Thiol-ene and thiol-yne chemistry in microfluidics: a straightforward method towards macroporous and nonporous functional polymer beads
R. Arun
Prasath
,
M. Talha
Gokmen
,
Pieter
Espeel
and
Filip E.
Du Prez
*
Polymer Chemistry Research Group, Department of Organic Chemistry, Ghent University, Krijgslaan 281 S4, 9000, Ghent, Belgium. E-mail: filip.duprez@ugent.be
First published on 13th May 2010
Abstract
Thiol-ene and thiol-yne reactions are explored as efficient pathways towards rapid production of diverse monodisperse macroporous and nonporous functional beads. In a straightforward method, polymer beads containing amine, hydroxyl and carboxyl groups have been prepared by reacting a tetrafunctional thiol with a range of mono and/or multifunctional -enes/-ynes containing the desired functional groups. The thiol-ene and thiol-yne reactions have been performed in a simple home-made microfluidic device utilizing thiol and ene/yne monomers at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiol to π-bond. The porous functional beads were prepared making use of a porogen in combination with a photoinitiator. The optical and scanning electron microscopy images demonstrated monodispersity of the beads with a spherical shape ranging in size from 210 to 600 μm. The beads were characterized in terms of glass transition temperature, surface area measurement and composition. The accessible amine and hydroxyl loading in the beads ranges from 0.23 to 0.69 mmol g−1 and 0.24 to 0.64 mmol g−1 respectively, as determined by the Fmoc method. This work demonstrates the applicability of thiol-ene and thiol-yne reactions in microfluidics as a powerful tool for the rapid design of functional beads for diverse applications.
1 ratio of thiol to π-bond. The porous functional beads were prepared making use of a porogen in combination with a photoinitiator. The optical and scanning electron microscopy images demonstrated monodispersity of the beads with a spherical shape ranging in size from 210 to 600 μm. The beads were characterized in terms of glass transition temperature, surface area measurement and composition. The accessible amine and hydroxyl loading in the beads ranges from 0.23 to 0.69 mmol g−1 and 0.24 to 0.64 mmol g−1 respectively, as determined by the Fmoc method. This work demonstrates the applicability of thiol-ene and thiol-yne reactions in microfluidics as a powerful tool for the rapid design of functional beads for diverse applications.
Introduction
Porous and gel-type (nonporous) polymer beads play an important role in various applications, i.e. solid supported catalysis,1 ion exchange,2 enzyme immobilization,3 solid phase extraction (SPE),4 high performance liquid chromatography (HPLC),5 gel permeation chromatography (GPC),6 scavenging,7 solid phase organic synthesis (SPOS)8 and solid phase peptide synthesis (SPPS).9 For solid phase synthesis and catalysis applications, beads with a diameter of 100–300 micrometres are preferred, whereas chromatographic applications require beads with only few micrometres in diameter.5,6,10 The monodispersity and the porosity are crucial factors for most of the applications mentioned above. On the other hand, beads bearing reactive functional groups are essential when aiming for chemical modification reactions such as in SPOS and SPPS. The functional groups in the beads are either introduced by the functionality of the monomers or via post-modification step(s). In general, beads with high rigidity, porosity, size monodispersity, and controllable amounts of reactive functional groups are needed for most of the above mentioned applications.The invention of chemical synthesis on a solid support11 resulted in revolutions in both peptide research and organic synthesis. As a result, drug discovery became much easier than before.12 The first resins used in SPPS were based on styrene(St)-divinylbenzene (DVB)11 and various other types of resins were introduced for SPPS thereafter.13,14 Gel type St-DVB based Merrifield resins are still much in use, together with the later developed hybrid TentaGels,15 which are composed of a rigid St-DVB core and a hydrophilic poly(ethylene glycol) shell. A typical loading for TentaGel resin is as low as 0.2 mmol g−1, whereas the loading of Merrifield resins can reach up to 1.5 mmol g−1. Although the latter swells only in hydrophobic solvents, TentaGel swells in a wide range of solvents ranging from aqueous to organic ones. For nonporous resins, swelling is indeed crucial for the reagents to access the inner reactive sites. On the contrary, swelling is not necessary for macroporous resins16,17 since their relatively large pores allow reagents and even non-solvents to penetrate and find the reactive sites.
Generally SPPS is performed in the C → N approach meaning that amino acids are attached to the resin from their carboxyl terminus with the amino terminus being protected. For the C → N approach, resins bearing –OH and –NH2 groups are the most popular ones.18 However, most of the time –NH2 groups are introduced on the beads by post-modification step(s)19–22 because the basic amine carrying monomers such as aminomethyl styrene, aminoethyl acrylate and aminoethyl methacrylate are commercially unavailable due to their instability. Nevertheless, examples of amine containing monomer preparation and subsequent polymerization are reported in literature.23 Furthermore, the well-known high reactivity of primary amine groups towards acrylates via Michael addition24 limits this strategy. Consequently a novel direct preparation strategy for amino, carboxyl and hydroxyl bearing beads in a controlled fashion is desired.
Traditional bead preparation is conducted via heterogeneous polymerization techniques that are all based on immiscibility of two or more phases. Suspension, emulsion, miniemulsion, dispersion and precipitation polymerizations are all well known for their advantages and disadvantages.25 Both emulsion and mini-emulsion processes can produce nanometre-sized beads but lacks porosity.26 On the contrary, suspension polymerization can produce porous27 micron sized beads, but the obtained beads are inherently polydisperse in their size28 and necessitate sieving afterwards. In the seeded version of suspension polymerization,5 monodisperse nanometre-sized seed particles (prepared by emulsion polymerization) are swollen by monomer, crosslinker and porogen in a second suspension media and polymerized thereafter. With this advanced technique, beads with high porosity and monodisperse character are obtained in a two step process. Precipitation polymerisation appears to be the most advantageous method since one can obtain monodisperse, porous beads by using suitable solvent mixtures6 in a single batch. However, like in seeded suspension polymerization the obtained beads are in the range of 0.1–10 micrometre, which is not suitable for solid phase synthesis and catalysis applications due to handling problems. Also, the incorporation of functional monomers can be problematic or impossible,29 because of the delicate mechanism of growing polymer chain precipitation during the polymerization.
In recent years there has been a growing interest to produce particles based on microfluidics technology.30 This is an advanced version of suspension polymerization where droplet formation is precisely controlled by linear flow instead of chaotic agitation. In a micro channel, continuous linear flow of a carrier phase drags the discrete monomer phase as monodisperse droplets due to viscous forces and interfacial tension.31 Solidification of these monodisperse droplets (generally on-flight) results in beads with a very narrow size distribution. Microfluidic emulsification also enables researchers to use porogens in order to obtain porous beads.32–34 In general, microfluidic systems are very tolerant to changes in ingredients, so one can easily change monomer, crosslinker and porogen(s) to look for the best combination without difficulty for the bead production. The capability of a one step synthesis to obtain monodisperse, functional, porous and nonporous beads makes microfluidics a quite attractive technique among the heterogeneous polymerizations. Moreover, microfluidics seems to be the most suitable approach for particles with various shapes such as nano to micro bead-in-bead assemblies,35 microcapsules,36 non-spherical particles,37 Janus particles,38 metal doped polymer rods,39 poly(HIPE) beads and rods.40
In this paper, we present a simple microfluidic approach for preparing both gel-type and macroporous amine, hydroxyl and carboxyl functionalized monodisperse beads via thiol-ene and thiol-yne polymerizations without any post-modification steps. Addition of thiols to carbon-carbon double bonds is century-old chemistry, however recently a couple of research groups41–44 have shown that the reaction of a thiol with a double bond, referred to as ‘thiol-ene’ chemistry, has many of the features of ‘click’ chemistry.45 Later, it has been shown that not only addition of thiols to alkenes (thiol-ene) but also to alkynes (thiol-yne; double addition) reaches high conversions in short times via photopolymerization.46,47
As a rapid photopolymerization is very attractive and efficient process, we decided to explore thiol-ene and thiol-yne approaches to create polymer beads using a simple microfluidic setup, which is composed of UV transparent tubing, needles and syringe pumps (Fig. 2).48,49 Also the orthogonality of thiol-ene/yne reactions in the presence of various functional monomers will be demonstrated. The fact that there are many functional thiols, alkenes and alkynes readily available further explains our interest to apply thiol-ene/yne photopolymerizations for the one-step creation of functional beads. Finally, the addition of porogen(s) to the discrete phase enabled us to prepare macroporous beads. These novel beads may have different performance characteristics compared to the commercial resins that are based on vinyl polymerizations.
Experimental section
Materials
The monomers pentaerythritol tetrakis(3-mercaptopropionate) [97%, tetra thiol, (TT)], diallyl phthalate (97%, DAP), 1,3,5,-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (98%, TTT), 1,7-octadiyne (98%, OY), mercaptoacetic acid (97%, MA), allylamine (98%, AA), pentaerythritol triallyl ether (70%, PTE) and propargylamine (98%, PA), the photoinitiator 2,2-dimethoxy-2-phenylacetophenone (99%, DMPA), the solvents acetonitrile (AN), tetrahydrofuran (THF), 1,4-dichlorobenzene (DCB), dibutyl phthalate (DBP), diethyl phthalate (DEP), dioctyl phthalate (DOP), o-xylene (Xy) and n-butyl acetate (BuAc), light mineral oil as carrier phase, the surfactant sodium dodecyl sulfate (95%, SDS), and the reagents, N,N′-diisopropylcarbodiimide (99%, DIC) N,N-diisopropylethylamine (99%, DIPEA), 4-(dimethylamino)pyridine (99%, DMAP), 5,5′′-dithiobis(2-nitrobenzoic acid) [99%, Ellman's reagent], (benzotriazol-1-yloxy)- tripyrrolidinophosphonium hexafluorophosphate (98%), piperidine (99%) were used as received from Sigma-Aldrich. Fmoc-glycine was purchased from Novabiochem and used as such. 2,2-Di(prop-2-ynyl)propane-1,3-diol (DPPD) and (2-{ethyl-[4-(4-nitro-phenylazo)-phenyl]-amino}-ethoxy)-acetic acid-4-nitro-phenyl ester (NF31) were synthesised in-house according to the reported literature.50,51 PVC [1.6 mm inner diameter, (ID)] and Tygon tubes (0.8 mm ID) were purchased from Sigma-Aldrich and VWR international respectively. 30G and 32G disposable needles were purchased from Becton Dickinson. A metalight UV box operating with 12 lamps (Philips 9W, intensity was ∼2.5 mW cm−2, 320–400 nm) was used. The surfactant ABIL EM-90 was kindly donated by Evonik Degussa. Solvents such as dimethylformamide, dimethyl sulfoxide, 1-methyl-2-pyrrolidinone were of analytical grade from Sigma-Aldrich. Methanol, dichloromethane, acetone and diethyl ether were of technical grade and used as received from Fiers (Belgium). MilliRO grade water was used for all purposes. Chemical structures of the thiols, -enes and -ynes used in this work are given in Fig. 1. | ||
| Fig. 1 Structures and acronyms for thiols, ene and yne compounds. | ||
Preparation and analysis
Beads were prepared using a home-made microfluidic setup as shown in Fig. 2. Typically, the reagent phase consists of thiol and ene/yne monomers at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiol to π-bond, photoinitiator DMPA 2–5 mol% to thiol and with or without 60/80(±2) wt% of porogen. For nonporous bead preparation, the continuous phase was light mineral oil containing 0.25 wt% surfactant (ABIL EM-90). In the case of porous bead preparation, the continuous carrier phase was an aqueous SDS solution (3 wt%). The continuous phase (oil or water) flows through the tubing (PVC or Tygon) and the reagent phase (dispersed phase) was injected into the continuous phase using a blunt bent needle (30G or 32G), which was inserted halfway the tubing (as shown in Fig. 2). The continuous phase was pumped using a syringe pump with a syringe of 150 mL capacity and the reagent phase was pumped using a second syringe pump with a syringe of 1 mL capacity. The flow rates (varied independently) of the continuous and reagent phase were between 60 to 90 mL h−1 and 0.1 to 0.3 mL h−1 respectively. The length of the tubing was varied between 2 and 4 metres and formed in the shape of helices inside the UV chamber, which allowed the formed droplets to be continuously irradiated to UV light. After collection, the beads were further exposed to UV irradiation for approximately one hour in favour of quantitative thiol-ene/yne reactions to occur in the beads. Beads collected from oil carrier phase were first washed with dichloromethane to remove the oil and ABIL, and subsequently with methanol, water, acetone and diethyl ether to remove any impurities from the beads. Beads collected from the aqueous carrier phase were first washed with water and subsequently with methanol, dichloromethane, acetone and diethyl ether. Finally beads were dried under high vacuum at 90 °C overnight.
1 ratio of thiol to π-bond, photoinitiator DMPA 2–5 mol% to thiol and with or without 60/80(±2) wt% of porogen. For nonporous bead preparation, the continuous phase was light mineral oil containing 0.25 wt% surfactant (ABIL EM-90). In the case of porous bead preparation, the continuous carrier phase was an aqueous SDS solution (3 wt%). The continuous phase (oil or water) flows through the tubing (PVC or Tygon) and the reagent phase (dispersed phase) was injected into the continuous phase using a blunt bent needle (30G or 32G), which was inserted halfway the tubing (as shown in Fig. 2). The continuous phase was pumped using a syringe pump with a syringe of 150 mL capacity and the reagent phase was pumped using a second syringe pump with a syringe of 1 mL capacity. The flow rates (varied independently) of the continuous and reagent phase were between 60 to 90 mL h−1 and 0.1 to 0.3 mL h−1 respectively. The length of the tubing was varied between 2 and 4 metres and formed in the shape of helices inside the UV chamber, which allowed the formed droplets to be continuously irradiated to UV light. After collection, the beads were further exposed to UV irradiation for approximately one hour in favour of quantitative thiol-ene/yne reactions to occur in the beads. Beads collected from oil carrier phase were first washed with dichloromethane to remove the oil and ABIL, and subsequently with methanol, water, acetone and diethyl ether to remove any impurities from the beads. Beads collected from the aqueous carrier phase were first washed with water and subsequently with methanol, dichloromethane, acetone and diethyl ether. Finally beads were dried under high vacuum at 90 °C overnight.
 | ||
| Fig. 2 A schematic drawing of the microfluidic setup. | ||
The size and morphology of the beads were analyzed by optical microscopy (OM, Nikon SMZ800 microscope) and scanning electron microscopy (SEM, Quanta 200FEG FEI). The glass transition temperature (Tg) of the beads was measured by differential scanning calorimetry (DSC Perkin Elmer 7). Specific surface area values were determined by measuring the adsorption and desorption isotherms of nitrogen on a Belsorp-mini II apparatus with a bath temperature of 77 K and evaluated with Bel Master Software (BET method). Fourier transform infrared (FTIR) spectra and Raman spectra were collected on a Nicolet Impact 400 D spectrometer and the Renishaw System-1000 Raman spectrometer respectively. Gravimetric analysis was carried out to determine the yield for the prepared nonporous and porous beads. For the –NH2 containing beads, amine loading was estimated via the Fmoc method.52 Similarly, hydroxyl loading in the porous and nonporous hydroxyl containing groups was estimated by the Fmoc method. In short, Fmoc-glycine was activated by reacting Fmoc-glycine (0.2 mol, in dry dichloromethane) with DIC (0.2 mol) for 20 min at room temperature. The activated Fmoc reagent was added (≥2 eq to hydroxyl loading) to the beads (25–30 mg) followed by DMAP addition (0.2 eq to hydroxyl loading) in a 5 ml vial. The vial was shaken on an orbital shaker for ∼2 h and then washed with DMF, MeOH, DCM and DEE (3× each) followed by vacuum drying. Approximately 20 mg of the beads was transferred in a round bottom flask. A solution of 20% piperidine/NMP (15 mL) was added to the flask and swirled occasionally. After 30 min, the solution was transferred to an UV cuvette and the absorption of the adduct piperidine-dibenzofulvene at 300 nm was measured with the same 20% piperidine/NMP solution as the blank solution. The hydroxyl loading was calculated using the Lambert–Beer Law. The beads were also subjected to a simple colorimetric test53 and Ellman's colorimetric test54 for qualitative detection and quantitative estimation of unreacted thiols.
Results and discussion
Pentaerythritol tetrakis(3-mercaptopropionate) was selected as the tetrafunctional thiol monomer (TT), which is capable to form crosslinked networks with di/multifunctional -ene monomers or with mono/multifunctional -yne monomers. DAP, TTT, and OY were used as multifunctional -ene monomers and -yne monomer respectively. Functional monomers such as MA (thiol-carboxyl), PTE (ene-hydroxyl), DPPD (yne-hydroxyl), AA (ene-amine) and PA (yne-amine) were selected to obtain polymer beads bearing reactive functional groups. Although PA is a monofunctional -yne monomer, it is capable of undergoing a double consecutive reaction with two thiol groups to form a dithioether crosslinking point.46 The thiol-yne reaction proceeds by the addition of a thiyl radical to an -yne with the formation of a carbon centred radical, which subsequently abstracts a hydrogen from another thiol. The generated vinyl sulfide moiety is capable of undergoing a further reaction through addition of a second thiyl radical, similar to the thiol-ene reaction. Scheme 1 gives the possible step-growth network formed in the bead by typical thiol-ene and thiol-yne reactions. Apart from the thiol-ene/yne reactions, other possible side reactions such as disulfide formations (thiyl radicals), intramolecular reactions (cyclization) and homopolymerizations (carbon radicals) may also take place. More detailed information about the thiol-ene and thiol-yne photoinitiated reaction mechanisms can be found in literature.46,55 | ||
| Scheme 1 An ideal representation of step-growth network formation in the bead by thiol-ene and thiol-yne reactions (F = –NH2, –OH, or –COOH). | ||
In general, monodisperse droplets were generated by adjusting the flow rate of both the continuous and reagent phase in the microfluidic system. The generated droplets are separated with an approximate distance of about 5 mm, thus avoiding the droplet coalescence inside the tubing. Also, the presence of a surfactant (ABIL in oil or SDS in water) facilitates the smooth motion of the droplets in the tubing and eliminates the possible problem of blockage. In addition, the surfactant provides stability to the droplets until they get photopolymerized in the UV chamber to form the final monodisperse beads. Typically, the time of the photopolymerization between the tetrafunctional thiol and various mono/multifunctional enes/ynes (at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiol to π-bond) to form highly crosslinked beads was varied from 30 to 180s. It was reported for the thiol-yne addition reaction that the first step, leading to the formation of the vinyl sulfide, is slower than the reaction between the thiol and vinyl sulfide.46 In our hands, a very rapid reaction was observed for both the thiol-ene and the thiol-yne formulations under the applied conditions. Moreover, each droplet generated in the microfluidic system has roughly the same exposure to UV irradiation, which was favoured by the spin movement of the droplets downstream of the tubing, thus facilitating homogenous curing in the droplets.
1 ratio of thiol to π-bond) to form highly crosslinked beads was varied from 30 to 180s. It was reported for the thiol-yne addition reaction that the first step, leading to the formation of the vinyl sulfide, is slower than the reaction between the thiol and vinyl sulfide.46 In our hands, a very rapid reaction was observed for both the thiol-ene and the thiol-yne formulations under the applied conditions. Moreover, each droplet generated in the microfluidic system has roughly the same exposure to UV irradiation, which was favoured by the spin movement of the droplets downstream of the tubing, thus facilitating homogenous curing in the droplets.
Nonporous beads
The nonporous functional beads were prepared using the following thiol-ene/yne formulations in bulk: TT/(DAP + AA), TT/PTE and TT/PA (see Fig. 1 for abbreviations). On the other hand, since DPPD is immiscible with TT, DMSO (∼25 wt%) was used as a solvent to prepare the TT/DPPD formulation. Fig. 3 shows representative optical and SEM images of the functional beads prepared in the absence of a porogen. The hexagonal close packing of the beads (Fig. 3a) clearly illustrates their monodispersity. For the TT/PA formulation, ‘satellite’ beads are also observed along with larger monodisperse beads (Fig. 3b). The average size varies between 285 μm and 600 μm for the various functional beads prepared (Table 1). The SEM images recorded for the amine and hydroxyl containing thiol-ene beads showed shrinkages of 20 and 26% respectively as compared to the corresponding optical images. This shrinkage phenomenon can be attributed to the low stiffness of the crosslinked networks, which is supported by the low Tg values, i.e. 0 and 3 °C for the amine and hydroxyl containing beads respectively (Table 1). In contrast, very little change in the dimensions was observed for the beads prepared from thiol-yne formulations, TT/PA and TT/DPPD. This is attributed to the higher stiffness in the crosslinked thiol-yne based beads as a result of the restricted rotation of dithioether linkages and their higher cross-link density compared to the beads prepared by thiol-ene chemistry. The Tg values for the amine and hydroxyl containing thiol-yne beads are 33 and 45 °C respectively (Table 1). The yields for the nonporous beads are in the range of 70 to 82%. | ||
| Fig. 3 Representative optical (top) and SEM (bottom) images of the nonporous functional beads prepared from the formulation of (a) TT/(DAP + AA), (b) TT/PA, (c) TT/PTE and (d) TT/DPPD. | ||
| Bead type (+ functional group) | Sample formulation | Functional monomer ratio | Size by OM/μm | Size by SEM/μm | T g/°C | Specific surface area/m2 g−1 | Yield (%) |
|---|---|---|---|---|---|---|---|
a Thiol-ene system.
b Thiol-yne system.
c DOP as the porogen.
d BuAc as the porogen.
e Xy as the porogen.
f Xy and BuAc as porogens (ratio of Xy to BAc is 4.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3.5).
g Ratio of thiol to π-bond 1 3.5).
g Ratio of thiol to π-bond 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25. 1.25.
|
|||||||
| Nonporous amine a | TT/(DAP + AA) | DAP)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA at 0.65 AA at 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
325 | 260 | 0 | — | 77 |
| Nonporous hydroxyl a | TT/PTE | — | 285 | 210 | 3 | — | 82 |
| Nonporous amine b | TT/PA | — | 350 | 340 | 33 | — | 73 |
| Nonporous hydroxyl b | TT/DPPD | — | 600 | 580 | 45 | — | 70 |
| Porous amine a | TT/(DAP + AA)c | DAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA at 0.65 AA at 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
375 | 255 | 3 | 0 | — |
| Porous amine a | TT/(DAP + AA)d | DAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA at 0.65 AA at 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
355 | 280 | 7 | 0 | 62 |
| Porous hydroxyl a | TT/PTEd | — | 265 | 240 | 4 | 0.7 | 75 |
| Porous carboxyl a | (TT + MA)/TTTd | TT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MA at 0.75 MA at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
330 | 325 | 40 | 4.9 | 71 |
| Porous amineb | TT/(OY + PA)d | OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA at 0.75 PA at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
280 | 240 | 38 | 35.6 | 66 |
| Porous amineb | TT/(OY + PA)e | OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA at 0.75 PA at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
320 | 300 | 44 | 3.5 | — |
| Porous amineb | TT/(OY + PA)d,g | OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA at 0.25 PA at 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
270 | 250 | 13 | 2.3 | — |
| Porous hydroxyl b | TT/(OY + DPPD)d | OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPD at 0.75 DPPD at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
305 | 255 | 40 | 9.3 | — |
| Porous hydroxyl b | TT/(OY + DPPD)f | OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPD at 0.75 DPPD at 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
340 | 300 | 42 | 2.2 | — |
Porous beads
The porous functional beads were prepared using the following formulations containing 60/80(±2) wt% of porogen: TT/(DAP + AA), (TT + MA)/TTT, TT/PTE, TT/(OY + PA), and TT/(OY + DPPD). Among the range of porogens studied (DCB, DBP, DEP, DOP, o-xylene, n-butyl acetate, and mixtures of porogens), n-butyl acetate performed the best in most of the cases in terms of the formation of beads with a spherical shape and porosity. Fig. 4 shows representative optical and SEM images of the porous functional beads prepared with the addition of a porogen to various thiol-ene formulations. The porous amine containing beads prepared in a first trial from the formulation TT/(DAP + AA) containing 60 wt% of the porogen DOP, showed a distorted, non-spherical surface (Fig. 4a). In contrast, well-defined spherical porous beads were prepared with the same formulation containing n-butyl acetate (60 wt%) as the porogen (Fig. 4b). Thus, the latter porogen was also used for the generation of hydroxyl (Fig. 4c) and carboxyl (Fig. 4d) containing beads. Porous thiol-ene beads with an average size between 330 to 375 μm (Fig. 4a1, 4b1 and 4d1) were prepared using a 30G needle at a pumping rate of 0.2 mL h−1 (reagent phase) and the average size was reduced to about 265 μm (Fig. 4c1) when using a 32G needle at a pumping rate of 0.15 mL h−1 (reagent phase). | ||
| Fig. 4 Representative optical (left) and SEM (middle and right) images of the porous functional beads prepared from the ‘thiol-ene’ formulation of (a) TT/(DAP + AA)c, (b) TT/(DAP + AA)d, (c) TT/PTEd and (d) (TT + MA)/TTTd. c DOP as the porogen; d BuAc as the porogen. | ||
The SEM images of the porous amine (Fig. 4b2) and hydroxyl (Fig. 4c2) containing thiol-ene beads showed nearly 33 and 9% shrinkage in their dimensions compared to the corresponding optical images. As explained earlier, the shrinkage can be attributed to the low rigidity of the crosslinked thiol-ene network, de-swelling due to removal of the porogen, and the nature of -ene monomer used. This is again well supported by their low Tg values (7 and 4 °C) for the amine and the hydroxyl containing beads respectively (Table 1). In order to have high Tg porous thiol-ene beads, we prepared carboxyl containing thiol-ene beads based on the formulation (TT + MA)/TTT, in which TTT has a rigid ring structure. As expected, a higher Tg value (about 40 °C) was observed. Furthermore, we have hardly seen any shrinkage in the carboxyl containing beads from the comparison of the SEM (Fig. 4d2) and optical microscopy images (Fig. 4d1). This illustrates that, by using appropriate -ene and/or thiol monomers having a rigid structure in a thiol-ene formulation, cross-linked beads with Tgs above room temperature can be produced.
The surface morphology of an individual bead shows a porous rough surface structure for most thiol-ene beads, although a smooth surface is observed for the hydroxyl containing porous beads (Fig. 4c2). The size of the pores on the surface of the beads ranges from 50 nm to 1–3 microns as observed from SEM images. The specific surface area using BET method could not be determined for the amine containing porous thiol-ene based beads, possibly due to their lower Tg (below room temperature) and thereby shrinkage of these beads after washing and drying. Also, a very low specific surface area of 0.7 m2 g−1 was measured for the hydroxyl containing porous thiol-ene based beads. On the other hand, the carboxylic acid (prepared using TTT that has high rigid ring structure) containing porous beads prepared by the thiol-ene strategy showed a specific surface area of 4.9 m2 g−1.
The representative optical and SEM images of the porous functional beads prepared using thiol-yne formulations containing n-butyl acetate as the porogen are shown in Fig. 5. Monodisperse beads were obtained for both amine (∼280 μm) and hydroxyl (∼305 μm) containing beads as shown by the optical images in Fig. 5a1 and 5b1. These beads show ∼13% shrinkage in their SEM images, which can be attributed to the removal of porogen from the highly cross-linked thiol-yne beads. The Tg values for these amine and hydroxyl containing thiol-yne systems exhibit higher values compared to the thiol-ene system, 38 and 40 °C respectively. The SEM images (Fig. 5a3 and 5b3) show a detailed surface morphology of the porous beads under high magnification. A closed-packing of interconnected microglobules with dimensions varying from 200 nm to 1 micron are observed. The specific surface area determined by the BET method is 35.6 and 9.3 m2 g−1 for the amine and hydroxyl containing porous beads (Tg values above room temperature) respectively.
 | ||
| Fig. 5 Representative optical and SEM images of the porous functional beads prepared from the ‘thiol-yne’ formulation of (a) TT/(OY + PA)d, (b) TT/(OY + DPPD)d. d BuAc as the porogen. | ||
Surprisingly, the specific surface area for the amine containing beads TT/(OY + PA)e based on the porogen xylene was very low, i.e. 3.5 m2 g−1. Fig. 6 shows representative SEM images of the crushed beads of TT/(OY + PA)d (Fig. 6a) and TT/(OY + PA)e (Fig. 6b) under high magnification. It is clear that the beads based on the porogen BuAc showed well interconnected nanoglobules with micro and mesopores, which in turn showed a high surface area of 35.6 m2 g−1. On the other hand, the porous amine beads based on the porogen xylene showed non-porous microglobules with ill-defined interconnections as a result of phase separation, explaining the low porosity of these beads.
 | ||
| Fig. 6 Representative SEM images under high magnification showing interior structure for amine containing porous (a) TT/(OY + PA)d and (b) TT/(OY + PA)e. d BuAc as the porogen, e Xy as the porogen. | ||
Also, a very low specific surface area of 2.3 m2 g−1 was determined for the amine containing porous thiol-yne beads [TT/(OY + PA)d,g] that have a Tg (13 °C) well below room temperature. The low Tg can be attributed to a reduced crosslink density of the beads owing to the presence of a large amount of monofunctional yne-amine monomer, PA (see Table 1). The effect of the porogen on the morphology of beads has been well studied in the literature for free radical copolymerization of vinyl monomers.33,56,57 In the previous section, it has already been shown that the nature of the porogen is also important for beads derived from thiol-ene formulations (Fig. 4). The output is of interest where xylene is used as the sole porogen or mixed with n-butyl acetate (Fig. 7) instead of using pure n-butyl acetate for the thiol-yne formulations given in Fig. 5. For both of the amine and hydroxyl monomers used, beads exhibited an inner structure composed of globules of almost the same size. As size monodisperse beads provide more reproducible and reliable results for chromatographic applications, commercial columns are packed with such size monodisperse particles.58 Thiol-yne beads prepared in this study, using xylene as the porogen, are not only size monodisperse but also possess almost monodisperse globules (Fig. 7). Such unique size and globule monodisperse beads may find chromatographic applications since separation efficiency is directly related with the structure of the column packing. However these beads prepared with Xy and mixture of solvents (Xy + BuAc) exhibited reduced values of surface area; 3.5 and 2.2 m2 g−1 respectively (Fig. 7a and 7b). This is not surprising as it is well known that macroporous beads exhibit lower surface area values compared to micro- and mesoporous beads.59 To the best of our knowledge, the highest reported surface area is below 30 m2 g−1 for macroporous beads produced in a microfluidic setup,33 although values over 400 m2 g−1 are reported when suspension polymerization is utilized for exactly the same monomers.60
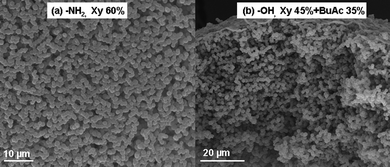 | ||
| Fig. 7 Representative SEM images of the porous functional beads prepared from the ‘thiol-yne’ formulation of (a) TT/(OY + PA)e, (b) TT/(OY + DPPD)f. e Xy as the porogen and f Xy + BuAc as the porogen. | ||
On the other hand, it has been demonstrated that the rate of the polymerization affects the final porous character of particles in suspension polymerization61 Thus, we believe that the fast UV initiated polymerizations may be responsible for the low surface area values for the beads obtained from the microfluidic setup, because this is the only difference between microfluidics and suspension polymerization approaches.
The yields for porous beads are in the range of 62 to 75%, which is lower when compared to nonporous beads. The possible reason for these lower yields, especially for porous amine containing beads, could be leaching of unreacted monofunctional monomers (e.g. AA and PA) along with the porogen during the initial reaction period and/or during curing time into the continuous phase.
Bead functionality
The presence of functional groups is confirmed by FTIR (Fig. 8). A very broad peak around 3450 cm−1 clearly illustrates –NH and –OH stretching in the amine (Fig. 8a) and hydroxyl (Fig. 8c) containing beads. In addition, the strong broad carbonyl stretching absorption at 1780 to 1710 cm−1 indicates the presence of carboxylic acid groups in the carboxyl beads (Fig. 8b). The FTIR spectra of the cured beads showed no detectable amount of carbon to carbon allyl or alkyne stretching around 1640 and 2150 cm−1 respectively. Also, Raman spectra (not shown) have not revealed any presence of unreacted groups (thiol or alkene/alkyne) in the beads. This indicates that the conversion of the reactive groups at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of thiol to π-bond is nearly quantitative. In Table 2, it is shown that the amine and hydroxyl loading in the porous beads is higher than that of the nonporous beads, which can be explained by the increased accessibility of functional groups due to the porous structure. However, the amine loading for the porous beads TT/(OY + PA)d is lower. This could be attributed to the decreased accessibility of amine groups as a result of the high cross-link density and low specific surface area (Table 1). Also, a very low hydroxyl group measurement for the nonporous bead (TT/DPPD) can be explained by its high cross-link density (thiol-yne). In general, the amine and hydroxyl loading determined by the Fmoc method is less than 50% compared to the initial loading. In general, the lower loading in the beads can be attributed to the restricted access in the highly crosslinked system on one hand and to the leaching of the some fraction of monofunctional monomers to the continuous aqueous phase on the other hand. It is worth noting that the highly sensitive colorimetric thiol test53 (with a detection limit as low as 5 μmol g−1) was positive for all the beads, which indicates the presence of unreacted free thiols. We further quantified free thiol groups of various functional beads via the Ellman's colorimetric test. The loading was found to be in the range of 1–7 μmol g−1, which is negligible.
1 ratio of thiol to π-bond is nearly quantitative. In Table 2, it is shown that the amine and hydroxyl loading in the porous beads is higher than that of the nonporous beads, which can be explained by the increased accessibility of functional groups due to the porous structure. However, the amine loading for the porous beads TT/(OY + PA)d is lower. This could be attributed to the decreased accessibility of amine groups as a result of the high cross-link density and low specific surface area (Table 1). Also, a very low hydroxyl group measurement for the nonporous bead (TT/DPPD) can be explained by its high cross-link density (thiol-yne). In general, the amine and hydroxyl loading determined by the Fmoc method is less than 50% compared to the initial loading. In general, the lower loading in the beads can be attributed to the restricted access in the highly crosslinked system on one hand and to the leaching of the some fraction of monofunctional monomers to the continuous aqueous phase on the other hand. It is worth noting that the highly sensitive colorimetric thiol test53 (with a detection limit as low as 5 μmol g−1) was positive for all the beads, which indicates the presence of unreacted free thiols. We further quantified free thiol groups of various functional beads via the Ellman's colorimetric test. The loading was found to be in the range of 1–7 μmol g−1, which is negligible.
 | ||
| Fig. 8 IR spectra for (a) TT/PA, (b) (TT + MA)/TTTd, and (c) TT/(OY + DPPD)d. dn-BA as the porogen. | ||
| Bead type | Sample formulation | Ratio of DAP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AA/OY AA/OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PA/OY PA/OY![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DPPD DPPD |
Initial amine/hydroxyl loading/mmol g−1 | Amine/hydroxyl loading by Fmoc method/mmol g−1 (%) |
|---|---|---|---|---|
a Thiol-ene system.
b Thiol-yne system.
c DOP as the porogen.
d
n-BA as the porogen.
e Ratio of thiol to π-bond is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.25. 1.25.
|
||||
| Nonporous amine a | TT(DAP + AA) | 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
1.56 | 0.55 (35) |
| Nonporous hydroxyl a | TT/PTE | — | 1.57 | 0.49 (30) |
| Nonporous amine b | TT/PA | — | 3.08 | 0.68 (22) |
| Nonporous hydroxyl b | TT/DPPD | 3.01 | 0.24 (8) | |
| Porous amine a | TT/(DAP + AA)c | 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
1.56 | 0.65 (42) |
| Porous amine a | TT/(DAP + AA)d | 0.65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.35 0.35 |
1.45 | 0.69 (48) |
| Porous hydroxyl a | TT/PTEd | — | 1.59 | 0.68 (42) |
| Porous amine b | TT/(OY + PA)d | 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
0.81 | 0.23 (28) |
| Porous hydroxyl b | TT/(OY + DPPD)d | 0.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 0.25 |
0.84 | 0.24 (29) |
| Porous amine b | TT/(OY + PA)d,e | 0.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
2.81 | 0.48 (17) |
Conclusion
In this work, it has been demonstrated that the rapid radical-mediated thiol-ene and thiol-yne photopolymerizations can be performed in a simple microfluidic setup to produce various macroporous and nonporous monodisperse functional beads without the need of any post-functionalization steps. The thiol-yne reaction results in an inherently higher cross-link density in the beads, resulting in higher glass transition temperatures compared to the beads produced by the thiol-ene reaction. It is realized that the nature of the porogen used plays an important role in the final porous character. Macroporosity has been proven by SEM images and for some cases low surface areas are obtained, which is expected for macroporous particles. Nevertheless, here we report a maximum value of 35.6 m2 g−1, surpassing the results of a previous detailed study.33 It is also important that we were able to obtain size and globule monodisperse particles as unique structures via using xylene as the porogen in thiol-yne formulations. Amine, hydroxyl and carboxyl groups on the beads have been detected by IR, while Fmoc-glycine is successfully coupled to amino and hydroxy beads proving the capability of these beads to be used as supports for amino acid coupling. This new type of nonporous and macroporous functional resins may find applications in the areas of SPPS, SPOS, scavenging, immobilization and catalysis.Acknowledgements
The authors acknowledge Ghent University for offering a ‘Special Research Fund’ to R.A.P. This work has been financially supported by a Marie Curie Early Stage Research Training Fellowship of the European Community's Sixth Framework Program under contract number 020643 (M.T.G, Sendichem project) and The Belgian Program on Interuniversity Attraction Poles initiated by the Belgian State, Prime Minister's office (Program P6/27).References
- R. Haag and S. Roller, Top. Curr. Chem., 2004, 242, 1–42 CAS.
- I. Bunia, V. Neagu and C. Luca, React. Funct. Polym., 2006, 66, 871–883 CrossRef CAS.
- N. Miletic, Z. Vukovic, A. Nastasovic and K. Loos, J. Mol. Catal. B: Enzym., 2009, 56, 196–201 CrossRef CAS.
- N. Fontanals, R. M. Marce, P. A. G. Cormack, D. C. Sherrington and F. Borrull, J. Chromatogr., A, 2008, 1191, 118–124 CrossRef CAS.
- M. Slater, M. Snauko, F. Svec and J. M. J. Frechet, Anal. Chem., 2006, 78, 4969–4975 CrossRef CAS.
- W. H. Li and H. D. H. Stover, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 1543–1551 CrossRef CAS.
- L. Leeb, P. Gmeiner and S. Löber, QSAR Comb. Sci., 2007, 26, 1145–1150 Search PubMed.
- A. R. Vaino and K. D. Janda, J. Comb. Chem., 2000, 2, 579–596 CrossRef CAS.
- M. Orzáez, P. Mora, L. Mondragón, E. Pérez-Payá and M. Vicent, Int. J. Pept. Res. Ther., 2007, 13, 281–293 Search PubMed.
- F. Limé and K. Irgum, Macromolecules, 2009, 42, 4436–4442 CrossRef CAS.
- R. B. Merrifield, J. Am. Chem. Soc., 1963, 85, 2149–2154 CrossRef CAS.
- D. C. Sherrington, Chem. Commun., 1998, 2275–2286 RSC.
- D. Hudson, J. Comb. Chem., 1999, 1, 333–360 CrossRef CAS.
- D. Hudson, J. Comb. Chem., 1999, 1, 403–457 CrossRef CAS.
- http://www.rapp-polymere.com/index_m.htm, accessed 08/01/2010.
- A. Basso, P. Braiuca, L. De Martin, C. Ebert, L. Gardossi, P. Linda, S. Verdelli and A. Tam, Chem.–Eur. J., 2004, 10, 1007–1013 CrossRef CAS.
- A. Akelah, S. B. Kingston and D. C. Sherrington, Polym. Commun., 1983, 24, 281–284 CAS.
- C. Blackburn, Biopolymers, 1998, 47, 311–351 CrossRef CAS.
- A. R. Mitchell, S. B. H. Kent, M. Engelhard and R. B. Merrifield, J. Org. Chem., 1978, 43, 2845–2852 CrossRef CAS.
- M. Meldal, in Methods in Enzymology, ed. G. B. Fields, Academic Press, 1997, vol. 289, pp. 83–104 Search PubMed.
- M. Renil and M. Meldal, Tetrahedron Lett., 1995, 36, 4647–4650 CrossRef CAS.
- C. C. Zikos and N. G. Ferderigos, Tetrahedron Lett., 1995, 36, 3741–3744 CrossRef CAS.
- M. Roice, S. F. Christensen and M. Meldal, Chem.–Eur. J., 2004, 10, 4407–4415 CrossRef CAS.
- J.-F. Morizur and L. J. Mathias, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 3182–3192 CrossRef CAS.
- R. Arshady, Colloid Polym. Sci., 1992, 270, 717–732 CrossRef CAS.
- M. Antonietti and K. Landfester, Prog. Polym. Sci., 2002, 27, 689–757 CrossRef CAS.
- C. Garcia-Diego and J. Cuellar, Ind. Eng. Chem. Res., 2005, 44, 8237–8247 CrossRef CAS.
- R. Kita, F. Svec and J. M. J. Frechet, J. Comb. Chem., 2001, 3, 564–571 CrossRef CAS.
- B. Saracoglu, E. Uguzdogan, C. Golgelioglu and A. Tuncel, Ind. Eng. Chem. Res., 2009, 48, 4844–4851 CrossRef CAS.
- C. A. Serra and Z. Chang, Chem. Eng. Technol., 2008, 31, 1099–1115 CrossRef CAS.
- A. S. Utada, L.-Y. Chu, A. Fernandez-Nieves, D. R. Link, C. Holtze and D. A. Weitz, MRS Bull., 2007, 32, 702–708 CAS.
- S. Xu, Z. Nie, M. Seo, P. Lewis, E. Kumacheva, H. A. Stone, P. Garstecki, D. B. Weibel, I. Gitlin and G. M. Whitesides, Angew. Chem., Int. Ed., 2005, 44, 724–728 CrossRef CAS.
- S. Dubinsky, H. Zhang, Z. Nie, I. Gourevich, D. Voicu, M. Deetz and E. Kumacheva, Macromolecules, 2008, 41, 3555–3561 CrossRef CAS.
- S. Dubinsky, J. I. Park, I. Gourevich, C. Chan, M. Deetz and E. Kumacheva, Macromolecules, 2009, 42, 1990–1994 CrossRef CAS.
- G.-R. Yi, S.-J. Jeon, T. Thorsen, V. N. Manoharan, S. R. Quake, D. J. Pine and S.-M. Yang, Synth. Met., 2003, 139, 803–806 CrossRef CAS.
- A. S. Utada, E. Lorenceau, D. R. Link, P. D. Kaplan, H. A. Stone and D. A. Weitz, Science, 2005, 308, 537–541 CrossRef.
- Z. Nie, S. Xu, M. Seo, P. C. Lewis and E. Kumacheva, J. Am. Chem. Soc., 2005, 127, 8058–8063 CrossRef CAS.
- T. Nisisako, T. Torii, T. Takahashi and Y. Takizawa, Adv. Mater., 2006, 18, 1152–1156 CrossRef CAS.
- G. A. Gross, C. Hamann, P. M. Günther and J. M. Köhler, Chem. Eng. Technol., 2007, 30, 341–346 CrossRef.
- M. T. Gokmen, W. Van Camp, P. J. Colver, S. A. F. Bon and F. E. Du Prez, Macromolecules, 2009, 42, 9289–9294 CrossRef CAS.
- A. Gress, A. Volkel and H. Schlaad, Macromolecules, 2007, 40, 7928–7933 CrossRef CAS.
- K. L. Killops, L. M. Campos and C. J. Hawker, J. Am. Chem. Soc., 2008, 130, 5062–5064 CrossRef CAS.
- J. W. Chan, B. Yu, C. E. Hoyle and A. B. Lowe, Chem. Commun., 2008, 4959–4961 RSC.
- N. Gupta, B. F. Lin, L. M. Campos, M. D. Dimitriou, S. T. Hikita, N. D. Treat, M. V. Tirrell, D. O. Clegg, E. J. Kramer and C. J. Hawker, Nat. Chem., 2010, 2, 138–145 Search PubMed.
- H. C. Kolb, M. G. Finn and K. B. Sharpless, Angew. Chem., Int. Ed., 2001, 40, 2004–2021 CrossRef CAS.
- B. D. Fairbanks, T. F. Scott, C. J. Kloxin, K. S. Anseth and C. N. Bowman, Macromolecules, 2009, 42, 211–217 CrossRef CAS.
- J. W. Chan, C. E. Hoyle and A. B. Lowe, J. Am. Chem. Soc., 2009, 131, 5751 CrossRef CAS.
- M. T. Gokmen, B. G. De Geest, W. E. Hennink and F. E. Du Prez, ACS Appl. Mater. Interfaces, 2009, 1, 1196–1202 Search PubMed.
- E. Quevedo, J. Steinbacher and D. T. McQuade, J. Am. Chem. Soc., 2005, 127, 10498–10499 CrossRef CAS.
- D. Fournier and F. Du Prez, Macromolecules, 2008, 41, 4622–4630 CrossRef CAS.
- A. Madder, N. Farcy, N. G. C. Hosten, H. De Muynck, P. J. De Clercq, J. Barry and A. P. Davis, Eur. J. Org. Chem., 1999, 2787–2791 CrossRef CAS.
- M. Hori, D. J. Gravert, P. Wentworth and K. D. Janda, Bioorg. Med. Chem. Lett., 1998, 8, 2363–2368 CrossRef CAS.
- J. Caroen and J. Van der Eycken, Tetrahedron Lett., 2009, 50, 41–44 CrossRef CAS.
- J. P. Badyal, A. M. Cameron, N. R. Cameron, D. M. Coe, R. Cox, B. G. Davis, L. J. Oates, G. Oye and P. G. Steel, Tetrahedron Lett., 2001, 42, 8531–8533 CrossRef CAS.
- N. B. Cramer, S. K. Reddy, A. K. O'Brien and C. N. Bowman, Macromolecules, 2003, 36, 7964–7969 CrossRef CAS.
- D. Horak, Z. Pelzbauer, F. Svec, J. Labsky and M. Bleha, Angew. Makromol. Chem., 1983, 117, 117–129 CrossRef CAS.
- T. Rohr, S. Knaus, H. Gruber and D. C. Sherrington, Macromolecules, 2002, 35, 97–105 CrossRef CAS.
- M. J. Benes, D. Horak and F. Svec, J. Sep. Sci., 2005, 28, 1855–1875 CrossRef.
- H. Zhang, G. C. Hardy, M. J. Rosseinsky and A. I. Cooper, Adv. Mater., 2003, 15, 78–81 CAS.
- D. Horak, F. Svec, M. Bleha and J. Kalal, Angew. Makromol. Chem., 1981, 95, 109–115 CrossRef CAS.
- F. Svec and J. M. J. Frechet, Macromolecules, 1995, 28, 7580–7582 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
