A facile method for synthesizing free-shaped and tough double network hydrogels using physically crosslinked poly(vinyl alcohol) as an internal mold
Tasuku
Nakajima
a,
Naoyuki
Takedomi
a,
Takayuki
Kurokawa
b,
Hidemitsu
Furukawa
ac and
Jian Ping
Gong
*ad
aGraduate School of Science, Hokkaido University, Kita-10jo-Nishi-8chome, Kita-Ku, Sapporo, Hokkaido, Japan
bCreative Research Institution, Hokkaido University, Kita-10jo-Nishi-8chome, Kita-Ku, Sapporo, Hokkaido, Japan
cGraduate School of Science and Technology, Yamagata University, Kita-10jo-Nishi-8chome, Kita-Ku, Sapporo, Hokkaido, Japan
dFaculty of Advanced Life Science, Hokkaido University, Kita-10jo-Nishi-8chome, Kita-Ku, Sapporo, Hokkaido, Japan. E-mail: gong@mail.sci.hokudai.ac.jp
First published on 29th April 2010
Abstract
The creation of double network hydrogels (DN gels), which show extremely high mechanical strength, enable hydrogels to be applied both in medical and industrial fields. However, one obstacle for various applications is the lack of formability of DN gels, owing to the brittleness of the first network PAMPS gels. In order to overcome this problem, we synthesized free-shaped DN gels (called PVA-DN gels) by using a physically cross-linked PVA gel as an “internal mold”. PVA-DN gels can form any complex shapes and their mechanical properties were comparable to those of conventional DN gels. This study may expand the application of tough hydrogels.
Introduction
Polymer hydrogels, consisting of hydrophilic polymer network with large amount of water, are unique materials. They show many interesting functions, such as volume phase transition,1 low surface friction,2 and permeability, which cannot be implemented in solid materials. Thus, hydrogels have attracted much attention as novel functional materials from both medical and industrial fields. Although the strength of conventional hydrogels is typically too low to apply as any load-bearing materials, recent advance of synthesis enables to improve the strength of hydrogels, such as slide-ring gels, nanocomposite gels, double network gels, click gels, and tetra-PEG gels.3–7 Of these hydrogels, the double network gels (DN gels), which were created by our group, show the highest fracture stress and toughness. DN gels are interpenetrating network (IPN) hydrogels consisting of tightly cross-linked rigid poly(2-acrylamido-2-methylpropanesulfonic acid) (PAMPS) gels as the first network and loosely cross-linked flexible polyacrylamide (PAAm) as the second network (even if a cross-linker is not used, PAAm in common DN gels is actually cross-linked8).Although the DN gels contain 90 wt% of water (and only 10 wt% of polymer chains), they show the excellent mechanical properties. The compressive fracture stress of the DN gels reaches several dozen MPa,5,9 which is comparable to that of articular cartilage (36 MPa),10 a common natural tough hydrogel. In addition, their fracture energy is up to 2200 J m−2,8,11–13 which is approximately 200 times larger than the theoretical value.11 Some DN gels also exhibit suitability for regenerative medicine. Yasuda's group (Hokkaido University) has reported that the modified DN gels are excellently biocompatible and do not degrade in a body.14,15 Additionally, they first succeeded in regenerating articular cartilage in vivo by embedding the biocompatible DN gels.16 Based on these excellent mechanical and medical properties, DN gels are supposed to become superior alternative materials for tough living organs, such as articular cartilages, blood vessels, and tendons.
Nevertheless, DN gels still have a problem – lack of formability, which is crucial for being extensively used as a material. For example, it is required for an artificial cartilage to form any specific complex shape dependent on the body of each patient. However, conventional DN gels can be formed only in the limited shapes, such as a sheet or a disc. The shapes of DN gels are determined by the first network PAMPS gels acting as a “skeleton” due to the two-step polymerization. Whereas PAMPS gels themselves can be formed any shapes by using molds, it is very difficult to eject convoluted PAMPS gels without breaking them due to their extremely poor mechanical properties, such as 0.08 MPa of tensile fracture stress, 0.36 of tensile fracture strain and 0.8 J m−2 of fracture energy.11 Incidentally, PAMPS gels or DN gels also cannot be cut or scraped into the desired shape after the synthesis.
We have solved this problem by applying a “double network” structure. As previously mentioned, the shape of DN gels is determined by the first network. Based on this fact, it is imagined that if PAMPS gels are synthesized in another kind of hydrogels which are strong enough to eject from molds, the shape of them can be modified easily. We call these shape-deciding hydrogels “internal molds”, as the opposite concept of common “external” molds. This time, we chose physically cross-linked poly(vinyl alcohol) gels (PVA gels) as internal molds because they are so flexible and relatively strong that they can form any complicated shapes.17–19 We performed the following experiments. Firstly, the PVA gels with complex shapes were synthesized. Secondly, the PAMPS network was polymerized in the PVA gels. Thirdly, the PAAm network was synthesized in the presence of the PVA/PAMPS double-network gels (PVA-PAMPS gels) and finally the free-shaped PVA/PAMPS/PAAm triple-network gels (PVA-DN gels) were obtained. These PVA-DN gels show the similar mechanical properties to the conventional DN gels.
Experimental
Materials
Poly(vinyl alcohol) (PVA), polymerization degree: 2000 (Nakalai Tesque, Ltd.) was used as received. Dimethyl sulfoxide (DMSO, Junsei Chemical Co., Ltd.) was used as received. 2-Acrylamido-2-methylpropanesulfonic acid (AMPS, Toa Gosei Co., Ltd.) was recrystallized from methanol. N,N′-methylenebis(acrylamide) (MBAA, Wako Pure Chemical Industries, Ltd.) was recrystallized from ethanol. 2-oxoglutaric acid (Wako Pure Chemical Industries, Ltd.) was used as received. Acrylamide (AAm, Junsei Chemical Co., Ltd.) was recrystallized from chloroform.Synthesis of PVA gels as internal molds
The physically cross-linked PVA gels were prepared by a quenching method.18 Firstly, the 10 wt% PVA (DMSO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O = 3
H2O = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, w/w) solution was prepared by heating a mixture of the PVA and the solvent for 1 h at 90 °C. Then, the PVA solution was poured into various shapes of molds made of silicone rubber or glass, and they were quenched for 24 h at −40 °C. After forming a network structure, the gels were immersed in pure water for more than 1 week to remove the DMSO.
1, w/w) solution was prepared by heating a mixture of the PVA and the solvent for 1 h at 90 °C. Then, the PVA solution was poured into various shapes of molds made of silicone rubber or glass, and they were quenched for 24 h at −40 °C. After forming a network structure, the gels were immersed in pure water for more than 1 week to remove the DMSO.
Synthesis of free-shaped double network gels
First, the AMPS aqueous solution was prepared from 1 M of AMPS as the monomer, 4 mol% of MBAA as cross-linker, and 0.1 or 0.6 mol% of 2-oxoglutaric acid as the photoinitiator (the molar percentages are with respect to the monomer). Then, the PVA gels with various shapes were immersed in this solution for at least 3 days. After immersing, the PVA gels containing AMPS were left in an argon blanket and sealed to prevent drying; then PAMPS network was synthesized in the presence of PVA gels by UV polymerization for 8 h. These PVA/PAMPS double-network gels (called PVA-PAMPS gels) were then immersed in 2 M of AAm and 0.01 mol% of 2-oxoglutaric acid aqueous solution for at least 2 days. Then, PAAm was synthesized in the presence of PVA-PAMPS gels by the same method. After the synthesis, PVA/PAMPS/PAAm triple-network gels, called PVA-DN gels, were immersed in pure water more than 1 week in order to remove any unreacted reagents. The swollen PVA-DN gels contained 90–92 wt% of water. It is important to synthesis the PVA-DN gels under a no oxygen atmosphere. If the synthesis proceeds with oxygen, the surface of the sample does not completely form the gel state due to chain growth inhibition by oxygen.20We also synthesized PAMPS/PAAm double-network gels (conventional DN gels), PVA/PAMPS double-network gels, and PVA/PAAm double-network gels as positive/negative controls with the same composition and by the same method, except that the second network of the PVA/PAAm gel was loosely cross-linked (0.02 mol% of MBAA was used). Table 1 shows the components of all the samples.
| Sample name | Internal mold | 1st network | 2nd network |
|---|---|---|---|
| PVA gel | — | PVA | — |
| PAMPS gel | — | PAMPS | — |
| PAAm gel | — | PAAm | — |
| PVA-PAMPS gel | PVA | PAMPS | — |
| PVA-PAAm gel | PVA | PAAm | — |
| DN gel | — | PAMPS | PAAm |
| PVA-DN gel | PVA | PAMPS | PAAm |
Mechanical strength measurements
The tensile-compressive tester (Tensilon RTC-1310A Orientic. Co.) was used for the measurement of mechanical properties. The compressive fracture stress σ and the Young's modulus E were measured by compressive test on cylindrical gels (5 mm in thickness and 9 mm in diameter); the compression rate is 10% min−1. The fracture energy G was measured by tearing test on trousers-shaped samples standardized as the JIS-K6252 1/2 size (5 mm in thickness, 50 mm in length (with 20 mm initial legs) and 7.5 mm in width). The tearing velocity was 250 mm min−1. G, defined as the energy required for creating a unit area of fracture surface in a sample, is calculated by G = Fave/w, where Fave is the average tearing resistance force, and w is the width of the gel.11 The loading curves were measured by a tensile test on dumbbell-shaped gels standardized as the JIS-K6251-7 size (length: 40 mm; width: 10 mm; thickness; 1.7–2.6 mm; gauge length: 12 mm; inner width; 2 mm). The tensile velocity was 100 mm min−1.Results and discussion
Fig. 1 shows the pictures of PVA-DN gels with various shapes. By this method, any complicated shapes can be formed, such as the bird, the fish, and the Chinese knot shape depending on the shape of the PVA gels used as the internal mold. In contrast, conventional PAMPS gels (and DN gels) cannot even form a simple ball-like shape. PAMPS gels were so fragile that they broke whenever they were removed from the external molds. Even though the PVA-DN gels have obtained the formability, their strength and toughness were still extremely high. Fig. 2 shows pictures of the sphere-shaped PVA-DN gel hit by a golf club (wood), recorded by a high-speed camera. In spite of the strong impact and the large deformation, the PVA-DN gel recovered its original shape without breaking. On the contrary, the PVA-PAMPS gel was catastrophically broken by the impact. It is noted that the PVA-DN gels look a little bit opaque in contrast with completely transparent common DN gels. This decrease of transparency is derived from the crystalline domain of PVA gels.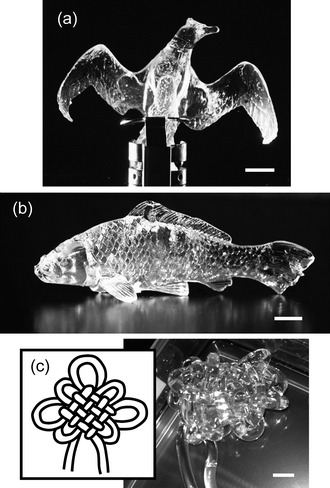 | ||
| Fig. 1 The pictures of the PVA-DN gels with the shape of (a) the bird, (b) the fish, and (c) the Chinese knot. Conventional PAMPS gels and DN gels cannot form such complicated shapes. The scale bars: 1 cm. | ||
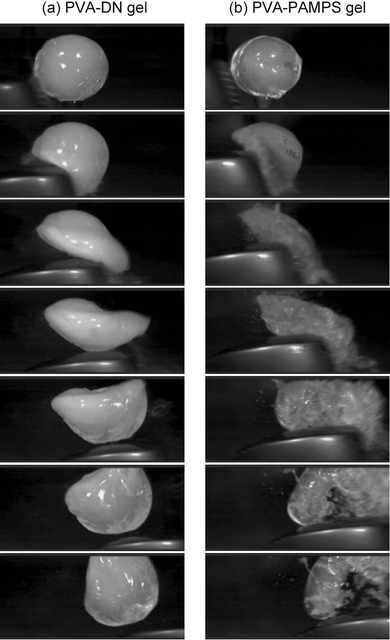 | ||
| Fig. 2 High-speed camera images of the spherical (a) PVA-DN gel and (b) PVA-PAMPS gel being hit by a golf club. The time interval between each picture is 3/4000 s. | ||
Here, we express the toughness of the PVA-DN gels by numeral values. Fig. 3 (filled bars) shows the fracture energy G of the PVA-DN, conventional DN, and PVA/PAAm DN gels. The G, which represents the toughness, of the PVA-DN gels was anomalously high and similar to that of the conventional DN gels. On the other hand, even though the PVA/PAAm DN gels also show formability, their fracture energy G was lower than that for the other DN gels. Fig. 4 shows the tensile stress-strain curves of the DN gels. The tensile fracture stress of the PVA-DN gels was less than that of DN gels; on the other hand, the fracture strain of those gels was similar to each other. This phenomenon might be caused by a lower PAMPS concentration in the PVA-DN or PVA-PAMPS gels than conventional PAMPS gels and DN gels. As the PVA gels contained 10 wt% of polymer, concentration of AMPS in the PVA-PAMPS gels did not reach to 1M in spite of the gel immersion in 1M AMPS solution. Using slightly higher concentration of AMPS solution may be useful to overcome the effect of PVA chains.
 | ||
| Fig. 3 The fracture energy G of the PVA-DN gels, the conventional DN gels, the PVA-PAAm gel, and the PVA gel. The filled bars represent the G of the DN gels before heating and the mesh bars represent the G after heating. As the PVA gel was dissolved by immersing in hot water, the G of the PVA gel after heating could not be measured. The tearing velocity was 250 mm min−1. PVA-DN denotes the PVA-DN gels, DN denotes the conventional DN gels, PVA/PAAm denotes the PVA/PAAm DN gels. The PAMPS gels and the PVA-PAMPS gels are too weak to be measured. The following number denotes the initiator concentration for the PAMPS network (mol%). | ||
 | ||
| Fig. 4 The tensile stress-strain curves of (a) the PVA-DN gels, the conventional DN gels (b) the PVA-PAAm gel, the PVA gel, and the PAMPS gel measured by the tensile test. The PVA-PAMPS gels are too weak to be measured. The tensile velocity was 100 mm min−1. | ||
Next, we try to clarify the effect of the PVA chains on the toughness of the PVA-DN gels. Physical PVA gels are cross-linked by the formation of hydrogen bonds18 and these bonds were easily destroyed by applying heat. Thus, the effect of the PVA network to the mechanical properties of PVA-DN gels can be removed by heating the samples. So we investigated the effect of PVA network by measuring the fracture energy G of the PVA-DN gels before and after heating. The DN gels, the PVA-DN gels, and the PVA gel were immersed in hot water at 70 °C for 8 h. Fig. 3 shows the fracture energy G of the DN gels before and after heating. The G of the conventional DN gels and the PVA-DN gels did not change by this operation. This result implies that PVA network of PVA-DN gels has no effect to the mechanical strength of DN gels. The PVA network works only as “internal mold”.
In order to explain why PVA-DN gels have both formability and toughness, we discuss the mechanical properties of the first network gels. First, we investigated the mechanical properties of as-prepared gels in order to discuss formability. Fig. 5 shows the tensile loading curves of the PVA gel, which consists of neutral polymer and show formability, and the as-prepared PAMPS gels and PVA-PAMPS gels, consisting of polyelectrolyte. The PVA gel showed high flexibility, indeed it did not break even if the strain reached 10. In addition, its tensile fracture stress is relatively high. Therefore, the PVA gels were not broken when they were ejected from complex molds. In contrast, the conventional PAMPS gels showed the completely different behavior, rigid and brittle. They were broken when the stress attained only 0.08 MPa; thus, they cannot be removed from the mold without being broken. These data imply that the flexibility is so important for the formability.
 | ||
| Fig. 5 (a) The tensile stress-strain curves of the PVA gel, the as-prepared PAMPS gels, and the as-prepared PVA-PAMPS gels. (b) The enlarged figure of the low-strain region. | ||
Second, we take notice of the mechanical properties of swollen first network gels. Our first paper about DN gels reports experimentally that the rigidness of the swollen first network PAMPS is enormously important for the strength of the DN gels.7 This point is also indicated theoretically by some other papers.21–25 As the index parameter of rigidness, we use modulus for simplification. Fig. 6 shows the compressive stress-strain curves and the modulus of the PVA/PAMPS gels, the conventional PAMPS gels and PVA gel in swollen state. The conventional PAMPS gels showed the highest moduli (∼0.25 MPa). The PVA-PAMPS gels also showed the high moduli (∼0.15 MPa), while the values were less than those of PAMPS gels. It is suggested that despite of the presence of PVA network, the properties of the swollen PVA/PAMPS gels are mainly dominated by rigid PAMPS network, caused by fully stretching of polyelectrolyte chains. This rigidness leads the toughening of DN gels; thus, both the PVA-DN gels and the DN gels showed the toughness. In contrast, the modulus of the swollen PVA gel is quite low (∼0.05 MPa) even though its polymer concentration is higher than that of the swollen PAMPS gels. Thus, the PVA/PAAm DN gels do not show the toughness.
 | ||
| Fig. 6 (a) The compressive stress-strain curves and (b) the Young's modulus of the PVA gel, the swollen PAMPS gels, and the swollen PVA-PAMPS gels. | ||
The essential points of this study are the following. Formability of (DN) gels is introduced by flexibility of as-prepared first network gels. On the other hand, the extremely high strength of DN gels is derived from the rigidness of swollen first network.4 Until now, there has been no rigid and flexible gel in accordance with our best knowledge; thus, tough DN gels with formability have not been synthesized. For example, PAMPS/PAAm DN gels are strong but not formable due to the rigid and “brittle” first network; on the other hand, PVA/PAAm DN gels show formability but weak due to the flexible and “soft” first network. This problem can be solved by using PVA/PAMPS gels as the first network. They are enough flexible (and soft) to form any shapes in as-prepared PVA single network state; in contrast, after synthesizing PAMPS network and swelling, their property changes to rigid (and brittle), which enables to toughen (PVA-)DN gels. This is the reason why PVA/PAMPS/PAAm (PVA-DN) gels have both formability and toughness.
Conclusions
Free-shaped double network hydrogels (PVA-DN gels) were synthesized by using PVA gels as internal molds. They could form any complicated shape, such as the fish and the bird. Additionally, the mechanical properties of PVA-DN gels were almost the same as those of the conventional DN gels. These formability and the strength are provided via the difference of the mechanical properties between the flexible as-prepared PVA gels and the rigid swollen PVA/PAMPS gels. This study will enable DN gels to be applied in biological and industrial fields, such as artificial cartilages adopted into individual patient, artificial blood-vessels with complicated shapes, and gel machines based on chemomechanical systems.26Acknowledgements
This work is supported by a Grant-in-Aid for the Specially Promoted Research (No. 18002002) from the Ministry of Education, Science, Sports and Culture of Japan.Notes and references
- T. Tanaka, Phys. Rev. Lett., 1978, 40, 820 CrossRef CAS.
- J. P. Gong, M. Higa, Y. Iwasaki, Y. Katsuyama and Y. Osada, J. Phys. Chem. B, 1997, 101, 5487 CrossRef CAS; J. P. Gong, T. Kurokawa, T. Narita, G. Kagata, Y. Osada, G. Nishimura and M. Kinjo, J. Am. Chem. Soc., 2001, 123, 5582 CrossRef CAS.
- Y. Okumura and K. Ito, Adv. Mater., 2001, 13, 485 CrossRef CAS.
- K. Haraguchi and T. Takeshita, Adv. Mater., 2002, 14, 1120 CrossRef CAS.
- J. P. Gong, Y. Katsuyama, T. Kurokawa and Y. Osada, Adv. Mater., 2003, 15, 1155 CrossRef CAS.
- M. Malkoch, R. Vestberg, N. Gupta, L. Mespouille, P. Dubois, A. Mason, J. Hedrick, Q. Liao, C. Frank, K. Kingsbury and C. Hawker, Chem. Commun., 2006,(26), 2774 RSC.
- T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama and U. Chung, Macromolecules, 2008, 41, 5379 CrossRef CAS.
- T. Nakajima, H. Furukawa, Y. Tanaka, T. Kurokawa, Y. Osada and J. P. Gong, Macromolecules, 2009, 42, 2184 CrossRef CAS.
- Y.-H. Na, T. Kurokawa, Y. Katsuyama, H. Tsukeshiba, J. P. Gong, Y. Osada, S. Okabe, T. Karino and M. Shibayama, Macromolecules, 2004, 37, 5370 CrossRef CAS.
- A. J. Kerin, M. R. Wisnom and M. A. Adams, Proc. Inst. Mech. Eng., Part H, 1998, 212, 273 Search PubMed.
- Y. Tanaka, R. Kuwabara, Y.-H. Na, T. Kurokawa, J. P. Gong and Y. Osada, J. Phys. Chem. B, 2005, 109, 11559 CrossRef CAS.
- H. Tsukeshiba, M. Huang, Y.-H. Na, T. Kurokawa, R. Kuwabara, Y. Tanaka, H. Furukawa, Y. Osada and J. P. Gong, J. Phys. Chem. B, 2005, 109, 16304 CrossRef CAS.
- M. Huang, H. Furukawa, Y. Tanaka, T. Nakajima, Y. Osada and J. P. Gong, Macromolecules, 2007, 40, 6658 CrossRef CAS.
- C. Azuma, K. Yasuda, Y. Tanabe, H. Taniguro, F. Kanaya, A. Nakayama, Y. M. Chen, J. P. Gong and Y. Osada, J. Biomed. Mater. Res. A, 2007, 81, 373 CrossRef.
- Y. Tanabe, K. Yasuda, C. Azuma, H. Taniguro, S. Onodera, A. Suzuki, Y. M. Chen, J. P. Gong and Y. Osada, J. Mater. Sci.: Mater. Med., 2008, 19, 1379 CrossRef CAS.
- K. Yasuda, N. Kitamura, J. P. Gong, K. Arakaki, H. J. Kwon, S. Onodera, Y. M. Chen, T. Kurokawa, F. Kanaya, Y. Ohmiya and Y. Osada, Macromol. Biosci., 2009, 9, 307 CrossRef CAS.
- S.-H. Hyon, W.-I. Cha and Y. Ikada, Polym. Bull., 1989, 22, 119 CrossRef CAS.
- N. A. Peppas and S. R. Stauffef, J. Controlled Release, 1991, 16, 305 CrossRef CAS.
- M. Ohta, A. Handa, H. Iwata, D. A. Rüfenacht and S. Tsutsumi, Tech. Health Care, 2004, 12, 225 Search PubMed.
- T. Kurokawa, J. P. Gong and Y. Osada, Macromolecules, 2002, 35, 8161 CrossRef CAS.
- Y.-H. Na, Y. Tanaka, Y. Kawauchi, H. Furukawa, T. Sumiyoshi, J. P. Gong and Y. Osada, Macromolecules, 2006, 39, 4641 CrossRef CAS.
- R. Webber, C. Creton, H. R. Brown and J. P. Gong, Macromolecules, 2007, 40, 2919 CrossRef CAS.
- H. R. Brown, Macromolecules, 2007, 40, 3815 CrossRef CAS.
- Y. Tanaka, Europhys. Lett., 2007, 78, 56005 CrossRef.
- Y. Tanaka, Y. Kawauchi, T. Kurokawa, H. Furukawa, T. Okajima and J. P. Gong, Macromol. Rapid Commun., 2008, 29, 1514 CrossRef CAS.
- Y. Osada, H. Okuzaki and H. Hori, Nature, 1992, 355, 242 CrossRef CAS; M. Otake, Y. Kagami, M. Inaba and H. Inoue, Rob. Auton. Syst., 2002, 40, 185 Search PubMed; R. Yoshida, T. Sakai, O. Tabata and T. Yamaguchi, Sci. Technol. Adv. Mater., 2002, 3, 95 CrossRef CAS; M. E. Harmon, M. Tang and C. W. Frank, Polymer, 2003, 44, 4547 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
