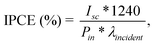A green neutral state donor–acceptor copolymer for organic solar cells
Serap
Günes
*a,
Derya
Baran
b,
Gorkem
Günbas
bc,
Asuman
Durmus
b,
Anita
Fuchsbauer
d,
Niyazi Serdar
Sariciftci
d and
Levent
Toppare
b
aYildiz Technical University, Faculty of Arts and Science, Department of Physics, Davutpasa Campus, 34210, Esenler, Istanbul, Turkey. E-mail: sgunes@yildiz.edu.tr
bMiddle East Technical University, Department of Chemistry, Ankara, Turkey
cDepartment of Chemistry, University of California Davis, One Shields Avenue, Davis, California 95616, USA
dLinz Institute for Organic Solar Cells (LIOS), Physical Chemistry, Johannes Kepler University Linz, Altenberger Strasse 69, A-4040, Linz, Austria
First published on 2nd July 2010
Abstract
We report on the photophysical and photovoltaic properties of a low band gap polymer bearing a quinoxaline moiety, poly(2,3-bis(3,4-bis(decyloxy)phenyl)-5,8-bis(2,3-dihydrothieno[3,4-b][1,4]dioxin-5-yl)quinoxaline) PDOPEQ, as an electron donor in bulk heterojunction solar cells blended with the acceptor 1-(3-methoxycarbonyl)propyl-1-phenyl-[6,6]-methanofullerene (PCBM). Devices were composed of PDOPEQ and varying amounts of PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w–w ratio). The components were spun cast from chlorobenzene (CB) and characterized by measuring current–voltage characteristics under simulated AM 1.5 conditions. The devices with 1
4 w–w ratio). The components were spun cast from chlorobenzene (CB) and characterized by measuring current–voltage characteristics under simulated AM 1.5 conditions. The devices with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 polymer to PCBM ratio exhibited short circuit current density (Jsc) of 0.8 mA cm−2, an open circuit voltage (Voc) of 0.2 V, and a fill factor (FF) of 0.3. Incident photon to current efficiency (IPCE) is also reported. The IPCE spectrum spans from 400 nm to 800 nm and exhibits a photocurrent contribution of ca. 5.5% at around 400 nm. The nanoscale morphology was investigated with atomic force microscopy (AFM). Photoinduced absorption spectroscopy confirms the photoinduced charge transfer in such donor acceptor blends.
3 polymer to PCBM ratio exhibited short circuit current density (Jsc) of 0.8 mA cm−2, an open circuit voltage (Voc) of 0.2 V, and a fill factor (FF) of 0.3. Incident photon to current efficiency (IPCE) is also reported. The IPCE spectrum spans from 400 nm to 800 nm and exhibits a photocurrent contribution of ca. 5.5% at around 400 nm. The nanoscale morphology was investigated with atomic force microscopy (AFM). Photoinduced absorption spectroscopy confirms the photoinduced charge transfer in such donor acceptor blends.
A Introduction
Due to advantages of low cost synthesis, easy integration into a wide variety of devices, organic semiconducting materials have gained increasing attention over the last decade.1–8 Organic semiconducting materials found applications in many fields such as organic photovoltaics (OPV), organic field effect transistors (OFETs), organic light emitting diodes (OLEDs) etc.7,8 Over the last few years, encouraging progress has been achieved in the field of solar cells using organic materials.9–11 Among organic semiconducting materials implemented into organic photovoltaics conjugated polymers (CPs) are of special interest.12–30 CPs are distinguished by their alternating single and double bonds between carbon atoms on the polymer backbone.3 Among the different proposed concepts, the bulk heterojunction approach which is formed by blending donor type CPs with acceptors like fullerenes has been the most attractive and successful one. Hence, this approach improves the power conversion efficiency of the OPV devices up to the 6–7% power conversion of efficient plastic solar cell.9–11 One of the possible improvements of organic bulk heterojunction solar cells is the use of new materials to absorb the red and near infrared part of the solar spectrum. These materials are regarded as low band gap semiconducting polymers.31 The synthesis and application of low band gap polymers which absorb light above 600 nm in organic solar cells have been reported by several groups.21,32–37 The band gap is defined as the difference between the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels in polymers neglecting the Coulomb interactions. Low band gap polymers are defined as a polymer with a band gap of approximately lower than 2 eV.38 One of the limiting parameters in plastic solar cells is the mismatch between the absorption of organic materials and the terrestrial solar spectrum.39 The optical band gap of the generally used CPs in organic solar cells have values around 2.0–2.2 eV.39 The use of low band gap polymers expands the spectral region of bulk heterojunction solar cells and is a viable route to enhance the number of photons absorbed.39 An ideal band gap of 1.3–1.6 eV for a bulk heterojunction device is described in a study by Scharber et al.40In this study, we report a novel low band gap polymer bearing a quinoxaline moiety, PDOPEQ, as an electron donor in bulk heterojunction solar cells blended with the acceptor 1-(3-methoxycarbonyl)propyl-1-phenyl-[6,6]-methanofullerene (PCBM).
Devices were composed of PDOPEQ (see Fig. 1) and varying amounts of PCBM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 w/w ratio). The components were spun cast from chlorobenzene (CB) and characterized by measuring current–voltage characteristics under simulated AM 1.5 conditions.
4 w/w ratio). The components were spun cast from chlorobenzene (CB) and characterized by measuring current–voltage characteristics under simulated AM 1.5 conditions.
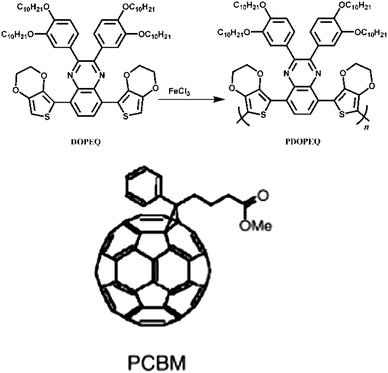 | ||
| Fig. 1 Structural formula of PDOPEQ and PCBM. | ||
B Experimental
The monomer has been previously synthesized by Gunbas et al.41 and polymerized both electrochemically and chemically. For the chemical polymerization of the monomer, 100 mg DOPEQ was dissolved in 2 mL of dry CHCl3 under argon atmosphere. 75 mg FeCl3 was suspended in 2 mL CHCl3 and slowly added to the monomer solution. The reaction mixture was stirred for 2 h and the mixture was added to 200 mL of methanol. The precipitate was dissolved in CHCl3 and extracted with water. The extracts were combined and evaporated under reduced pressure. The residue was dissolved in 50 mL THF and 50 mL hydrazine was added. The mixture was stirred for 24 h to undergo a complete dedoping process. As reported previously, GPC results reveal Mn: 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800, Mw: 110
800, Mw: 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 028.41 In this study, we investigated the photovoltaic properties of chemically synthesized polymer PDOPEQ by blending with a fullerene.
028.41 In this study, we investigated the photovoltaic properties of chemically synthesized polymer PDOPEQ by blending with a fullerene.
For photoinduced absorption studies drop cast pristine PDOPEQ and PDOPEQ/PCBM blend films were mounted into a homemade cold finger cryostat and held at liquid nitrogen temperature. The vacuum during the measurements was < 10−6 mbar. The excitation source for the PIA measurements was a diode laser with an output wavelength of 664 nm and an output intensity of 45 mW. The pump beam was modulated by a mechanical chopper (Stanford SR 540) with a frequency of 218 Hz. PIA measurements were carried out by probing the absorption of light from a tungsten halogen lamp during the laser excitation. The white light from the lamp was focused on the sample and on the entrance slit of a monochromator. In front of the entrance a set of cut off filters were mounted. The transmission (T) and the photoinduced changes in the transmission ΔT was recorded by a Si (up to 1100 nm) and an InGaAsSb (1100–2200 nm) which is mounted on the exit slit of the monochromator. The signal is amplified and detected by a Standford Model SR 830 lock-in-amplifier.
For solar cell preparation, as substrates, glass sheets of 1.5 cm × 1.5 cm coated with ITO (Merck KG Darmstadt) were used with an ITO thickness of about 120 nm and sheet resistance < 15 Ω cm−2. The ITO was patterned by etching with an acid mixture of HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3
HNO3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (4.6
H2O (4.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5) for 30 min. The part of the substrate which forms the contact is covered with a scotch tape to prevent etching. The tape was removed after etching and the substrate was then cleaned using acetone and isopropanol in an ultrasonic bath.
5) for 30 min. The part of the substrate which forms the contact is covered with a scotch tape to prevent etching. The tape was removed after etching and the substrate was then cleaned using acetone and isopropanol in an ultrasonic bath.
An aqueous solution of poly(3,4-ethylenedioxythiophene) doped with poly(styrenesulfonate) (PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS) from Clevios was spin coated on the glass–ITO substrate, and dried under a dynamic vacuum leading to ca. 100 nm films.
PSS) from Clevios was spin coated on the glass–ITO substrate, and dried under a dynamic vacuum leading to ca. 100 nm films.
The blends for the active layer with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 1
3 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (w
4 (w![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) w) ratios of PDOPEQ/PCBM, prepared by dissolving 5 mg of PDOPEQ and 10 mg of PCBM (in the case of 1
w) ratios of PDOPEQ/PCBM, prepared by dissolving 5 mg of PDOPEQ and 10 mg of PCBM (in the case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/ml of chlorobenzene (CB) and stirring at 50 °C overnight. For the top electrodes, 0.6 nm of lithium fluoride (LiF) and 100 nm of aluminium (Al) were thermally evaporated.
2)/ml of chlorobenzene (CB) and stirring at 50 °C overnight. For the top electrodes, 0.6 nm of lithium fluoride (LiF) and 100 nm of aluminium (Al) were thermally evaporated.
All current–voltage (I–V) characteristics of the PV devices were measured using a Keithley SMU 236 under nitrogen in a dry glove box. A Steuernagel solar simulator for AM1.5 conditions was used as the excitation source with an input power of 100 mW cm−2 white-light illumination which was calibrated using a standard crystalline silicon diode. The solar cells were illuminated through the ITO side.
UV-Vis absorption spectra of the solid thin films were obtained using Varian Carry spectrophotometer. Thin films for UV-Vis measurements were spun cast on glass from chlorobenzene (CB) solutions containing 5 mg of PDOPEQ and 10 mg of PCBM (in the case of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2)/ml.
2)/ml.
The spectrally resolved photocurrent was measured with an EG&G Instruments 7260 lock-in amplifier. The samples were illuminated with monochromatic light of a Xenon lamp. The incident photon to current efficiency (% IPCE) was calculated according to the following equation:
Atomic force microscopy studies were performed using Digital Instruments DIMENSION 3100 in the tapping mode.
C Results and discussion
UV-Vis absorption spectrum of the solid thin films of pristine polymer is shown in Fig. 2. The polymer individually exhibits two absorptions in the visible region centered at 415 nm and 690 nm which correspond to two π → π* transitions due to its donor–acceptor nature. Since the absorption bands are centered at around 400 nm and 700 nm, the polymer reveals a green color in its neutral state which has been of interest for applications involving conducting polymers. PDOPEQ is a processable neutral state green polymer with a transmissive oxidized stated synthesized by Gunbas et al.41 Processability and the green color of the polymer in the neutral state encouraged us to investigate the photovoltaic cell properties since green color means absorptions in the visible region leading to a better match with the electromagnetic spectrum of sun. The synthesis and application of low band gap polymers have been reported by several groups.21,33–36,42 It has been shown that insertion of donor–acceptor units on the polymer backbone leads to a considerable decrease in band gap because of the increased double bond character in the structure.43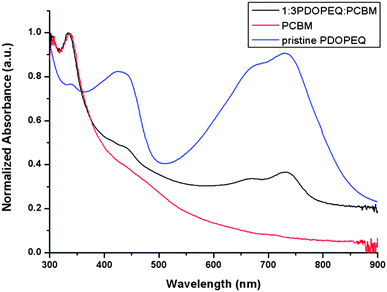 | ||
| Fig. 2 Normalized absorption spectrum of pristine PDOPEQ, pristine PCBM and blend films of PDOPEQ and PCBM. | ||
The PL quenching studies indicate a photoinduced charge transfer from PDOPEQ to PCBM (see Fig. 3).
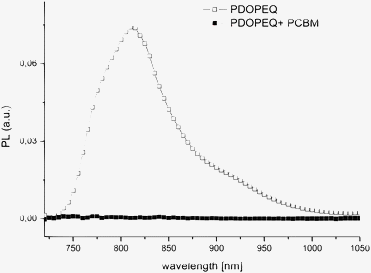 | ||
| Fig. 3 PL spectra of pristine PDOPEQ and PDOPEQ/PCBM blend. | ||
The highest molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of PDOPEQ were determined using cyclic voltammetry. The details of cyclic voltammetry can be found in ref. 41. The onset values for oxidation and reduction were determined at 0.4 V and −1.3 V vs. Ag wire pseudo reference electrode respectively (corresponds to 0.5 V and −1.1 V versus NHE).44 This leads to an electrochemical band gap Eg of ca. 1.6 eV. The band edges for the HOMO and the LUMO can be estimated as −5.1 eV and −3.7 eV, respectively. It was shown in literature that at least 0.3 to 0.4 eV offset between LUMO of the donor and LUMO of the acceptor is necessary to make sure an effective charge transfer from the photoexcited polymer to PCBM.39,45 In our case, the LUMO of the PDOPEQ lies higher than LUMO of PCBM.
The PIA spectra are depicted in Fig. 4. The PIA spectrum of pristine PDOPEQ film shows a maximum peak at approximately 0.93 eV. The features around 1.7 eV are residual uncompensated photoluminescence. The modulation frequency dependence was done in the region between 20 and 3600 Hz. Due to limited frequency range of the measurement the mean life time can be estimated to be lower than around 0.27 ms. In general, the dependence of the PIA signal on the modulation frequency can be used to estimate the recombination time of the species. The frequency response of the signal is given by the following formula (see eqn (1,2) and 3).
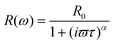 | (1) |
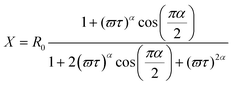 | (2) |
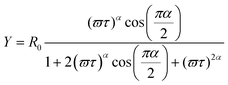 | (3) |
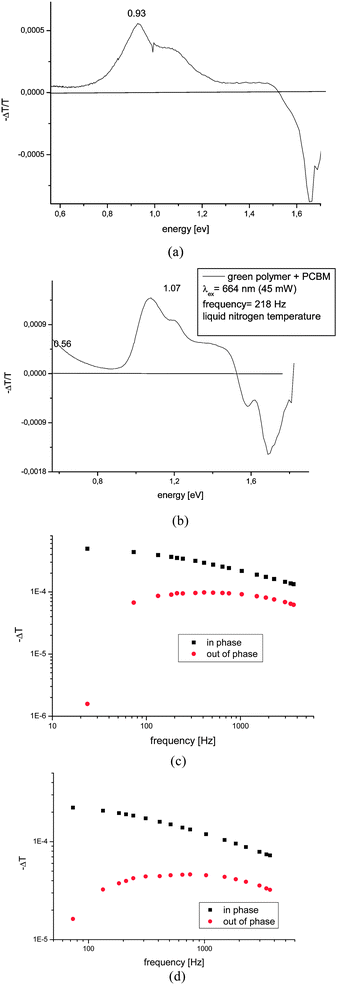 | ||
| Fig. 4 Photoinduced absorption spectra of (a) pristine PDOPEQ films (b) PDOPEQ/PCBM blend films. (c) Modulation frequency dependence of 1.07 eV blend signal. (d) Modulation frequency dependence of 0.56 eV blend signal. | ||
Experimental results were fitted with the second equation (for in phase signal) and the third equation above (for out of phase signal) simultaneously. Form this fit we can derive the mean lifetime τ and the dispersive factor α. The in phase signal X is decreasing with ω, whereas Y shows a maximum.
Additionally, the dependence of the PIA signal upon increasing the laser pump power, I, shows a power law dependence Ik with k = 0.9. The PIA of the PDOPEQ/PCBM blend shows absorption features at 1.07 eV and as well as a second absorption arising below 0.56 eV. The peak at 0.56 eV can be assigned to low energy PIA and the one at 1.07 eV to a high energy PIA of charged polarons.46 The negative peak at around 1.7 eV is assigned to photobleaching of the linear absorption. The modulation frequency dependence for the two features at 0.56 and 1.07 eV were done separately (see Fig. 4 (c) and (d)). Our fitting to the above formulas has resulted in not reliable fit parameters. This is due to limited frequency range of the measurement. Since the measurement can not be extended to higher frequencies with the given experimental setup the estimation for the lifetime was done from the maximum of the out of phase data in Fig. 4 (c) and (d). A characteristic mean lifetime of around 2.4 ms for the peak at 1.07 eV and of 1.3 ms for the peak at 0.56 eV was found. The pump power dependence shows a near square root dependence (k ∼ 0.5), indicating a bimolecular recombination (see Fig. 5).
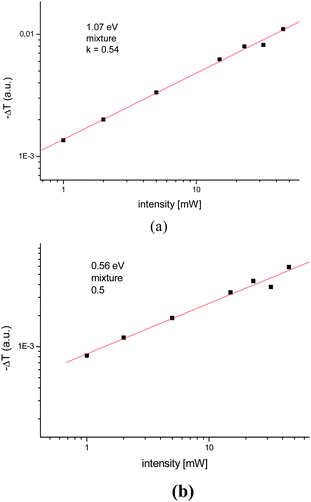 | ||
| Fig. 5 The pump power dependence of the PIA (a) peak at 1.07 eV (b) peak at 0.56 eV. | ||
In summary, for pristine films of PDOPEQ, a single PIA peak at 0.93 eV was found from the PIA measurements. Such PIA is commonly assigned to triplet–triplet absorption.
After blending with the electron acceptor PCBM, the PL is quenched (see Fig. 3) and two PIA peaks are observed at 0.56 eV and 1.07 eV. Such PIA spectra with two photoinduced transitions in the bandgap, are commonly assigned to polaronic charge carriers on a conjugated polymer.47
Fig. 6 shows the photovoltaic characterization of PDOPEQ/PCBM blend solar cells. The bulk heterojunction device with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 polymer to PCBM ratio has a short circuit current density (Jsc) of 0.8 mA cm−2, an open circuit voltage (Voc) of 0.2 V, and a fill factor (FF) of 0.3. The low rectification ratio of approximately 10 can be attributed to the rather poor film formation of the active layer (diode, “dark” curve). However, the overall efficiency of the device is mainly hindered by very low Voc. Generally, Voc is related to the difference between the HOMO level of the donor (PDOPEQ) and the LUMO level of the acceptor (PCBM). Thus, HOMO of the PDOPEQ lying close to the LUMO of PCBM might have diminished the Voc.
3 polymer to PCBM ratio has a short circuit current density (Jsc) of 0.8 mA cm−2, an open circuit voltage (Voc) of 0.2 V, and a fill factor (FF) of 0.3. The low rectification ratio of approximately 10 can be attributed to the rather poor film formation of the active layer (diode, “dark” curve). However, the overall efficiency of the device is mainly hindered by very low Voc. Generally, Voc is related to the difference between the HOMO level of the donor (PDOPEQ) and the LUMO level of the acceptor (PCBM). Thus, HOMO of the PDOPEQ lying close to the LUMO of PCBM might have diminished the Voc.
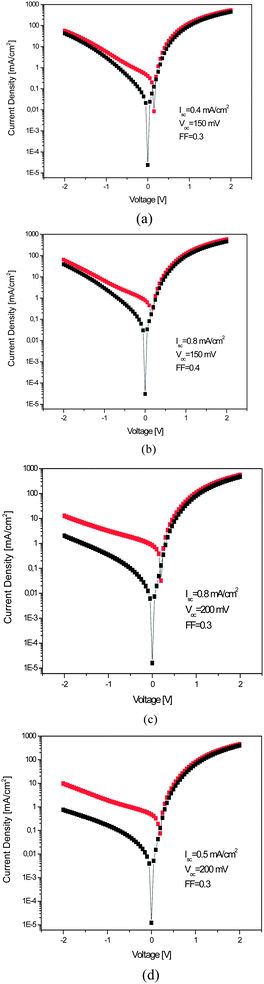 | ||
Fig. 6 Current–voltage characteristics of PDOPEQ/PCBM blend solar cells with (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (b) 1 1 (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (c) 1 2 (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (d) 1 3 (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 polymer to blend ratio. 4 polymer to blend ratio. | ||
By means of IPCE measurements, the contribution of organic species present in the device to the total photocurrent generation can be investigated. The shape of the IPCE spectrum provides information about which part of the photovoltaic device is active. The IPCE spectrum here spans from 400 nm to 800 nm and exhibits a photocurrent contribution of ca. 5.5% at around 400 nm (Fig. 7). As can be seen from the figure, the shape of the IPCE spectrum is mainly dominated by the absorption spectrum of PDOPEQ. This is an indication that PDOPEQ contributes to the photocurrent generation in the organic device. Also, we observed that with a higher PCBM concentration, the IPCE response slightly moves to higher wavelengths.
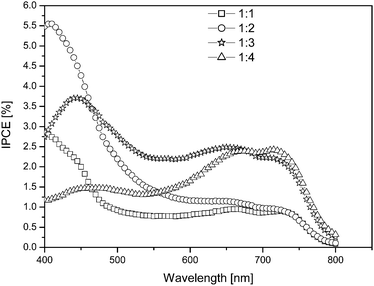 | ||
| Fig. 7 IPCE curve of the PDOPEQ/PCBM solar cells. | ||
The behavior of solar cells can better be analyzed via understanding the nanomorphology of the active layer.5,23,48–51 The challenge in bulk heterojunction solar cells is to organize donor and acceptor materials in a nanometre scale. On one hand their interfacial area is to be maximized,22,36,52,53 while typical dimensions of phase separation are within the exciton diffusion length (in the order of 10–20 nm). On the other hand, continuous, preferably undisturbed pathways for transport of charge carriers to the electrodes must be ensured (interpenetrating networks).22 To correlate the morphology of the solar cells to the photovoltaic performance, we performed an AFM study on the PDOPEQ/PCBM blends. Fig. 8 shows the AFM images of PDOPEQ/PCBM films for four different compositions (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 1
3, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) that are spun cast from CB.
4) that are spun cast from CB.
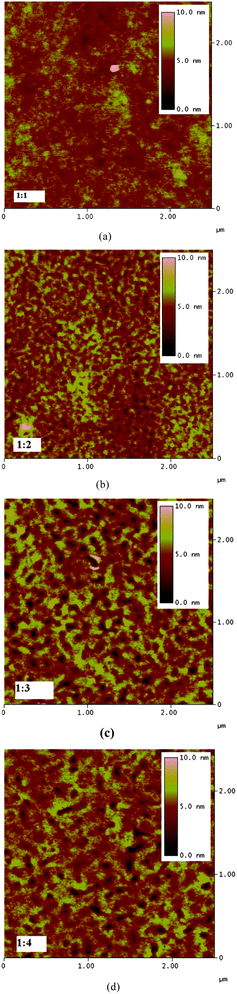 | ||
Fig. 8 AFM images of PDOPEQ/PCBM blends spin cast from dichlorobenzene; (a) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (b) 1 1 (b) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (c) 1 2 (c) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (d) 1 3 (d) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios. 4 ratios. | ||
AFM gives information about the surface morphology of the active layers. For the transport of charges to the appropriate electrodes there should be continuous pathways or the phase separation between the donor and acceptor should be avoided. As is seen from the figures, PDOPEQ/PCBM films with different PCBM ratios all indicate rather smooth surfaces which was derived from the height scale values (10 nm). However, the films prepared from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, 1
2, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 1
3 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratios show increasingly grained nanostructures with increasing fullerene content and also hole like structures on the surface which might be an indication of film formation problems leading to low filling factors.
4 ratios show increasingly grained nanostructures with increasing fullerene content and also hole like structures on the surface which might be an indication of film formation problems leading to low filling factors.
Conclusions
In conclusion, we have reported on the photovoltaic and photophysical properties of a processable low band gap polymer. We observed that blending PDOPEQ with PCBM leads to an active layer that absorbs photons in the entire visible region. Such property shows that low band gap polymers are potential candidates for the development of organic solar cells. However, further optimization is required such as the choice of the solvent, metal contacts, active layer optimization (spinning speed, concentration and annealing).Acknowledgements
Serap Günes acknowledges the financial support of European Science Foundation (ESF) summer exchange grant with exchange grant number 1408.References
- Handbook of Conducting Polymers, ed. T. A. Skotheim, R. L. Elsenbaumer and J. R. Reynolds, Marcel Dekker, Inc, New York, 1998 Search PubMed.
- C. N. Hoth, P. Schilinsky, S. A. Choulis and C. J. Brabec, Nano Lett., 2008, 8, 2806 CrossRef CAS.
- Y. Li and Y. Zou, Adv. Mater., 2008, 20, 2952 CrossRef CAS.
- M. Shahinpoor, K. J. Kim, M. Mojarrad, Artificial Muscles, Chapter 8, Conductive or Conjugated Polymers as Artificial Muscles, Taylor and Francis, 2007, 323 Search PubMed.
- H. Hoppe and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 3462 RSC.
- N. S. Sariciftci, Mater. Today, 2004, 7, 36 CrossRef CAS.
- M. Al'Ibrahim, R. H. Klaus, M. Schroedner, A. Kalvin, U. Zhokhavets, G. Gobsch, P. Scharff and S. Sensfuss, Org. Electron., 2005, 6, 65 CrossRef CAS.
- T. Erb, S. Raleva, U. Zhokhavets, G. Gobsch, B. Stühn, M. Spode and O. Ambacher, Thin Solid Films, 2004, 450, 97 CrossRef CAS.
- S. H. Park, A. Roy, S. Beaupré, S. Cho, N. Coates, J. S. Moon, D. Moses, M. Leclerc, K. Lee and A. J. Heeger, Nat. Photonics, 2009, 3, 297 Search PubMed.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T. Q. Nguyen, M. C. Dante and A. J. Heeger, Science, 2007, 317, 222 CrossRef CAS.
- Y. Liang, Z. Xu, J. Xia, S.-T. Tsa, T. Wu, G. Li, C. Ray and L. Yu, Adv. Mater., 2010, 22, E135 CrossRef CAS.
- S. Shaheen, R. Radspinner, N. Peyghamberian and G. Jabbour, Appl. Phys. Lett., 2001, 79, 2996 CrossRef CAS.
- D. A. M. Egbe, L. H. Nguyen, K. Schmidtke, A. Wild, S. Günes, C. Sieber and N. S. Sariciftci, J. Polym. Sci., Part A: Polym. Chem., 2007, 45, 1619 CrossRef CAS.
- J. A. Hauch, P. Schilinsky, S. A. Choulis, R. Childer, M. Biele and C. J. Brabec, Sol. Energy Mater. Sol. Cells, 2008, 92, 727 CrossRef CAS.
- H. Hoppe and N. S. Sariciftci, in Advamces in Polymer Science, ed. S. R. Marder and K.-S. Lee, Springer, Berlin-Heidelberg, 2008, vol. 214, pp. 1–86 Search PubMed.
- R. Pacios, J. Nelson, D. D. C. Bradley and C. J. Brabec, Appl. Phys. Lett., 2003, 83, 4764 CrossRef CAS.
- A. J. Breeze, Z. Schlesinger, S. A. Carter, H. Tillmann and H. H. Hörhold, Sol. Energy Mater. Sol. Cells, 2004, 83, 263 CrossRef CAS.
- C. J. Brabec, V. Dyakonov, J. Parisi and N. S. Sariciftci, Organic Photovoltaics: Concepts and Realization, SpringerNew York, USA, 2003 Search PubMed.
- C. Winder and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 1077 RSC.
- F. Padinger, R. Rittberger and N. S. Sariciftci, Adv. Funct. Mater., 2003, 13, 85 CrossRef CAS.
- L. Campos, A. Tontcheva, S. Günes, G. Sönmez, H. Neugebauer, N. S. Sariciftci and F. Wudl, Chem. Mater., 2005, 17, 4031 CrossRef CAS.
- C. J. Brabec, C. Winder, N. S. Sariciftci, J. C. Hummelen, A. Dhanabalan, P. A. Van Hal and R. A. J. Janssen, Adv. Funct. Mater., 2002, 12, 709 CrossRef CAS.
- W. Ma, C. Yang, X. Gong, K. Lee and A. J. Heeger, Adv. Funct. Mater., 2005, 15, 1617 CrossRef CAS.
- F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2009, 93, 394 CrossRef CAS.
- T. Ameri, G. Dennler, C. Lungenschmidt and J. C. Brabec, Energy Environ. Sci., 2009, 2, 347 RSC.
- I. Gonzales-Valls and M. Lira-Cantu, Energy Environ. Sci., 2009, 2, 19 RSC.
- M. Helgesen, R. Søndergaard and F. C. Krebs, J. Mater. Chem., 2010, 20, 36 RSC.
- F. C. Krebs, S. A. Gevorgyan and J. Alstrup, J. Mater. Chem., 2009, 19, 5442 RSC.
- F. C. Krebs, M. Jørgensen, K. Norrman, O. Hagemann, J. Alstrup, D. Nielsen, J. Fyenbo, K. Larsen and J. Kristensen, Sol. Energy Mater. Sol. Cells, 2009, 93, 422 CrossRef CAS.
- F. C. Krebs and S. A. Gevorgyan, et al, Sol. Energy Mater. Sol. Cells, 2009, 93, 1968 CrossRef CAS.
- H. Spanggaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2004, 83, 125 CrossRef CAS.
- E. Perzon, X. Wang, S. Admassie, O. Inganäs and M. R. Andersson, Polymer, 2006, 47, 4261 CrossRef CAS.
- A. Cravino, M. Loi, M. C. Scharber, C. Winder, H. Neugebauer, P. Denk, H. Meng, Y. Chen, F. Wudl and N. S. Sariciftci, Synth. Met., 2003, 137, 1435 CrossRef CAS.
- M. Wienk, M. Struijk and R. A. J. Janssen, Chem. Phys. Lett., 2006, 422, 488 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Macromolecules, 2006, 39, 2823 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2007, 91, 1019 CrossRef CAS.
- C. Winder, G. Matt, J. C. Hummelen, R. A. J. Janssen, N. S. Sariciftci and C. Brabec, Thin Solid Films, 2002, 403–404, 373 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Sol. Energy Mater. Sol. Cells, 2007, 91, 954 CrossRef CAS.
- C. Winder, G. Matt, J. C. Hummelen, R. A. J. Janssen, N. S. Sariciftci and C. J. Brabec, Thin Solid Films, 2002, 403–404, 373 CrossRef CAS.
- M. C. Scharber, D. Mühlbacher, M. Koppe, P. Denk, C. Waldauf, A. J. Heeger and C. J. Brabec, Adv. Mater., 2006, 18, 789 CrossRef CAS.
- G. Günbas, A. Durmus and L. Toppare, Adv. Funct. Mater., 2008, 18, 2026 CrossRef CAS.
- E. Bundgaard and F. C. Krebs, Macromolecules, 2006, 39, 2823 CrossRef CAS.
- A. Pennisi, F. Simone, G. Barletta, G. Di Marco and L. Lanza, Electrochim. Acta, 1999, 44, 3237 CrossRef CAS.
- B. C. Thompson, Y. G. Kim and J. R. Reynolds, Macromolecules, 2005, 38, 5359 CrossRef CAS.
- A. J. Mozer, P. Denk, M. C. Scharber, H. Neugebauer, N. S. Sariciftci, P. Wagner, L. Lutsen and D. Vanderzande, J. Phys. Chem. B, 2004, 108, 5235 CrossRef CAS.
- R. A. J. Janssen, D. Moses and N. S. Sariciftci, J. Chem. Phys., 1994, 101, 9519 CrossRef CAS.
- C. Winder and N. S. Sariciftci, J. Mater. Chem., 2004, 14, 1077 RSC.
- X. Yang, J. Loos, S. C. Veenstra, W. J. H. Verkees, M. Wienk, J. M. Kroon, M. H. J. Michels and R. A. J. Janssen, Nano Lett., 2005, 5, 579 CrossRef CAS.
- G. Li, V. Shrotriya, J. Huang, Y. Yao, T. Mariorty, K. Emery and Y. Yang, Nat. Mater., 2005, 4, 864 CAS.
- S. Shaheen, C. J. Brabec, N. S. Sariciftci, F. Padinger and J. C. Hummelen, Appl. Phys. Lett., 2001, 78, 841 CrossRef CAS.
- D. Chirvaze, J. Parisi, J. C. Hummelen and V. Dyakonov, Nanotechnology, 2004, 15, 1317 CrossRef CAS.
- M. T. Rispens, A. Meetsma, R. Rittberger, C. J. Brabec, N. S. Sariciftci and J. C. Hummelen, Chem. Commun., 2003, 2116 RSC.
- M. Wienk, J. M. Kroon, W. J. H. Verhees, J. C. Hummelen, P. A. Vanhal and R. A. J. Janssen, Angew. Chem., Int. Ed., 2003, 42, 3371 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |