |||||
| 1 | 10 | 98 | 54000 | 60000 | 1.22 |
| 2 | 5 | 94 | 52000 | 57000 | 1.26 |
| 3 | — | 94 | 52000 | 57000 | 1.30 |
As expected, an increase of the surfactant loading involved a decrease of the particles size. Indeed, microspheres with a diameter of 100μm coexisted with larger elongated particles when 5 wt% of surfactant (Fig. 1B) was used. On the other hand, well defined PMMA microspheres with an average diameter of 16 ± 2 μm were obtained in the presence of 10 wt% of PFDA-SG1 (Fig. 2). For sake of comparison, in the absence of stabilizer, PMMA was collected as a single chunk of polymer.
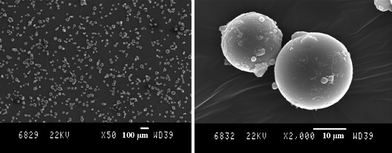 | ||
| Fig. 2 SEM characterization of PMMA particles (Mn,exp = 60000 g mol−1, PDI = 1.22, Table 2, entry 1) obtained by dispersion NMP in scCO2 at 70 °C and 300 bar in the presence of 10 wt% of PFDA-SG1 of Mn = 70000 g mol−1. | ||
Such particle stabilization is due to the in situ formation of PFDA-b-PMMA diblock copolymer from PFDA-SG1 macroinitiator that acts as a stabilizer for the growing PMMA chains initiated by the BlocBuilder. In order to further evidence the formation of the block copolymer, PMMA microspheres (Mn = 57000 g mol−1, Table 1 entry 6) were solubilised by a selective solvent of PMMA (tetrahydrofurane, THF). Centrifugation allowed to isolate some insoluble PDFA chains which however represented only about 0.5 wt% of initial PFDA-SG1 used for the polymerization. 1H NMR analysis of this residue revealed the presence of 3 MMA units in average at the ω-chain ends. This result demonstrates that more than 99% of the stabilizer remains in the solution. As PFDA-SG1 used for the dispersion polymerization is not soluble in THF, the PFDA-b-PMMA diblock copolymer was therefore expected to be present in the solution as micelles or small aggregates. This assumption was demonstrated by transmission electron microscopy (TEM) of a drop of the solution dried on a TEM grid that evidenced the presence of spherical objects with a mean diameter of about 100 to 300 nm (Fig. S3†). Due to the high stabilizing capability of the diblock formed in situ, all our attempts to isolate the copolymer were unsuccessful.
As a conclusion, dispersion NMP of MMA in the presence of 8.8 mol% styrene was successfully implemented in scCO2 using BlocBuilder as both the initiator and control agent in the presence of a CO2-soluble perfluorinated polymer end-capped by an alkoxyamine (PFDA-SG1) that generates in situ the stabilizer for the growing PMMA chains. Moreover, after depressurisation of the cell, depending on the targeted molecular weight, PMMA was collected as a white free flowing powder consisting of small sized microspheres, quasi spherical particles or microspheres/elongated particles mixture, thanks to the stabilizing properties of the PFDA-b-PMMA block copolymer formed in situ. Importantly, all steps of this process, including the synthesis of the stabiliser precursor, are implemented in scCO2. The challenge is now to prepare various block copolymers of practical interest such as the Nanostrength® (PMMA-b-PnBuA-b-PMMA and PMMA-b-PnBuA) using this efficient green approach.
Acknowledgements
The authors of Liege are indebted to the “Belgian Science Policy” for general support in the frame of the “Interuniversity Attraction Poles Programme (IAP 6/27) – Functional Supramolecular Systems” and to the “Fonds National pour la Recherche Scientifique” (F.R.S.-FNRS) for financial support. B.G. thanks the “Région Wallonne” and the FEDER for their financial support in the frame of the POLYTISS project. The authors thank Arkema for having kindly provided BlocBuilder and SG1. T.P. DB. and D.G. acknowledge University of Provence and CNRS for financial support.Reference and notes
- B. Charleux, J. Nicolas and O. Guerret, Macromolecules, 2005, 38, 5485 CrossRef CAS.
- V. Sciannamea, R. Jerome and C. Detrembleur, Chem. Rev., 2008, 108(3), 1104 CrossRef CAS.
- C. Detrembleur, Ph. Teyssie and R. Jerome, Macromolecules, 2002, 35, 1611 CrossRef CAS.
- C. Detrembleur, P. Teyssié and R. Jérôme, e-Polym., 2002, 004 Search PubMed.
- C. Detrembleur, M. Claes, R. Jérôme, ACS Symposium Series, 2003, American Chemical Society (ACS), vol. 854, pp. 496–518 Search PubMed.
- Y. Guillaneuf, D. Gigmes, S. R. A. Marque, P. Astolfi, L. Greci, P. Tordo and D. Bertin, Macromolecules, 2007, 40(9), 3108 CrossRef CAS.
- A. C. Greene and R. B. Grubbs, Macromolecules, 2009, 42, 4388 CrossRef CAS.
- J. Nicolas, C. Dire, L. Mueller, J. Belleney, B. Charleux, S. Marque, D. Bertin, S. Magnet and L. Couvreur, Macromolecules, 2006, 39, 8274 CrossRef CAS.
- C. Dire, S. Magnet, L. Couvreur and B. Charleux, Macromolecules, 2009, 42(1), 95 CrossRef CAS.
- B. Lessard, C. Tervo, S. De Wahl, F. J. Clerveaux, K. K. Tang, S. Yasmine, S. Andjelic, A. D'Alessandro and M. Maric, Macromolecules, 2010, 43, 868 CrossRef CAS.
- B. Grignard, C. Jerome, C. Calberg, R. Jerome, W. Wang, S. M. Howdle and C. Detrembleur, Macromolecules, 2008, 41, 8575 CrossRef CAS.
- B. Grignard, C. Jerome, C. Calberg, R. Jerome, W. Wang, S. M. Howdle and C. Detrembleur, Chem. Commun., 2008,(3), 314 RSC.
- B. Grignard, C. Jerome, C. Calberg, W. Wang, S. M. Howdle and C. Detrembleur, Chem. Commun., 2008,(44), 5803 RSC.
- J. Xia, T. Johnson, S. G. Gaynor, K. Matyjaszewski and J. DeSimone, Macromolecules, 1999, 32, 4802 CrossRef CAS.
- S. Villarroya, J. Zhou, C. J. Duxbury, A. Heise and S. M. Howdle, Macromolecules, 2006, 39, 633 CrossRef CAS.
- J. Zhou, S. Villarroya, W. Wang, M. F. Wyatt, C. J. Duxbury, K. J. Thurecht and S. M. Howdle, Macromolecules, 2006, 39(16), 5352 CrossRef CAS.
- K. J. Thurecht, A. M. Gregory, W. Wang and S. M. Howdle, Macromolecules, 2007, 40(9), 2965 CrossRef CAS.
- H. Lee, E. Terry, M. Zong, N. Arrowsmith, S. Perrier, K. J. Thurecht and S. M. Howdle, J. Am. Chem. Soc., 2008, 130(37), 12242 CrossRef CAS.
- K. J. Thurecht, A. M. Gregory, S. Villarroya, J. Zhou, A. Heise and S. M. Howdle, Chem. Commun., 2006,(42), 4383 RSC.
- M. Zong, K. J. Thurecht and S. M. Howdle, Chem. Commun., 2008,(45), 5942 RSC.
- T. Arita, S. Beuermann, M. Buback and P. Vana, Macromol. Mater. Eng., 2005, 290(4), 283 CrossRef CAS.
- P. Odell and G. Hamer, Polym. Mater.: Sci. Eng., 1996, 74, 404 CAS.
- R. McHale, F. Aldabbagh, P. B. Zetterlund and M. Okubo, Macromol. Chem. Phys., 2007, 208(16), 1813 CrossRef CAS.
- R. McHale, F. Aldabbagh, P. B. Zetterlund, H. Minami and M. Okubo, Macromolecules, 2006, 39(20), 6853 CrossRef CAS.
- F. Aldabbagh, P. B. Zetterlund and M. Okubo, Macromolecules, 2008, 41(7), 2732 CrossRef CAS.
- R. McHale, F. Aldabbagh, P. B. Zetterlund and M. Okubo, Macromol. Rapid Commun., 2006, 27(17), 1465 CrossRef CAS.
- J. Ryan, F. Aldabbagh, Per B. Zetterlund and M. Okubo, Polymer, 2005, 46(23), 9769 CrossRef CAS.
- D. G. Ramirez-Wong, C. A. Posada-Velez, E. Saldivar-Guerra, J. G. Luna-Barcenas, C. Ott and U. S. Schubert, Macromol. Symp., 2009, 283–284(1), 120 CrossRef CAS.
- F. Aldabbagh, P. B. Zetterlund and M. Okubo, Eur. Polym. J., 2008, 44, 4037 CrossRef CAS.
- P. O'Connor, P. B. Zetterlund and F. Aldabbagh, Macromolecules, 2010, 43, 914 CrossRef CAS.
- F. Chauvin, P.-E. Dufils, D. Gigmes, Y. Guillaneuf, S. R. A. Marque, P. Tordo and D. Bertin, Macromolecules, 2006, 39, 5238 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, Table S1 and Fig. S1–S3. See DOI: 10.1039/c0py00066c |
| This journal is © The Royal Society of Chemistry 2010 |
