Synthesis, electrical properties, and nanocomposites of poly(3,4-ethylenedioxythiophene) nanorods
James D.
Mendez
ab and
Christoph
Weder
*ab
aAdolphe Merkle Institute and Fribourg Center for Nanomaterials, University of Fribourg, Route de l'Ancienne Papeterie CP 209, CH-1723, Marly 1, Switzerland. E-mail: christoph.weder@unifr.ch; Fax: +41 (0)26 300 9624
bDepartment of Macromolecular Science and Engineering, Case Western Reserve University, 2100 Adelbert Rd., Cleveland, OH 44106-7202, USA
First published on 28th June 2010
Abstract
Cellulose nanofibers or “whiskers” derived from tunicates were coated with PEDOT:PSS to produce high-aspect-ratio nanorods with high electrical conductivity. Films made from these PEDOT:PSS whiskers have surface resistivities as low as 97 Ω/□. The nanorods were found to disperse well in polar organic solvents such as dimethylformamide, which allowed the fabrication of nanocomposites with inert host polymers such as poly(methyl methacrylate) and polystyrene. These materials display surface resistivities as low as 760 Ω/□. Due to the high aspect ratio of the PEDOT:PSS-coated whiskers (64) and the formation of well-dispersed whisker networks within the polymer matrices, these materials display a percolation threshold as low as ∼2% v/v. Using a sol–gel process, electrically conducting gels and aerogels with a density of 6.4 mg cm−3 and a surface resistivity of ∼200 kΩ/□ were also produced.
Introduction
Nanocomposites involving electrically (semi)conducting conjugated polymers are of interest, because their (opto)electronic properties can, in many cases, be widely manipulated through combination of two or more different types of materials. A plethora of studies have focused on materials in which conjugated polymers are used as the continuous phase into which inorganic nanoparticles,1–3 organic nanocrystals,4–7 carbon nanotubes,8–10 and other nanomaterials are integrated (note that a clear distinction is made between nanocomposites and phase-separated blends, whose morphology is often very difficult to control11). Less work has addressed materials in which the nanoparticle used is a conjugated polymer,12 and even fewer studies have investigated nanocomposites that comprise conjugated polymer particles of high aspect ratio, i.e. nanofibers, -rods, or -whiskers.13 The one-dimensional nature of such nanofillers imparts several interesting features, including percolation at low concentration14,15 (of interest are e.g. nanocomposites of conducting polymers with ‘inert’ matrix polymers for antistatic applications, electromagnetic shielding16–18), the possibility to create anisotropic materials,19–21 and nanocomposites with high internal surface areas (which are useful in sensor applications,22,23 rapidly switchable electrochromic devices,24 and heterojunction photovoltaic devices4–7). However, few broadly applicable approaches are known, which allow the fabrication of well-individualized and well-defined nanofibers of conjugated polymers by simple processes. Usually, very specific methods are required, which are only applicable to one particular polymer. Examples include templated synthesis25 and the synthesis of polyaniline rods under confining conditions.26–28 Direct synthesis often yields ill-defined rod-like shapes, instead of well-defined wires with high aspect ratio.29,30 Other techniques rely on shaping already synthesized conjugated polymers, such as the preparation of nanorods or nanotubes using a porous membrane as a template,31–33 phase separation of block copolymers,34 or electrospinning.35–38 However, many of these methods are intricate or do not afford well-individualized fibers that can be incorporated as the percolating phase into a matrix polymer by simple mixing.Poly(3,4-ethylenedioxythiophene) (PEDOT) is one of the technologically most relevant conducting polymers due to its combination of its high electrical conductivity as well as it high transparency in the visible spectrum.39,40 Yet, current methods for the preparation of high-aspect ratio PEDOT nanofibers are limited. These current methods can be categorized into three general areas that yield pure PEDOT nanorods,41 coated fibers (commonly electrospun),42–44 and coated nanorods (commonly carbon nanotubes).45–47 PEDOT nanorods without any scaffold retain the excellent properties of bulk PEDOT, but most of these nanorods have relatively low aspect ratios.48
We here report the synthesis of PEDOT nanofibers by the polymerization of ethylenedioxythiophene (EDOT) in the presence of cellulose nanofibers as a scaffold.49 This general approach was introduced by Flandin et al. for the preparation of polypyrrole rods.12 We previously used a modified version of this framework to fabricate polyaniline and poly(p-phenylene ethynylene) nanofiber composites.50 These materials were prepared by the adsorption of pre-formed polymers onto the cellulose template, rather than in situ polymerization. The potential problems of the latter reside in the difficultly of obtaining a smooth and thin layer of the conjugated polymer on the template,12 the aggregation of the conducting nanofibers during synthesis, the fact that the polymerization also proceeds remote from the template, and the formation of nanoparticles on the nanofibers, as we reported recently for silver, gold, copper, and platinum.51 However, we show here that, under appropriate reaction conditions, the process allows the formation of high-aspect-ratio nanorods with nm-thin layers of a highly conductive coating, which can readily be processed into conducting films, gels, aerogels, and polymer nanocomposites with low percolation threshold.
Results and discussion
Cellulose nanofibers or “whiskers” can be obtained from a range of renewable bio-sources, including tunicates, wood, cotton, and sisal.63 For this study we employed whiskers isolated from tunicates by hydrolysis with sulfuric acid according to methods reported elsewhere.52,53 The whiskers obtained from these sea creatures exhibit high stiffness (tensile modulus ∼130 GPa) and a very high aspect ratio (average dimensions 26 nm × 2.2 μm, Fig. 1A).54 Due to the high density of strongly interacting surface hydroxyl groups, cellulose whiskers have a strong tendency for aggregation. However, if sulfuric acid is used for the hydrolysis, a small number of sulfate groups is introduced, which imparts good dispersibility in water and a range of polar organic solvents, such as dimethylformamide (DMF).52,53,55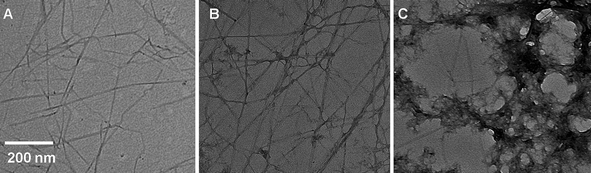 | ||
Fig. 1 Transmission electron microscopy (TEM) images of (A) cellulose whiskers isolated from tunicates; (B) cellulose whiskers coated with PEDOT:PSS by synthesis with an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2 whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w); and (C) cellulose whiskers coated with PEDOT:PSS by synthesis with an EDOT 1 (w/w); and (C) cellulose whiskers coated with PEDOT:PSS by synthesis with an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 9 whisker ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). 1 (w/w). | ||
PEDOT-coated whiskers were prepared by the oxidative polymerization of EDOT in the presence of the whiskers (Scheme 1). We first attempted the synthesis of p-toluenesulfonate-doped PEDOT (PEDOT:TS) with iron(III) p-toluenesulfonate as the oxidant (Scheme 1A), since this PEDOT variant exhibits high electrical conductivity.56 The synthesis of PEDOT:TS is preferably conducted in aliphatic alcohols, but these solvents do not disperse cellulose whiskers.52 On the other hand, DMF is a good dispersant for the whiskers, but not suitable for the polymerization of EDOT. Therefore, our initial protocol used equal amounts of water and methanol along with EDOT and cellulose whiskers in a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). Unfortunately, the reaction afforded large aggregates that appeared to be PEDOT, which could not be dispersed by soniciating the isolated reaction product in water (Fig. 2A). The EDOT
1 (w/w). Unfortunately, the reaction afforded large aggregates that appeared to be PEDOT, which could not be dispersed by soniciating the isolated reaction product in water (Fig. 2A). The EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio was varied between 1
whisker ratio was varied between 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), but the outcome was similar. We hypothesized that binding of the iron ions to the whiskers might be the cause for aggregation (due to either cross-linking between the whiskers or electrostatic shielding). Support for this was provided in a control experiment where iron(III) p-toluenesulfonate was added to a dispersion of neat cellulose whiskers in water; this caused the immediate precipitation of cellulose whiskers. The amount of methanol was therefore reduced to the minimum needed to solubilize the EDOT. These conditions resulted in products, in which the macroscopic PEDOT aggregates were no longer visible and the resistivity of solution cast films was as low as 57 Ω/□. However, closer inspection with transmission electron microscopy (TEM) (Fig. 2B) revealed the formation of nanoscale PEDOT aggregates and the whiskers were inhomogeneously coated.
1 (w/w), but the outcome was similar. We hypothesized that binding of the iron ions to the whiskers might be the cause for aggregation (due to either cross-linking between the whiskers or electrostatic shielding). Support for this was provided in a control experiment where iron(III) p-toluenesulfonate was added to a dispersion of neat cellulose whiskers in water; this caused the immediate precipitation of cellulose whiskers. The amount of methanol was therefore reduced to the minimum needed to solubilize the EDOT. These conditions resulted in products, in which the macroscopic PEDOT aggregates were no longer visible and the resistivity of solution cast films was as low as 57 Ω/□. However, closer inspection with transmission electron microscopy (TEM) (Fig. 2B) revealed the formation of nanoscale PEDOT aggregates and the whiskers were inhomogeneously coated.
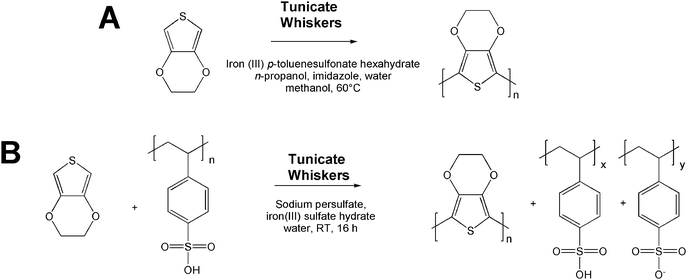 | ||
| Scheme 1 Synthesis of PEDOT-coated cellulose whiskers via the oxidative polymerization of EDOT with p-toluenesulfonate (A) and poly(styrenesulfonate) (B) as the dopant, in the presence of cellulose whiskers isolated from tunicates. | ||
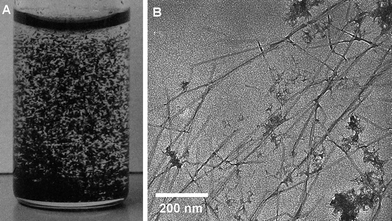 | ||
| Fig. 2 Photograph of PEDOT:TS-coated cellulose whiskers prepared at high concentration dispersed in water (A) and a transmission electron microscopy (TEM) image of PEDOT:TS-coated whiskers prepared under dilute concentration (B). | ||
We next explored poly(styrene sulfonic acid) (PSS) as a counterion (Scheme 1B), since PEDOT:PSS is normally formed and processed as a dispersion in water,39 with sodium persulfate as the main oxidant. Only a catalytic amount of iron is used, i.e. far less than required for the toluene sulfonate system described above. Initial experiments were carried out with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) EDOT
1 (w/w) EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio. Upon termination of the reaction, the product readily formed a homogeneous dispersion in either water or DMF upon sonication for 15 min. TEM images (Fig. 1) reveal individually coated whiskers with an average overall thickness of 30 nm, i.e. slightly thicker than the uncoated whiskers (23 nm). The coating appears to be homogeneous and the images are largely free of PEDOT and/or whisker aggregates. The thickness comparison suggests that the PEDOT:PSS-coated whiskers comprise similar volume fractions of electrically conducting coating and cellulose core. Dispersions of such whiskers in both DMF and water (2–5 mg mL−1) could readily be cast into thin films. Samples with a thickness of 0.5 μm produced in this manner had an average surface resistivity of 97 (±27) Ω/□ and a bulk conductivity of 7.9 (±2.4) S cm−1. These values are comparable to those of films of neat PEDOT:PSS made in a similar fashion, as well as literature values for PEDOT:PSS (200 Ω/□, 10 S cm−1).39,57,58 The choice of solvent (DMF or water) used to redisperse the whiskers and cast the films did not have an appreciable effect on either the film quality or properties.
whisker ratio. Upon termination of the reaction, the product readily formed a homogeneous dispersion in either water or DMF upon sonication for 15 min. TEM images (Fig. 1) reveal individually coated whiskers with an average overall thickness of 30 nm, i.e. slightly thicker than the uncoated whiskers (23 nm). The coating appears to be homogeneous and the images are largely free of PEDOT and/or whisker aggregates. The thickness comparison suggests that the PEDOT:PSS-coated whiskers comprise similar volume fractions of electrically conducting coating and cellulose core. Dispersions of such whiskers in both DMF and water (2–5 mg mL−1) could readily be cast into thin films. Samples with a thickness of 0.5 μm produced in this manner had an average surface resistivity of 97 (±27) Ω/□ and a bulk conductivity of 7.9 (±2.4) S cm−1. These values are comparable to those of films of neat PEDOT:PSS made in a similar fashion, as well as literature values for PEDOT:PSS (200 Ω/□, 10 S cm−1).39,57,58 The choice of solvent (DMF or water) used to redisperse the whiskers and cast the films did not have an appreciable effect on either the film quality or properties.
To further investigate the effect of the reaction conditions on the properties of films or coatings made from PEDOT:PSS-coated whiskers, the EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio was systematically varied. The PEDOT:PSS-coated whiskers thus produced were dispersed in DMF and cast into films with a thickness from 0.3 to 1.2 μm. The surface resistivity of these samples varied greatly with the EDOT:whisker ratio. It decreased dramatically from 25 MΩ/□ for an EDOT
whisker ratio was systematically varied. The PEDOT:PSS-coated whiskers thus produced were dispersed in DMF and cast into films with a thickness from 0.3 to 1.2 μm. The surface resistivity of these samples varied greatly with the EDOT:whisker ratio. It decreased dramatically from 25 MΩ/□ for an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 0.5
whisker ratio of 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) to 97 Ω/□ for an EDOT
1 (w/w) to 97 Ω/□ for an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) and remained constant at higher EDOT concentrations (Fig. 3). However, at EDOT
1 (w/w) and remained constant at higher EDOT concentrations (Fig. 3). However, at EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratios of >2
whisker ratios of >2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w), low-aspect-ratio aggregates—presumably PEDOT:PSS—formed, as seen in the TEM images (Fig. 1). Interestingly, the thickness of the PEDOT:PSS coating on the whiskers changed little between the different experimental conditions. Thus, it appears that a thin layer of PEDOT:PSS absorbs readily onto the whiskers, but the growth of the coating is not sustained. This might be due to attractive interactions between the iron ions and the whiskers, which might promote that the synthesis occurs predominantly at the whisker surface. After formation of an initial PEDOT:PSS layer, this effect is suppressed, leading (in case of an excess of EDOT) to conventional polymerization and the formation of PEDOT:PSS aggregates apart from the whiskers. In view of these observations, all subsequent experiments were conducted with whiskers coated with PEDOT:PSS by synthesis with an EDOT
1 (w/w), low-aspect-ratio aggregates—presumably PEDOT:PSS—formed, as seen in the TEM images (Fig. 1). Interestingly, the thickness of the PEDOT:PSS coating on the whiskers changed little between the different experimental conditions. Thus, it appears that a thin layer of PEDOT:PSS absorbs readily onto the whiskers, but the growth of the coating is not sustained. This might be due to attractive interactions between the iron ions and the whiskers, which might promote that the synthesis occurs predominantly at the whisker surface. After formation of an initial PEDOT:PSS layer, this effect is suppressed, leading (in case of an excess of EDOT) to conventional polymerization and the formation of PEDOT:PSS aggregates apart from the whiskers. In view of these observations, all subsequent experiments were conducted with whiskers coated with PEDOT:PSS by synthesis with an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w).
1 (w/w).
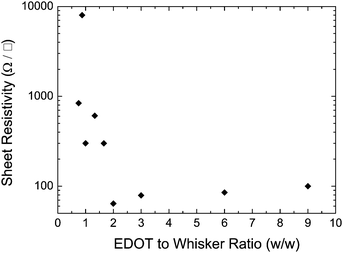 | ||
| Fig. 3 Surface resistivity of PEDOT:PSS-coated cellulose whisker films as a function of EDOT:whisker ratio in the reaction mixture. | ||
The nature of the coatings on the whiskers produced with the above protocol was investigated by infrared spectroscopy (Fig. 4). The characteristically weak PEDOT signals59 mostly overlap with those of the cellulose whiskers. However, the C–S stretch (829 cm−1) and the C–O–C stretch (1209 cm−1) are visible upon close inspection, suggesting that the conductive coating is indeed doped PEDOT.
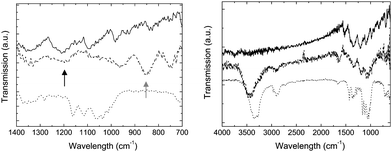 | ||
Fig. 4 Infrared spectra of PEDOT:PSS (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ), cellulose whiskers (⋯), and PEDOT:PSS-coated cellulose whiskers ( ), cellulose whiskers (⋯), and PEDOT:PSS-coated cellulose whiskers (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ). The black arrow points to the C–O–C stretch signal around 1207 cm−1 while the grey arrow points to the C–S stretch signal around 829 cm−1, which are both present in PEDOT but not cellulose. ). The black arrow points to the C–O–C stretch signal around 1207 cm−1 while the grey arrow points to the C–S stretch signal around 829 cm−1, which are both present in PEDOT but not cellulose. | ||
One important feature of PEDOT is its high transparency in the visible region. This makes PEDOT useful for several applications where low absorption is necessary, for example antistatic coatings on lenses and films.60 Ultra-violet visible spectroscopy was used to determine the transparency of films of the PEDOT:PSS-coated whiskers (Fig. 5). Significant absorption was not seen on a 250 nm thin film, i.e., one that has a thickness that is above the level where surface resistivity has levelled off (200 nm). A thick film (1200 nm) was also cast and displays distinctive absorption spectrum of PEDOT with a low around 400 nm confirming that the coating is indeed doped PEDOT.
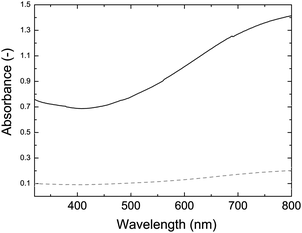 | ||
Fig. 5 UV-vis absorption spectra of a thick (1200 nm, solid) and thin (250 nm, dashed) PEDOT:PSS-coated cellulose whisker film (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w EDOT 1 w/w EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio). whisker ratio). | ||
Individually coated whiskers that can be dispersed in organic solvents such as DMF open up many processing options. One attractive feature is the high aspect ratio that leads to a very low percolation threshold when incorporating them into an insulating polymer. Theoretically this benefit can be seen in the power law for electrical conductivity:61,62
| σ = σo[φ − φc(α)]t(α) | (1) |
| ρ = ρo[φ − φc(α)]−t(α) | (2) |
To probe the usefulness of the present PEDOT:PSS-coated whiskers as the conducting nanofiller in electrically inert matrices, composite films were made by combining varying concentrations of whiskers and two different matrix polymers in a common solvent; water and DMF were combined with solutions of poly(ethylene oxide) (PEO) and polystyrene (PS) respectively. Films were then solution-cast and surface resistivity measurements were made (Fig. 6). The percolation threshold was calculated by dividing the known value for an aspect ratio of 1 (0.6)63 by the aspect ratio for the coated cellulose whiskers (64). A comparison between experimental and theoretical data (Fig. 6) shows an excellent agreement for both systems.
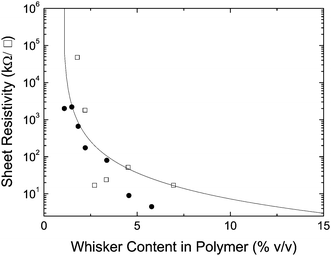 | ||
Fig. 6 Surface resistivity of nanocomposites of PEDOT:PSS-coated cellulose whiskers (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) EDOT 1 (w/w) EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio) and polystyrene (●) or poly(ethylene oxide) (□). The solid line represents the theoretical conductivity calculated by eqn (2). whisker ratio) and polystyrene (●) or poly(ethylene oxide) (□). The solid line represents the theoretical conductivity calculated by eqn (2). | ||
Our group has recently shown that neat cellulose whiskers can be gelled via a solvent-exchange method, e.g. by substituting water for acetone. Elimination of water as a competitive hydrogen bonding agent switches the strong interactions between the whiskers on, which causes the formation of percolating whisker networks, resulting in stable gels. This approach was here applied to PEDOT:PSS-coated whiskers with much success (Fig. 7). The materials made by gelating an aqueous dispersion by solvent exchange with acetone were dimensionally stable and electrically conductive. In a wet state, i.e. swollen with acetone, the gels have a surface resistivity of approximately 200 kΩ/□ and a bulk conductivity of ca. 6.6 e−6 S cm−1. After drying the gels into (rather inhomogeneous) films, the surface resistivity was found to be very similar to that of films made by casting PEDOT:whiskers, i.e. of the order of 100 Ω/□ (note that due to the irregular dimensions it was not possible to determine this value with high accuracy).
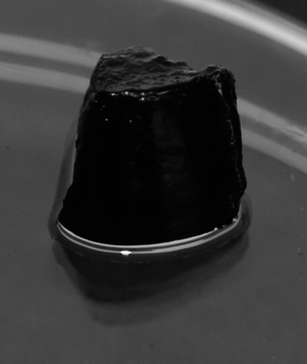 | ||
Fig. 7 A picture of a PEDOT:PSS-coated cellulose whisker gel produced from an 8 mg mL−1 aqueous dispersion of whiskers (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w EDOT 1 w/w EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio) via solvent-exchange with acetone. whisker ratio) via solvent-exchange with acetone. | ||
We have shown earlier that cellulose nanofibers can be processed into aerogels (either individually, as hybrids with clay, and optionally with a polymer binder) by lyophilization of aqueous dispersions, and we anticipated that it should be possible to process the present PEDOT:PSS-coated whiskers in a similar manner.66 Thus, exploratory experiments were conducted with cellulose whiskers that were coated with PEDOT:PSS by synthesis with an EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w). Aqueous dispersions with an initial whisker concentration of 6 mg mL−1 were thus freeze-dried as described in the Experimental section, resulting in mechanically stable, self-supporting, foam-like structures with a density of 6.4 mg cm−3. These aerogels (Fig. 8A) have a very similar appearance to aerogels made from the neat (uncoated) cellulose whiskers, except for the dark blue color imparted by the PEDOT:PSS coating. SEM images (Fig. 8B) of these aerogels revealed a layered architecture, in which the whiskers are assembled into thin individual sheets that are separated by large voids. The sheets follow the direction of the ice crystal growth. Overall, the images are very similar to those of aerogels produced from the neat cellulose whiskers.66 However, while the latter are electrical insulators, the present materials are electrically conductive. Surface resistivity measurements on the face of the aerogel yielded values of approximately 200 kΩ/□. The conductivity of the aerogels investigated here are similar to those of recently reported aerogels based on entangled cellulose nanofibers, which have been rendered conductive by coating with doped polyaniline.67
1 (w/w). Aqueous dispersions with an initial whisker concentration of 6 mg mL−1 were thus freeze-dried as described in the Experimental section, resulting in mechanically stable, self-supporting, foam-like structures with a density of 6.4 mg cm−3. These aerogels (Fig. 8A) have a very similar appearance to aerogels made from the neat (uncoated) cellulose whiskers, except for the dark blue color imparted by the PEDOT:PSS coating. SEM images (Fig. 8B) of these aerogels revealed a layered architecture, in which the whiskers are assembled into thin individual sheets that are separated by large voids. The sheets follow the direction of the ice crystal growth. Overall, the images are very similar to those of aerogels produced from the neat cellulose whiskers.66 However, while the latter are electrical insulators, the present materials are electrically conductive. Surface resistivity measurements on the face of the aerogel yielded values of approximately 200 kΩ/□. The conductivity of the aerogels investigated here are similar to those of recently reported aerogels based on entangled cellulose nanofibers, which have been rendered conductive by coating with doped polyaniline.67
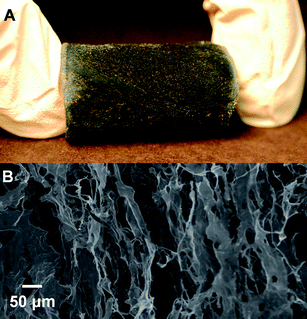 | ||
Fig. 8 (A) An image of a PEDOT:PSS-coated cellulose whisker aerogel produced from a 6 mg mL−1 aqueous dispersion of whiskers (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (w/w) EDOT 1 (w/w) EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio). (B) A SEM image of the same material. whisker ratio). (B) A SEM image of the same material. | ||
Conclusions
A potentially broadly useful technique for the manufacture of high aspect ratio conductive nanofibers was explored. It was found that by polymerizing EDOT in the presence of cellulose whiskers, individually coated whiskers could be synthesized. In a bulk state, these coated whiskers have a surface resistivity very similar to PEDOT. These coated whiskers can be incorporated into other polymer matrices and demonstrate a very low percolation threshold yielding electrically conductive composites at as low as 2% loading. The coated whiskers can also be made into a variety of gels. Using a sol–gel method, gels can be formed that are electrically conductive. Aerogels can also be made from coated whiskers via a freeze-drying method. These aerogels have a low density around 6.4 mg cm−3 while maintaining a surface resistivity of approximately 200 kΩ/□. These are just a few examples of the many possible uses for coated whiskers. These coated whiskers, made from very abundant and renewable cellulose, make it possible to achieve the same properties as PEDOT with much less material.Experimental
Materials and methods
All materials and reagents were of highest available purity and were purchased from Sigma Aldrich. 3,4-Ethylenedioxythiophene was further purified by vacuum distillation.Ultraviolet–visible (UV–vis) absorption spectra were obtained on a Perkin Elmer Lambda 800 spectrometer. Transmission electron micrographs (TEMs) were acquired using a JEOL 1200EX Transmission Electron Microscope. All TEM samples were prepared by deposition of 20 μL of the whisker dispersions onto carbon-coated copper grids. Scanning electron micrographs (SEMs) were acquired with a Philips model XL-30 Environmental SEM without any additional coating. Whisker diameter and length were averaged for at least 50 whiskers over 3 images. For ultrasonication of whisker dispersions a Fischer Scientific FS60H ultrasonic bath was employed. For surface resistivity measurements a Keithley 2400 series source measure unit was used in combination with prefabricated copper electrodes, consisting of self-adhesive copper strips fixed onto a microscope slide with spacing of 0.3 cm. For electrical conductivity measurements the samples (film deposited by solution casting onto glass microscopy slides) were pressed against the prefabricated electrode, using a metal clamp. IR spectra were recorded using an ABB Bomen MB series FTIR spectrometer. Freeze-drying was done in a VirTis AdVantage® EL-85 freeze-dryer.
Preparation of PEDOT-coated cellulose whiskers
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 water–methanol mixture and the dispersion was again centrifuged. This procedure was repeated twice; in the final step water was employed instead of a water–methanol mixture. The resulting dark blue, wet paste was re-dispersed in either water (20 mL) or DMF (30 mL) by ultrasonication for 2 h. The volume of the DMF dispersion was subsequently reduced to 20 mL under vacuum to remove the remaining water. The concentrations of both aqueous and DMF dispersions (approx. 1–5 mg mL−1) were determined by solution-casting a known volume of the dispersion, drying and weighing the resulting film.
1 water–methanol mixture and the dispersion was again centrifuged. This procedure was repeated twice; in the final step water was employed instead of a water–methanol mixture. The resulting dark blue, wet paste was re-dispersed in either water (20 mL) or DMF (30 mL) by ultrasonication for 2 h. The volume of the DMF dispersion was subsequently reduced to 20 mL under vacuum to remove the remaining water. The concentrations of both aqueous and DMF dispersions (approx. 1–5 mg mL−1) were determined by solution-casting a known volume of the dispersion, drying and weighing the resulting film.
The above procedure for a EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w was adapted to EDOT/whisker ratios of between 0.25
1 w/w was adapted to EDOT/whisker ratios of between 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1
1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9 w/w by appropriately adjusting the amount of EDOT, sodium persulfate, and iron(III) chloride, while the amounts of cellulose whiskers and water were kept unchanged.
9 w/w by appropriately adjusting the amount of EDOT, sodium persulfate, and iron(III) chloride, while the amounts of cellulose whiskers and water were kept unchanged.
The above procedure was adapted to a lower methanol concentration with all the same reactants but at different concentrations with a slightly different protocol. Iron(III) p-toluenesulfonate hexahydrate (0.60 g, 0.90 mmol), cellulose whiskers (0.025 g), and water (50.0 mL) were combined in one flask. EDOT (0.050 g, 0.33 mmol) was dissolved in a small amount of methanol (∼1 mL) and the mixture was added to the reaction flask; the reaction mixture was then stirred for 16 h at 60 °C. The resulting dispersion was centrifuged, the supernatant solution was decanted, the residue was washed with methanol, and the dispersion was again centrifuged. This procedure was repeated twice; in the final step water was employed instead of methanol. The resulting wet paste was re-dispersed in water (20 mL) by ultrasonication for 4 h. The concentration (approx. 1–5 mg ml−1) of the dispersion was obtained by solution casting a known volume and weighing the resulting film. The overall concentration of the reaction was also varied by changing the amount of methanol from 10 mL to 100 mL.
Both above procedures for an EDOT/whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w were adapted to EDOT/whisker ratios of between 0.5
1 w/w were adapted to EDOT/whisker ratios of between 0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 4
1 and 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w by appropriately adjusting the amount of EDOT and iron(III) p-toluenesulfonate hexahydrate, while the amounts of cellulose whiskers and water were kept unchanged.
1 w/w by appropriately adjusting the amount of EDOT and iron(III) p-toluenesulfonate hexahydrate, while the amounts of cellulose whiskers and water were kept unchanged.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) in DMF (0.6–1.0% w/w) were prepared by diluting stock dispersions and sonicated for an additional 10 min in 20 mL glass vials. Acetone was then carefully added to the vials with care being taken not to disturb the surface of the whisker layer. The top layer of acetone was exchanged twice a day for three days against fresh acetone. After this time the excess solvent was decanted off and the gel that had formed was released from the vial.
1 w/w) in DMF (0.6–1.0% w/w) were prepared by diluting stock dispersions and sonicated for an additional 10 min in 20 mL glass vials. Acetone was then carefully added to the vials with care being taken not to disturb the surface of the whisker layer. The top layer of acetone was exchanged twice a day for three days against fresh acetone. After this time the excess solvent was decanted off and the gel that had formed was released from the vial.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) dispersed in water (0.6% w/w) were placed into PET or glass vials and the dispersions were frozen in a dry ice/ethanol bath. The samples were then freeze dried using a VirTis AdVantage EL-85 lyophilizer with an initial shelf temperature of −10 °C, condenser temperature of −87 °C and eventual vacuum of <10 μbar. The sample tray was placed on the shelf and the vacuum was turned on and allowed to reach 1 mbar before the shelf began heating to 25 °C. Samples were left in the lyophilizer for 4 days to ensure complete drying; they were subsequently stored in a desiccator.
1 w/w) dispersed in water (0.6% w/w) were placed into PET or glass vials and the dispersions were frozen in a dry ice/ethanol bath. The samples were then freeze dried using a VirTis AdVantage EL-85 lyophilizer with an initial shelf temperature of −10 °C, condenser temperature of −87 °C and eventual vacuum of <10 μbar. The sample tray was placed on the shelf and the vacuum was turned on and allowed to reach 1 mbar before the shelf began heating to 25 °C. Samples were left in the lyophilizer for 4 days to ensure complete drying; they were subsequently stored in a desiccator.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) were added to 1 mL of the polymer solution under vigorous mixing. The resulting mixture was then ultrasonicated for 10 min. before being cast onto a glass slide in several 1 mL portions. The films were allowed to dry at ambient temperature for approximately 3 h. After this time, copper strips (with 0.3 cm spacing) were thermally evaporated onto the films (greater than 5 μm) to serve as contact points for resistivity measurements.
1 w/w) were added to 1 mL of the polymer solution under vigorous mixing. The resulting mixture was then ultrasonicated for 10 min. before being cast onto a glass slide in several 1 mL portions. The films were allowed to dry at ambient temperature for approximately 3 h. After this time, copper strips (with 0.3 cm spacing) were thermally evaporated onto the films (greater than 5 μm) to serve as contact points for resistivity measurements.
Polystyrene nanocomposites were made in a similar fashion. Polystyrene was dissolved in dimethylformamide (DMF) at a concentration of 20 mg mL−1. Between 0.02 and 0.4 mL of a dispersion of PEDOT:PSS-coated cellulose whiskers (5 mg mL−1, EDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) whisker ratio of 2
whisker ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w) were added to 1 mL of the polymer solution under vigorous mixing. The resulting mixture was then ultrasonicated for 10 min. before being cast onto a glass slide in several 1 mL portions. The films were allowed to dry at ambient temperature for approximately 5 h. After this time, copper strips (with 0.3 cm spacing) were thermally evaporated onto the films (greater than 5 μm) to serve as contact points for resistivity measurements.
1 w/w) were added to 1 mL of the polymer solution under vigorous mixing. The resulting mixture was then ultrasonicated for 10 min. before being cast onto a glass slide in several 1 mL portions. The films were allowed to dry at ambient temperature for approximately 5 h. After this time, copper strips (with 0.3 cm spacing) were thermally evaporated onto the films (greater than 5 μm) to serve as contact points for resistivity measurements.
The volume fraction of PEDOT-coated whiskers in these nanocomposites was calculated from the weight fraction, the densities of the neat polymers (poly(ethylene oxide): 1.13 g cm−3, polystyrene: 1.05 g cm−3), and the density of the PEDOT-coated cellulose whiskers (1.29 g cm−3), which was estimated from the density of crystalline cellulose (1.58 g cm−3) and PEDOT (1.0 g cm−3).
Acknowledgements
This material is based upon work supported by the National Science Foundation under Grant No. DMR-0804874. We thank Mathew Gawryla for help with acquisition of the SEM images and Ben Elder for assistance with the initial experiments.References
- D. Y. Godovsky, A. E. Varfolomeev, D. F. Zaretsky, R. L. N. Chandrakanthi, A. Kündig, C. Weder and W. Caseri, J. Mater. Chem., 2001, 11, 2465–2469 RSC.
- W. U. Huynh, X. G. Peng and A. P. Alivisatos, Adv. Mater., 1999, 11, 923 CrossRef CAS.
- B. O’Regan and M. Gratzel, Nature, 1991, 353, 737–740 CrossRef CAS.
- G. Yu, J. Gao, J. C. Hummelen, F. Wudl and A. J. Heeger, Science, 1995, 270, 1789–1791 CrossRef CAS.
- J. J. M. Halls, C. A. Walsh, N. C. Greenham, E. A. Marseglia, R. H. Friend, S. C. Moratti and A. B. Holmes, Nature, 1995, 376, 498–500 CrossRef CAS.
- N. C. Greenham, X. G. Peng and A. P. Alivisatos, Phys. Rev. B: Condens. Matter, 1996, 54, 17628–17637 CrossRef CAS.
- J. Y. Kim, K. Lee, N. E. Coates, D. Moses, T. Q. Nguyen, M. Dante and A. J. Heeger, Science, 2007, 317, 222–225 CrossRef CAS.
- P. M. Ajayan and J. M. Tour, Nature, 2007, 447, 1066–1068 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105–1136 CrossRef CAS.
- P. M. Ajayan, L. S. Schadler, C. Giannaris and A. Rubio, Adv. Mater., 2000, 12, 750 CrossRef CAS.
- C. Schmitz, P. Pösch, M. Thelakkat, H. W. Schmidt, A. Montali, K. Feldman, P. Smith and C. Weder, Adv. Funct. Mater., 2001, 11, 41–46 CrossRef CAS.
- L. Flandin, G. Bidan, Y. Brechet and J. Y. Cavaille, Polym. Compos., 2000, 21, 165–174 Search PubMed.
- M. Ginic-Markovic, J. G. Matisons, R. Cervini, G. P. Simon and P. M. Fredericks, Chem. Mater., 2006, 18, 6258–6265 CrossRef CAS.
- S. Kirkpatrick, Rev. Mod. Phys., 1973, 45, 574–588 CrossRef.
- D. Stauffer and A. Aharony, An Introduction to Percolation Theory, Taylor & Francis, Washington, 1991 Search PubMed.
- O. T. Ikkala, J. Laakso, K. Vakiparta, E. Virtanen, H. Ruohonen, H. Jarvinen, T. Taka, P. Passiniemi, J. E. Osterholm, Y. Cao, A. Andreatta, P. Smith and A. J. Heeger, Synth. Met., 1995, 69, 97–100 CrossRef CAS.
- M. Omastova, I. Chodak and J. Pionteck, Synth. Met., 1999, 102, 1251–1252 CrossRef CAS.
- A. Bhattacharya and A. De, J. Macromol. Sci., Rev. Macromol. Chem. Phys., 1999, C39, 17–56 Search PubMed.
- H. Sirringhaus, R. J. Wilson, R. H. Friend, M. Inbasekaran, W. Wu, E. P. Woo, M. Grell and D. D. C. Bradley, Appl. Phys. Lett., 2000, 77, 406–408 CrossRef CAS.
- C. Weder, C. Sarwa, A. Montali, G. Bastiaansen and P. Smith, Science, 1998, 279, 835–837 CrossRef CAS.
- C. Weder, C. Sarwa, C. Bastiaansen and P. Smith, Adv. Mater., 1997, 9, 1035 CrossRef CAS.
- J. Huang, S. Virji, B. H. Weiller and R. B. Kaner, Chem.–Eur. J., 2004, 10, 1314–1319 CrossRef CAS.
- R. J. Tseng, J. X. Huang, J. Ouyang, R. B. Kaner and Y. Yang, Nano Lett., 2005, 5, 1077–1080 CrossRef CAS.
- S. I. Cho, W. J. Kwon, S. J. Choi, P. Kim, S. A. Park, J. Kim, S. J. Son, R. Xiao, S. H. Kim and S. B. Lee, Adv. Mater., 2005, 17, 171 CrossRef CAS.
- S. J. Choi and S. M. Park, Adv. Mater., 2000, 12, 1547–1549 CrossRef CAS.
- J. X. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817–5821 CrossRef CAS.
- J. X. Huang and R. B. Kaner, J. Am. Chem. Soc., 2004, 126, 851–855 CrossRef CAS.
- L. H. Meng, Y. Lu, X. D. Wang, J. Zhang, Y. Q. Duan and C. X. Li, Macromolecules, 2007, 40, 2981–2983 CrossRef CAS.
- Y. Y. Wang, X. L. Jing and J. H. Kong, Synth. Met., 2007, 157, 269–275 CrossRef CAS.
- X. Sun and M. Hagner, Macromolecules, 2007, 40, 8537–8539 CrossRef CAS.
- K. B. Jirage, J. C. Hulteen and C. R. Martin, Science, 1997, 278, 655–658 CrossRef CAS.
- M. Steinhart, S. Zimmermann, P. Goring, A. K. Schaper, U. Gosele, C. Weder and J. H. Wendorff, Nano Lett., 2005, 5, 429–434 CrossRef CAS.
- M. Steinhart, S. Murano, A. K. Schaper, T. Ogawa, M. Tsuji, U. Gösele, C. Weder and J. H. Wendorff, Adv. Funct. Mater., 2005, 15, 1656–1664 CrossRef CAS.
- J. S. Liu, E. Sheina, T. Kowalewski and R. D. McCullough, Angew. Chem., Int. Ed., 2002, 41, 329 CrossRef CAS.
- I. D. Norris, M. M. Shaker, F. K. Ko and A. G. MacDiarmid, Synth. Met., 2000, 114, 109–114 CrossRef CAS.
- S. Y. Jang, V. Seshadri, M. S. Khil, A. Kumar, M. Marquez, P. T. Mather and G. A. Sotzing, Adv. Mater., 2005, 17, 2177–2180 CrossRef CAS.
- Y. Q. Wang, J. S. Park, J. P. Leech, S. Miao and U. H. F. Bunz, Macromolecules, 2007, 40, 1843–1850 CrossRef CAS.
- S. Changsarn, J. D. Mendez, C. Weder and P. Supaphol, Macromol. Mater. Eng., 2008, 293, 952–963 CrossRef CAS.
- B. L. Groenendaal, F. Jonas, D. Freitag, H. Pielartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481–494 CrossRef CAS.
- F. Louwet, L. Groenendaal, J. Dhaen, J. Manca, J. Van Luppen, E. Verdonck and L. Leenders, Synth. Met., 2003, 135–136, 115–117 CrossRef CAS.
- J. L. Duvail, P. Retho, S. Garreau, G. Louarn, C. Godon and S. Demoustier-Champagne, Synth. Met., 2002, 131, 123–128 CrossRef CAS.
- M. H. Bolin, K. Svennersten, X. J. Wang, I. S. Chronakis, A. Richter-Dahlfors, E. W. H. Jager and M. Berggren, Sens. Actuators, B, 2009, 142, 451–456 CrossRef.
- O. Martinez, A. G. Bravo and N. J. Pinto, Macromolecules, 2009, 42, 7924–7929 CrossRef CAS.
- S. Nair, E. Hsiao and S. H. Kim, Chem. Mater., 2009, 21, 115–121 CrossRef CAS.
- S. Bhandari, M. Deepa, A. K. Srivastava, C. Lal and R. Kant, Macromol. Rapid Commun., 2008, 29, 1959–1964 CrossRef CAS.
- H. T. Ham, Y. S. Choi, M. G. Chee, M. H. Cha and I. J. Chung, Polym. Eng. Sci., 2008, 48, 1–10 CrossRef CAS.
- G. F. Wang, X. M. Tao and R. X. Wang, 2008.
- W. Baik, W. Q. Luan, R. H. Zhao, S. Koo and K. S. Kim, Synth. Met., 2009, 159, 1244–1246 CrossRef CAS.
- M. M. D. Lima and R. Borsali, Macromol. Rapid Commun., 2004, 25, 771–787 CrossRef.
- O. van den Berg, M. Schroeter, J. R. Capadona and C. Weder, J. Mater. Chem., 2007, 17, 2746–2753 RSC.
- S. Padalkar, J. R. Capadona, S. J. Rowan, C. Weder, Y.-H. Won, L. A. Stanciu and R. J. Moon, Langmuir, 2010, 26, 8497 CrossRef CAS.
- O. van den Berg, J. R. Capadona and C. Weder, Biomacromolecules, 2007, 8, 1353–1357 CrossRef CAS.
- J. R. Capadona, O. van den Berg, L. A. Capadona, M. Schroeter, S. J. Rowan, D. J. Tyler and C. Weder, Nat. Nanotechnol., 2007, 2, 765–769 CrossRef CAS.
- A. Sturcova, G. R. Davies and S. J. Eichhorn, Biomacromolecules, 2005, 6, 1055–1061 CrossRef CAS.
- J. R. Capadona, K. Shanmuganathan, S. Trittschuh, S. Seidel, S. J. Rowan and C. Weder, Biomacromolecules, 2009, 10, 712–716 CrossRef CAS.
- Y. H. Ha, N. Nikolov, S. K. Pollack, J. Mastrangelo, B. D. Martin and R. Shashidhar, Adv. Funct. Mater., 2004, 14, 615–622 CrossRef CAS.
- G. A. Sotzing, J. R. Reynolds and P. J. Steel, Adv. Mater., 1997, 9, 795 CrossRef CAS.
- A. N. Aleshin, S. R. Williams and A. J. Heeger, Synth. Met., 1998, 94, 173–177 CrossRef CAS.
- J. W. Choi, M. G. Han, S. Y. Kim, S. G. Oh and S. S. Im, Synth. Met., 2004, 141, 293–299 CrossRef CAS.
- S. Kirchmeyer and K. Reuter, J. Mater. Chem., 2005, 15, 2077–2088 RSC.
- C. S. Lu and Y. W. Mai, J. Mater. Sci., 2008, 43, 6012–6015 CrossRef CAS.
- H. Randriamahazaka, F. Vidal, P. Dassonville, C. Chevrot and D. Teyssie, Synth. Met., 2002, 128, 197–204 CrossRef CAS.
- D. S. McLachlan, M. Blaszkiewicz and R. E. Newnham, J. Am. Ceram. Soc., 1990, 73, 2187–2203.
- Y. C. Wang and C. Anderson, Macromolecules, 1999, 32, 6172–6179 CrossRef CAS.
- Q. Yuan and D. Y. Wu, J. Appl. Polym. Sci., 2010, 115, 3527–3534 CrossRef CAS.
- M. D. Gawryla, O. van den Berg, C. Weder and D. A. Schiraldi, J. Mater. Chem., 2009, 19, 2118–2124 RSC.
- M. Paakko, J. Vapaavuori, R. Silvennoinen, H. Kosonen, M. Ankerfors, T. Lindstrom, L. A. Berglund and O. Ikkala, Soft Matter, 2008, 4, 2492–2499 RSC.
- V. Favier, H. Chanzy and J. Y. Cavaille, Macromolecules, 1995, 28, 6365–6367 CrossRef CAS.
- V. Favier, G. R. Canova, S. C. Shrivastava and J. Y. Cavaille, Polym. Eng. Sci., 1997, 37, 1732–1739 CrossRef CAS.
- V. Favier, G. R. Canova, J. Y. Cavaille, H. Chanzy, A. Dufresne and C. Gauthier, Polym. Adv. Technol., 1995, 6, 351–355 CrossRef CAS.
- H. H. Yuan, Y. Nishiyama, M. Wada and S. Kuga, Biomacromolecules, 2006, 7, 696–700 CrossRef CAS.
- E. M. Arndt, M. D. Gawryla and D. A. Schiraldi, J. Mater. Chem., 2007, 17, 3525–3529 RSC.
| This journal is © The Royal Society of Chemistry 2010 |
