Solvent-driven swelling and shrinking of poly(NIPAM) gels crosslinked by tris-methacrylated phloroglucinol derivatives
Toshio
Itahara
*,
Takahiro
Tsuchida
and
Mayumi
Morimoto
Faculty of Engineering, Kagoshima University, Korimoto, Kagoshima, 890-0065, Japan. E-mail: itahara@be.kagoshima-u.ac.jp; Fax: +81 099-285-8208; Tel: +81 099-285-8208
First published on 20th May 2010
Abstract
Poly(N-isopropylacrylamide) gels, crosslinked by tris-methacrylated derivatives of phloroglucinol, were prepared. The gels did not exhibit large water absorption, but absorbed a large amount of ethanol and acetic acid. When the gels, swollen with ethanol and acetic acid, were placed in water at 40 °C, they quickly shrank.
Introduction
Gels are characterized by their three-dimensional microscopic network structure. Much attention has been denoted to gels' ability to modify their shapes in response to external stimuli,1–11 and the ability is related to the network structure. Multi-functional compounds are important crosslinkers for the preparation of the networked polymer gels. Here, we synthesized new tris-methacrylated derivatives of phloroglucinol, 1,3,5-tris-(ω-methacryloyloxyalkyloxy)benzenes (TMAOBn). This work is motivated by a presumption that TMAOBn can result in crosslinking towards three specific directions, forming an orderly three-dimensional network. On the other hand, poly(N-isopropylacrylamide) [poly(NIPAM)] is a well-known thermoresponsive polymer with a lower critical solution temperature of around 32 °C,12 and is an increasingly interesting candidate for applications to sensors, actuators, and drug delivery systems among others in recent years.13–28 Therefore, poly(NIPAM) crosslinked by TMAOBn, poly(N-Tn), was prepared. The polymeric material did not exhibit large water absorption, but absorbed a large amount of alcohols and acetic acid, swelling into a multiple of its initial size and weight. Interestingly, the gels swollen with ethanol and acetic acid quickly shrank when placed in water. In this paper we report simple and efficient swelling and shrinking behaviors of poly(N-Tn) gels driven by solvent and temperature.Results and discussion
Tris-methacrylated compounds TMAOBn (n = 4, 5 and 6) were synthesized according to the procedure shown in Scheme 1. The compounds were soluble in many organic solvents such as benzene, toluene and THF. Therefore, TMAOBn may be applicable to the crosslinker for various polymeric materials. Radical polymerization of NIPAM containing TMAOBn (2.5, 5, 10, 20, and 30 wt%) in toluene gave poly(N-Tn-2.5%, 5%, 10%, 20%, and 30%) (n = 4, 5 and 6).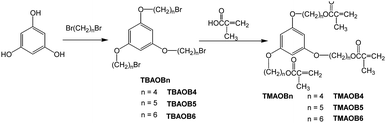 | ||
| Scheme 1 Synthesis of TMAOBn (n = 4, 5 and 6). | ||
Fig. 1a shows a comparison of the swelling behaviors of poly(N-T6-2.5%, 5%, 10%, 20% and 30%), which are the copolymers of NIPAM and TMAOB6 (2.5, 5, 10, 20, and 30 wt%), in water, methanol, ethanol, 1-propanol, 2-propanol, 1-butanol and acetic acid at room temperature after 24 hours. The swelling behaviors of poly(N-T4) and poly(N-T5) were similar to those of poly(N-T6). Measured values of water uptake (Q) of poly(N-Tn-2.5%) (n = 4, 5 and 6) were between 4.5 and 4.8, in contrast with the Q of poly(N-Tn-30%), revolving around 0.7–0.8. Poly(N-Tn-20% and 30%) absorbed only a small amount of water. The values of Q in water were remarkably dependent on the amounts of TMAOBn. In addition, we prepared poly(NIPAM)s crosslinked by trimethylpropane trimethacrylate (TMPTMA) and pentaerythriol tetraacrylate (PETTA) (5 and 10 wt%), and compared their swelling behaviors with those of poly(N-Tn-5% and 10%). We measured the values of Q of the copolymers crosslinked by TMPTMA and PETTA in water to be approximately 5 times larger than those of poly(N-Tn). These results suggest that the presence of TMAOBn has a significant influence on the swelling behaviors of poly(N-Tn) in water. The results may be explained on the basis of a moderate hydrophobic property of the aromatic core in the crosslinking agent. In addition, we think that the 1,3,5-trifunctional aromatic core results in crosslinking towards three specific directions and disperses into the polymer matrix. Therefore, the water uptake is remarkably dependent on the ratio of TMAOBn to NIPAM. On the other hand, TMPTMA and PETTA may bring about crosslinking in random directions and not exert an obvious influence on the characteristic of poly(NIPAM).
 | ||
| Fig. 1 Swelling behaviors of poly(N-T6) at room temperature. (a) Solvent uptake (Q) of poly(N-T6-2.5%, 5%, 10%, 20% and 30%) for 24 hours. (b) Solvent uptake (Q) as a function of time for swelling behaviors of poly(N-T6-2.5%) in water (▲), ethanol (■) and acetic acid (●). (c) Dependence of the solvent uptake (Q) on ROH concentration (vol%) for poly(N-T6-2.5%) in mixtures of ROH and water for 24 hours. ROH = ethanol (■). ROH = acetic acid (●). | ||
Poly(N-Tn) could absorb alcohols and acetic acid much better than they could absorb water. However, we did not observe any notable differences in the swelling behaviors among the alcohols used for this experiment. The values of Q in alcohols and acetic acid were dependent on the ratio of TMAOBn to NIPAM. Specifically, increasing the amount of TMAOBn resulted in a decrease in the gels' swelling ability. For poly(N-T6-2.5%), we measured Q = 26.1 in ethanol, yet the swollen gel was sticky after a 24 hour immersion. On the other hand, the respective values of Q of poly(N-T6-20%) and poly(N-T6-30%) were 13.2 and 11.5 in ethanol, and the swollen gels were slightly sticky. The gels maintained their shapes in the swollen state for more than 7 days. The value of Q of poly(N-T6-2.5%) in acetic acid reached 31.2 after 24 hours, although the swollen gel was very sticky. Fig. 1b shows solvent uptake (Q)–time plots for the swelling behaviors of poly(N-T6-2.5%) in water, ethanol and acetic acid. The gels markedly swelled for 1 hour. Furthermore, we studied the solvent dependence of the swelling behaviors of poly(N-T6-2.5%) in mixtures of ROH (ethanol and acetic acid) and water (Fig. 1c). The addition of 20% of water into ROH decreased the values of Q in about half of those in ROH, and the swelling behaviors of the mixtures of ROH (40%) and water (60%) were similar to those observed in water.
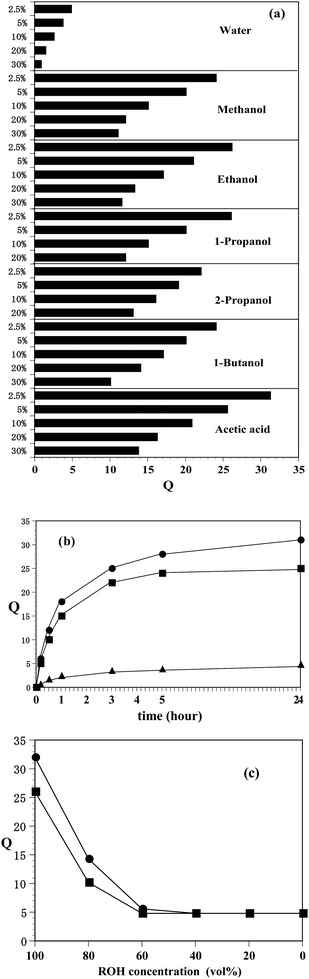
The temperature dependence of the swelling ratio (%) of poly(N-T6-5%) gels swollen with water, ethanol, and acetic acid is shown in Fig. 2. Here, the temperature was increased at a rate of 1.0 °C min−1. As can be seen from the pictures shown in Fig. 2, a moderate amount of water was expelled from the hydrogel upon increasing the temperature above 32 °C. The hydrogel shrank quickly as water was expelled. Furthermore, to make sure the influence of the thickness of the hydrogel on the temperature dependence, we prepared poly(N-T6-5% and 10%) samples that were sliced thinly. Water was released from the thin samples upon increasing the temperature above 31 °C. Recently increasing interest is being shown in rapid shrinking behaviors of poly(NIPAM) hydrogels driven by temperature in connection with drug delivery systems.20–28Fig. 2 shows an efficient shrinking behavior of poly(N-T6-5%) driven by temperature. On the other hand, the organogels swollen with ethanol and acetic acid hardly shrank at a 22 °C to 42 °C temperature change. The difference between hydrogels and organogels was confirmed by DSC thermograms at a 1.0 °C min−1 heating rate (Fig. 3). The temperatures at onset points of the DSC endotherm of poly(N-T6-2.5%) and poly(N-T6-30%) hydrogels were 30.5 °C and 30.0 °C, respectively. On the basis of these data, it may be concluded that the lower critical solution temperature of poly(N-T6) hydrogel is 30–31 °C. However, we were not able to identify the lower critical solution temperature on the DSC thermograms of poly(N-Tn) (n = 4, 5 and 6) organogels swollen with ethanol and acetic acid at a 22 °C to 60 °C temperature change.
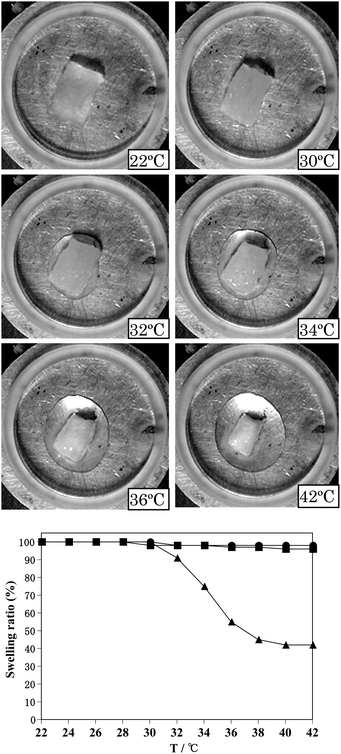 | ||
| Fig. 2 Temperature dependence of swelling ratio (%) of poly(N-T6-5%) gels swollen with water (▲), ethanol (■) and acetic acid (●). The temperature increase rate was 1.0 °C min−1. The swelling ratios (%) were determined by the areas of the gels in those at 22 °C. Pictures show the hydrogel at 22 °C, 30 °C, 32 °C, 34 °C, 36 °C and 42 °C. | ||
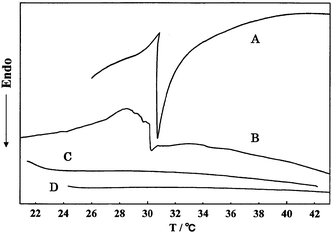 | ||
| Fig. 3 DSC thermograms of poly(N-T6) gels at a heating rate of 1.0 °C min−1. A: Poly(N-T6-2.5%) hydrogel. B: Poly(N-T6-30%) hydrogel. C: Poly(N-T6-5%) organogel swollen with ethanol. D: Poly(N-T6-5%) organogel swollen with acetic acid. | ||
When the organogels, swollen in ethanol and acetic acid, were placed in water, they quickly shrank. The shrinking process occurred at a much faster rate than swelling in ethanol and acetic acid. Fig. 4 shows the changes in Q of swelling in ROH (ethanol and acetic acid) and shrinking in water for poly(N-T6-10%). A dry poly(N-T6-10%) (A in Fig. 4) was immersed in acetic acid at room temperature for 24 hours. When the resulting organogel (B in Fig. 4) (Q = 20.8) was placed in water at 40 °C, it shrank almost completely in 10 minutes (D in Fig. 4) (Q = 1.7). On the other hand, when the organogel was immersed in water at room temperature, the value of Q of the shrunken gel after 10 minutes was 3.5. We observed the gel to become very foamy in the shrinking process in water (C in Fig. 4), because of a rapid solvent exchange between ROH and water in the gel. The gel swollen with ethanol (Q = 17.1) also quickly shrank when placed in water at 40 °C.
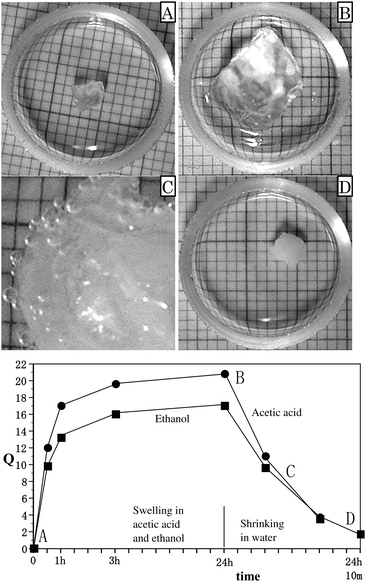 | ||
| Fig. 4 Solvent uptake (Q) of swelling in ROH at room temperature and shrinking in water at 40 °C as a function of time plots for poly(N-T6-10%). ROH = ethanol (■). ROH = acetic acid (●). (A) A dried gel. B: Sample A swollen in acetic acid at room temperature for 24 hours. C: Sample B in the shrinking process in water after 5 minutes (enlarged picture). D: Sample B shrunken in water at 40 °C for 10 minutes. | ||
Given the aforementioned results, it was interesting to explore the application of poly(N-Tn) gels to actuators driven by solvents. We repeatedly tested the swelling behaviors of poly(N-T6-20%) in ROH (ethanol and acetic acid) for 10 minutes, followed by shrinking in water for 10 minutes at room temperature (Fig. 5). A dry gel was immersed in ethanol for 10 minutes. The resulting swollen gel was placed in water and allowed to shrink for 10 minutes. The swelling behavior of poly(N-T6) in ROH and the shrinking behavior of the resultant organogel in water could be repeated over many iterations. Although the gels did not absorb a large amount of ethanol and acetic acid for 10 minutes, the gel maintained its shape with good fidelity in both swollen and shrunken states. Given their properties, especially the remarkable shrinking behaviors, such gels may have numerous applications to actuators, sensors, and drug delivery systems driven by solvents and temperature.
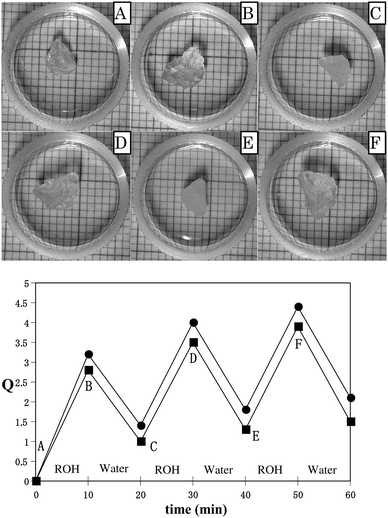 | ||
| Fig. 5 Solvent-driven swelling and shrinking of poly(N-T6-20%) at room temperature. ROH = ethanol (■). ROH = acetic acid (●). Pictures B–F show the swollen and shrunken gels in ethanol and water. | ||
Experimental
The 1H NMR spectra (400 MHz) were obtained with a JEOL GSX 400 spectrometer. The chemical shifts (δ values) were measured in parts per million (ppm) downfield from tetramethylsilane as an internal reference. The IR spectra were recorded with a JASCO FT/IR-420 spectrometer. The measurements in CDCl3 were made with a 0.1 mm KBr cell. FAB mass data were obtained with a Hitachi JMS-700 spectrometer. Differential scanning calorimetry (DSC) measurements were carried out with a Shimadzu DSC-60 calorimeter. Heating experiments were performed with Linkam LK-600PH. NIPAM, TMPTMA and PETTA were obtained from Aldrich Chemical Co.1,3,5-Tris-(ω-bromoalkyloxy)benzene (TBAOBn)
A mixture of phloroglucinol (1.0 mmol) and Br(CH2)nBr (n = 4, 5 and 6) (4.0 mmol) in N,N-dimethylformamide (50 mL) in the presence of K2CO3 (4.0 mmol) was stirred for 24 hours at room temperature. The reaction mixture was evaporated to give a residue, which was chromatographed over silica gel. Elution of a mixture of hexane and chloroform (4 : 6) gave TBAOBn (n = 4, 5 and 6).TBAOB4: oil, 20% yield based on the amount of phloroglucinol used. 1H NMR (CDCl3) δ = 6.05 (s, 3H, Ph), 3.95 (t, 6H, J = 6 Hz, CH2O), 3.48 (t, 6H, J = 6 Hz, CH2Br), 2.06 (quintet, 6H, J = 6 Hz, CH2), 1.92 (quintet, 6H, J = 6 Hz, CH2). IR (CDCl3) 2945, 2877, 1601, 1464, 1387, 1248, 1153, 1067 cm−1.
TBAOB5: oil, 18% yield based on the amount of phloroglucinol used. 1H NMR (CDCl3) δ = 6.05 (s, 3H, Ph), 3.92 (t, 6H, J = 7 Hz, CH2O), 3.44 (t, 6H, J = 7 Hz, CH2Br), 1.93 (quintet, 6H, J = 7 Hz, CH2), 1.79 (quintet, 6H, J = 7 Hz, CH2), 1.61 (quintet, 6H, J = 7 Hz, CH2). IR (CDCl3) 2941, 2862, 1599, 1462, 1387, 1244, 1157, 1063 cm−1.
TBAOB6: oil, 18% yield based on the amount of phloroglucinol used. 1H NMR (CDCl3) δ = 6.05 (s, 3H, Ph), 3.91 (t, 6H, J = 7 Hz, CH2O), 3.42 (t, 6H, J = 7 Hz, CH2Br), 1.89 (quintet, 6H, J = 7 Hz, CH2), 1.77 (quintet, 6H, J = 7 Hz, CH2), 1.49 (m, 12H, CH2CH2). IR (CDCl3) 2941, 2862, 1595, 1462, 1387, 1244, 1157, 1063 cm−1.
1,3,5-Tris-(ω-methacryloyloxyalkyloxy)benzene (TMAOBn)
A mixture of TBAOBn (1.0 mmol) and methacrylic acid (4.0 mmol) in N,N-dimethylformamide (100 mL) in the presence of K2CO3 (4.0 mmol) was stirred for 24 hours at room temperature. The reaction mixture was evaporated to give a residue, which was chromatographed over silica gel. An elution with chloroform gave TMAOBn.TMAOB4: oil, 31% yield based on the amount of TBAOB4 used. 1H NMR (CDCl3): δ = 6.11 (m, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.05 (s, 3H, Ph), 5.56 (quintet, J = 1.2 Hz, 3H, CH2
), 6.05 (s, 3H, Ph), 5.56 (quintet, J = 1.2 Hz, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 4.22 (broad t, J = 6 Hz, 6H, CH2OCO), 3.95 (broad t, J = 6 Hz, 6H, CH2O), 1.95 (m, 9H, CH3), 1.86 (broad m, 12H, CH2CH2). IR (CDCl3): ν = 2958, 2877, 1712 (C
), 4.22 (broad t, J = 6 Hz, 6H, CH2OCO), 3.95 (broad t, J = 6 Hz, 6H, CH2O), 1.95 (m, 9H, CH3), 1.86 (broad m, 12H, CH2CH2). IR (CDCl3): ν = 2958, 2877, 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1601, 1464, 1387, 1325, 1298, 1159, 1066 cm−1. FABMS: m/z (%): 548 (9), 547 (26) [M + 1], 546 (14), 141 (70), 69 (100). HRFABMS: m/z calcd for C30H43O9 547.2904, found 547.2906.
C), 1601, 1464, 1387, 1325, 1298, 1159, 1066 cm−1. FABMS: m/z (%): 548 (9), 547 (26) [M + 1], 546 (14), 141 (70), 69 (100). HRFABMS: m/z calcd for C30H43O9 547.2904, found 547.2906.
TMAOB5: oil, 33% yield based on the amount of TBAOB5 used. 1H NMR (CDCl3) δ = 6.10 (m, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.05 (s, 3H, Ph), 5.55 (quintet, J = 1.2 Hz, 3H, CH2
), 6.05 (s, 3H, Ph), 5.55 (quintet, J = 1.2 Hz, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 4.17 (t, J = 7 Hz, 6H, CH2OCO), 3.92 (t, J = 7 Hz, 6H, CH2O), 1.94 (m, 9H, CH3), 1.81 (quintet, J = 7 Hz, 6H, CH2), 1.75 (quintet, J = 7 Hz, 6H, CH2), 1.55 (quintet, J = 7 Hz, 6H, CH2). IR (CDCl3): ν = 2951, 2873, 1712 (C
), 4.17 (t, J = 7 Hz, 6H, CH2OCO), 3.92 (t, J = 7 Hz, 6H, CH2O), 1.94 (m, 9H, CH3), 1.81 (quintet, J = 7 Hz, 6H, CH2), 1.75 (quintet, J = 7 Hz, 6H, CH2), 1.55 (quintet, J = 7 Hz, 6H, CH2). IR (CDCl3): ν = 2951, 2873, 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1601, 1464, 1389, 1323, 1298, 1158, 1063 cm−1. FABMS: m/z (%): 590 (24), 589 (67) [M + 1], 588 (24), 503 (13), 69 (100). HRFABMS: m/z calcd for C33H49O9 589.3374, found 589.3377.
C), 1601, 1464, 1389, 1323, 1298, 1158, 1063 cm−1. FABMS: m/z (%): 590 (24), 589 (67) [M + 1], 588 (24), 503 (13), 69 (100). HRFABMS: m/z calcd for C33H49O9 589.3374, found 589.3377.
TMAOB6: oil, 30% yield based on the amount of TBAOB6 used. 1H NMR (CDCl3) δ = 6.10 (m, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 6.05 (s, 3H, Ph), 5.55 (quintet, J = 1.2 Hz, 3H, CH2
), 6.05 (s, 3H, Ph), 5.55 (quintet, J = 1.2 Hz, 3H, CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) ), 4.15 (t, J = 7 Hz, 6H, CH2OCO), 3.91 (t, J = 7 Hz, 6H, CH2O), 1.94 (m, 9H, CH3), 1.77 (quintet, J = 7 Hz, 6H, CH2), 1.71 (quintet, J = 7 Hz, 6H, CH2), 1.47 (m, 12H, CH2CH2). IR (CDCl3): ν = 2943, 2862, 1712 (C
), 4.15 (t, J = 7 Hz, 6H, CH2OCO), 3.91 (t, J = 7 Hz, 6H, CH2O), 1.94 (m, 9H, CH3), 1.77 (quintet, J = 7 Hz, 6H, CH2), 1.71 (quintet, J = 7 Hz, 6H, CH2), 1.47 (m, 12H, CH2CH2). IR (CDCl3): ν = 2943, 2862, 1712 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), 1637 (C
O), 1637 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C), 1599, 1462, 1389, 1323, 1298, 1164, 1063 cm−1. FABMS: m/z (%): 632 (30), 631 (78) [M + 1], 630 (23), 69 (100). HRFABMS: m/z calcd for C36H55O9 631.3843, found 631.3843.
C), 1599, 1462, 1389, 1323, 1298, 1164, 1063 cm−1. FABMS: m/z (%): 632 (30), 631 (78) [M + 1], 630 (23), 69 (100). HRFABMS: m/z calcd for C36H55O9 631.3843, found 631.3843.
Poly(N-Tn)
NIPAM (200 mg) and TMAOBn (n = 4, 5 and 6) (5, 10, 20, 40 or 60 mg) were dissolved in toluene (2 mL), and AIBN (20 mg) was added as initiator. The reaction mixture was flushed with nitrogen for 10 minutes and placed in an aluminium block dry bath at 70 °C under nitrogen atmosphere to initiate polymerization. The reaction was terminated after 24 hours by addition of methanol (5 mL) to give a swollen gel. The resulting gel was washed with methanol, and then air-dried at room temperature to give poly(N-Tn-2.5%, 5%, 10%, 20% or 30%).Swelling and shrinking measurements
The cross-linked solid polymer sample was placed in the solvents at room temperature. After immersion for 10 minutes to 24 hours, we measured Q (solvent uptake) of the sample, which is defined by as following equation:| Q = (Wwet − Wdry)/Wdry |
In order to study the solvent uptake (Q)–time plots for the swelling behaviors (Fig. 1b), the solid material of poly(N-T6-2.5%) was divided into six samples, which were kept immersed during 10 minutes, 30 minutes, 1 hour, 3 hours, 5 hours and 24 hours in the solvent, respectively. The value of Q of each swollen sample was measured. The solvent uptake (Q)–time plots for the swelling and shrinking behaviors of poly(N-T6-10%) (Fig. 4) were studied similarly.
Conclusions
New stimuli-responsive polymers, poly(N-Tn)s, were prepared. They could absorb alcohols and acetic acid much better than they could absorb water. The swelling in water were remarkably dependent on the amounts of TMAOBn. The presence of TMAOBn had a great influence on the swelling behaviors of poly(N-Tn) gels in water. When poly(N-Tn) gels, swollen in ethanol and acetic acid, were placed in water at 40 °C, they quickly shrank. The swelling behavior of poly(N-Tn) in acetic acid or ethanol and the shrinking behavior of the resultant organogel in water could be repeated over many iterations.Acknowledgements
The authors thank Dr Yasuko Tanaka, Kyushu University, for the FAB mass data of TMAOBn.Notes and references
- E. S. Gil and S. M. Hudson, Prog. Polym. Sci., 2004, 29, 1173 CrossRef CAS.
- N. A. Peppas, J. Z. Hilt, A. Khademhosseini and R. Langer, Adv. Mater., 2006, 18, 1345 CrossRef CAS.
- S. Chaterji, I. K. Kwon and K. Park, Prog. Polym. Sci., 2007, 32, 1083 CrossRef CAS.
- A. Kumar, A. Srivastava, I. Y. Galaev and B. Mattiasson, Prog. Polym. Sci., 2007, 32, 1205 CrossRef CAS.
- P. Calvert, Adv. Mater., 2009, 21, 743 CrossRef CAS.
- H.-J. Schneider and R. M. Strongin, Acc. Chem. Res., 2009, 42, 1489 CrossRef CAS.
- D. Kuckling, Colloid Polym. Sci., 2009, 287, 881 CrossRef CAS.
- I. Tokarev and S. Minko, Soft Matter, 2009, 5, 511 RSC.
- V. Kozlovskaya, E. Khariampieva, I. Erel and S. A. Sukhishvili, Soft Matter, 2009, 5, 4077 RSC.
- Q. Meng and J. Hu, Composites, Part A, 2009, 40, 1661 CrossRef.
- M. Motornov, Y. Roiter, I. Tokarev and S. Minko, Prog. Polym. Sci., 2010, 35, 174 CrossRef CAS.
- H. G. Schild, Prog. Polym. Sci., 1992, 17, 163 CrossRef CAS.
- J. Kopecek, Biomaterials, 2007, 28, 5185 CrossRef CAS.
- C. He, S. W. Kim and D. S. Lee, J. Controlled Release, 2008, 127, 189 CrossRef CAS.
- T.-Y. Liu, S.-H. Hu, D.-M. Liu, S.-Y. Chen and I.-W. Chen, Nano Today, 2009, 4, 52 CrossRef CAS.
- H. Wei, S.-X. Cheng, X.-Z. Zhang and R.-X. Zhuo, Prog. Polym. Sci., 2009, 34, 893 CrossRef CAS.
- K. Nagase, J. Kobayashi and T. Okano, J. R. Soc. Interface, 2009, 6, S293 CrossRef CAS.
- I. Tokarev and S. Minko, Adv. Mater., 2009, 21, 241 CrossRef CAS.
- F. Liu and M. W. Urban, Prog. Polym. Sci., 2010, 35, 3 CrossRef CAS.
- T. Suzuki, T. Karino, F. Ikkai and M. Shibayama, Macromolecules, 2008, 41, 9882 CrossRef CAS.
- J.-T. Zhang and K. D. Jandt, Macromol. Rapid Commun., 2008, 29, 593 CrossRef CAS.
- M. Klis, M. Karbarz, Z. Stojek, J. Rogalski and R. Bilewicz, J. Phys. Chem. B, 2009, 113, 6062 CrossRef CAS.
- X. Yang and J.-C. Kim, Colloid Polym. Sci., 2009, 287, 893 CrossRef CAS.
- G. Fundueanu, M. Constantin and P. Ascenzi, Acta Biomater., 2009, 5, 363 CrossRef CAS.
- M. Karbarz, M. Gniadek and Z. Stojek, Electroanalysis, 2009, 21, 1363 CrossRef CAS.
- S. Chai, J. Zhang, T. Yang, J. Yuan and S. Cheng, Colloids Surf., A, 2010, 356, 32 CrossRef CAS.
- A. de Sousa, D. A. Maria, R. G. de Sousa and E. M. B. de Sousa, J. Mater. Sci., 2010, 45, 1478 CrossRef CAS.
- R. C. Mundargi, N. B. Shelke, V. R. Babu, P. Patel, V. Rangaswamy and T. M. Aminabhavi, J. Appl. Polym. Sci., 2010, 116, 1832 CAS.
| This journal is © The Royal Society of Chemistry 2010 |
