Acoustical parameters of polyacrylamide with sodium (meta) silicate and potassium silicate solution at 303 K
S.
Ravichandran
*a and
K.
Ramanathan
b
aDepartment of Physics, Sathyabama University, Chennai, 600 119, Tamilnadu, India. E-mail: rs_ravichandran@yahoo.com
bDepartment of Physics, Thiagarajar College of Engg, Madurai, 15, Tamilnadu, India
First published on 15th February 2010
Abstract
The ultrasonic velocity (U) and density (ρ) have been measured in mixtures of polyacrylamide solution in sodium meta silicate and potassium silicate solutions in different concentrations at 303 K. Based on the data obtained, the adiabatic compressibility (βad), intermolecular free length (Lf), acoustic impedance (Z) and relative association have been calculated. Variations of the acoustical parameters in polyacrylamide are studied with silicate solutions. The results have been discussed in terms of various interactions present in these mixtures and relative associations in the components.
1. Introduction
Ultrasound has been extensively used to determine the ion-solvent interactions in aqueous solutions containing electrolytes.1,2 Acoustical parameters have been determined to study the ion-solvent interactions in alkali and alkaline earth metal ions.Das and Jha employed the ultrasonic velocity and viscosity measurements to determine the co-ordination of Co2+ ions in aqueous solutions containing different anions.3 They found that the number of water molecules present in the co-ordination sphere varies with the anion. Acoustical parameters have been determined to study the ion-solvent interactions in alkali and alkaline earth metal ions.4 In spite of having industrial and biological importance, so far no systematic studies have been done on the acoustical properties of solutions containing transition of metal ions. This paper deals with the calculation of acoustical parameters such as adiabatic compressibility, free length, acoustic impedance and relative association from the measured ultrasonic velocity and density.
2. Experimental
The ultrasonic velocities were measured in aqueous solutions of polyacrylamide (PAA) (MERCK-MW-5000000) with sodium meta silicate and potassium silicate solutions by ultrasonic interferometer (Mittel Enterprises, New Delhi) operating at 2 MHz. Doubly distilled water was used in all cases. Polyacrylamide solution was prepared at a concentration of 0.1%. The densities were measured by specific gravity bottle and are accurate to three decimal places. All the experiments were carried out at 303 K.Sodium meta silicate powder (GR grade, MW = 284.20 g mol−1) and potassium silicate solution were employed. From the standard solution, 1%, 0.25 N and 0.5 N solutions were prepared. The polyacrylamide solution and sodium meta silicate solution were mixed at different volume ratios and ultrasonic studies were reported.
3. Results
Ultrasonic velocities and densities are measured for the mixture of polyacrylamide with sodium meta silicate and potassium silicate solutions at different volume ratios and the data are shown in Tables 1–3. The other acoustical parameters are calculated by using as usual formulae.5,6Volume ratio of PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na meta Si/ml Na meta Si/ml |
ρ/kg m−3 | U/m s−1 | Cm of Na Si | β × 10−10/kg m−2 S−1 | L f × 10−12/m | Z/kg m−2 S−1 | RA |
|---|---|---|---|---|---|---|---|
| 0.5% of PAA vs. 1% of Na meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1000 | 1513 | 0.00000 | 4.3684 | 1.324 | 1513000 | 1.000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1008 | 1528 | 0.99980 | 4.2497 | 1.305 | 1540010 | 1.005 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1004 | 1521 | 0.99995 | 4.3044 | 1.314 | 1527434 | 1.003 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1006 | 1517 | 0.99998 | 4.3209 | 1.316 | 1525500 | 1.005 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1006 | 1514 | 0.99999 | 4.3352 | 1.319 | 1523467 | 1.006 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1006 | 1520 | 0.99997 | 4.3005 | 1.313 | 1529534 | 1.005 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
1008 | 1521 | 0.99999 | 4.2854 | 1.311 | 1533866 | 1.007 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1012 | 1526 | 1.00000 | 4.2449 | 1.305 | 1543957 | 1.009 |
| 0.5% of PAA vs. 0.25 N of Na meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1000 | 1513 | 0.000000 | 4.3684 | 1.324 | 1513000 | 1.000 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1005 | 1523 | 0.999981 | 4.2899 | 1.312 | 1530590 | 1.003 |
80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1010 | 1530 | 0.999985 | 4.2298 | 1.302 | 1545311 | 1.006 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1014 | 1533 | 0.999991 | 4.1943 | 1.297 | 1554822 | 1.009 |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1021 | 1537 | 0.999994 | 4.1460 | 1.290 | 1569339 | 1.012 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1022 | 1542 | 0.999996 | 4.1183 | 1.285 | 1574727 | 1.015 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 60 60 |
1025 | 1546 | 0.999997 | 4.0802 | 1.279 | 1585289 | 1.018 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70 70 |
1028 | 1551 | 0.999999 | 4.0424 | 1.273 | 1594680 | 1.020 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80 80 |
1032 | 1557 | 0.999999 | 3.9985 | 1.266 | 1606655 | 1.022 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90 90 |
1033 | 1563 | 1.000000 | 3.9624 | 1.261 | 1614473 | 1.022 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1038 | 1567 | 1.000000 | 3.9259 | 1.255 | 1626016 | 1.026 |
Volume ratio of PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na meta Si/ml Na meta Si/ml |
ρ/kg m−3 | U/m s−1 | Cm of Na M Si | β × 10−10/kg m−2 S−1 | kg m−2 S−1 m | Z/kg m−2 S−1 | RA |
|---|---|---|---|---|---|---|---|
| 0.5% of PAA vs. 0.5 N of Na meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1000 | 1513 | 0.00000 | 4.3684 | 1.324 | 1513000 | 1.000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1014 | 1536 | 0.999992 | 4.1828 | 1.295 | 1556672 | 1.008 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1024 | 1550 | 0.999997 | 4.0656 | 1.277 | 1586994 | 1.016 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1038 | 1567 | 0.999998 | 3.9239 | 1.254 | 1626127 | 1.026 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1048 | 1579 | 0.999999 | 3.8282 | 1.239 | 1654768 | 1.033 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1062 | 1600 | 1.000000 | 3.6784 | 1.215 | 1699340 | 1.042 |
| 0.5% of PAA vs. 1 N of Na meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1000 | 1513 | 0.000000 | 4.3684 | 1.324 | 1513000 | 1.000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1028 | 1549 | 0.999996 | 4.0582 | 1.276 | 1591332 | 1.020 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1049 | 1578 | 0.999998 | 3.8189 | 1.238 | 1655417 | 1.034 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1071 | 1610 | 0.999999 | 3.6021 | 1.202 | 1724326 | 1.049 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1090 | 1629 | 0.999999 | 3.4587 | 1.178 | 1775088 | 1.063 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1116 | 1667 | 1.000000 | 3.2254 | 1.137 | 1860331 | 1.081 |
Volume ratio of PAA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) potassium Si/ml potassium Si/ml |
ρ/kg m−3 | U/m s−1 | Cm of K Si | β × 10−10/kg m−2S−1 | Lf × 10−12/m | Z/kg m−2 S−1 | RA |
|---|---|---|---|---|---|---|---|
| 0.1% of PAA vs. 0.5% of potassium meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1002 | 1513 | 0.000000 | 4.3596 | 1.322 | 1516026 | 1.000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1046 | 1525 | 0.999992 | 4.1112 | 1.284 | 1595025 | 1.043 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1089 | 1547 | 0.999997 | 3.8395 | 1.241 | 1684139 | 1.081 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1126 | 1560 | 0.999998 | 3.6508 | 1.209 | 1756200 | 1.115 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1164 | 1579 | 0.999999 | 3.4475 | 1.176 | 1837502 | 1.148 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1205 | 1595 | 1.000000 | 3.2621 | 1.144 | 1921975 | 1.184 |
| 0.25% of PAA vs. 0.5% of potassium meta silicate | |||||||
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00 00 |
1004 | 1507 | 0.000000 | 4.3857 | 1.326 | 1513028 | 1.000 |
40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 10 |
1045 | 1526 | 0.999996 | 4.1105 | 1.284 | 1594451 | 1.041 |
30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
1084 | 1542 | 0.999998 | 3.8823 | 1.248 | 1670986 | 1.077 |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 30 |
1124 | 1562 | 0.999999 | 3.6486 | 1.209 | 1755182 | 1.108 |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 40 |
1159 | 1577 | 0.999999 | 3.4703 | 1.179 | 1827511 | 1.139 |
00![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
1207 | 1603 | 1.000000 | 3.2250 | 1.137 | 1934604 | 1.179 |
The density of polyacrylamide with sodium meta silicate and potassium silicate solution is measured and given in Tables 1–3. It is observed that the density of a solution varies linearly with the addition of salt solution. The rate of variation of density is observed with increasing the concentration of a solution. But, in the case of 1% dilute solution of sodium meta silicate, non-linear variations are observed. It indicates the ion-solvent interactions in a mixed solution of polyacrylamide and sodium meta silicate or potassium silicate solution.6
The velocity of mixed solution of polyacrylamide with sodium meta silicate (Fig. 1) and potassium meta silicate solutions are measured at low concentrations. The velocity is increased with increasing the concentration of sodium meta silicate and potassium silicate solution (Fig. 2). However, the rate of variation of velocity increases as the concentration of salt solution increases. This again supports the close packing of molecules at higher concentration. This indicates good solubility of solvent and ion-solvent interaction increases with concentration,7 while in the case of polyacrylamide with 1% of sodium meta silicate solution, the velocity increases non-linearly with concentration. The velocity decreases to minimum value and again it increases with the increased concentration. These observations indicate that there are weaker ion-solvent interactions in the mixed solutions of polyacrylamide with 1% of sodium meta silicate solution.8 This non-linear variation of velocity indicates the presence of dipole-ion interaction in the system.
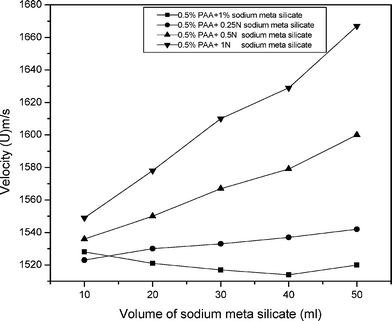 | ||
| Fig. 1 A graph of the ultrasonic velocity and volume of sodium meta silicate in polyacrylamide mixed solutions. | ||
 | ||
| Fig. 2 A graph of the ultrasonic velocity and volume of potassium silicate in polyacrylamide mixed solutions. | ||
The computed values of adiabatic compressibility of mixed solution of polyacrylamide with sodium meta silicate solution and potassium silicate solution are shown in Fig. 3 and 4. The adiabatic compressibility decreases with increasing concentration of the solution. But in the case of PAA with 1% of sodium meta silicate solution non-linear variations are obtained. The decrease in the values of compressibility in the present study indicates significant interaction between polyacrylamide and molecules of sodium meta silicate/potassium silicate solution.7,9
 | ||
| Fig. 3 A graph of the adiabatic compressibility and volume of sodium meta silicate in polyacrylamide mixed solutions. | ||
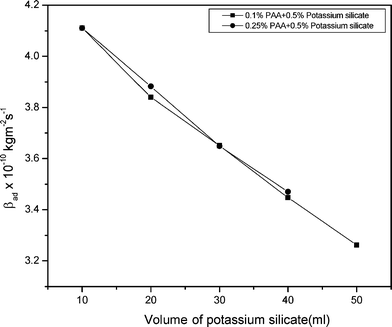 | ||
| Fig. 4 A graph of the adiabatic compressibility and volume of potassium silicate in polyacrylamide mixed solutions. | ||
The computed values of free length of the solutions are presented in Tables 1–3. The free length is a thermodynamic property measured by the ultrasonic velocity and it is the distance between the surfaces of the neighboring molecules. Even a small decrease in free length on addition of salt solution explains the fact that the molecules of polyacrylamide and the molecules of sodium meta silicate or potassium silicate become closer. The variations of intermolecular free length of a mixed solution are shown in Fig. 3 and 6. The closer packing of the molecules indicates that there are dipole–dipole interactions between the polyacrylamide and sodium meta silicate and potassium silicate solution.10 But the increase in free length after the addition of 1% of sodium meta silicate solution is due to weak interactions and it indicates complex formation between unlike molecules.6
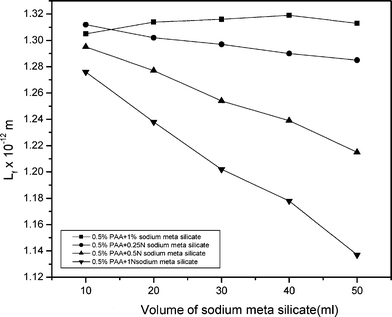 | ||
| Fig. 5 A graph of the intermolecular free length and volume of sodium meta silicate in polyacrylamide mixed solution. | ||
 | ||
| Fig. 6 A graph of the intermolecular free length and volume of potassium silicate in polyacrylamide mixed solution. | ||
The derived acoustic parameters and acoustic impedance of sodium meta silicate and potassium silicate salt solution in polyacrylamide are given in Tables 1–3. Acoustic impedance is the ratio of the sound pressure to the vibrational velocity of the particles of the medium. In all of the solutions of polyacrylamide with sodium meta silicate and potassium silicate, acoustic impedance increases with concentration, whereas the non-linear variations are observed in the 1% of sodium meta silicate solution. The acoustic impedance is minimal for the pure sodium meta silicate solution as well as for the volume ratio 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The acoustic impedance being the function of velocity and density, increases with the increase in concentration for a good interaction and in the former case, the interaction is poor.
10. The acoustic impedance being the function of velocity and density, increases with the increase in concentration for a good interaction and in the former case, the interaction is poor.
4. Discussion
The ultrasonic velocity of the mixed solutions of polyacrylamide with sodium meta silicate solutions in low concentrations was measured and results are presented in Table 1. Non-linear variations are observed. It increases in the beginning followed by a decrease and then it increases again. This observation suggests that sodium meta silicate is not thoroughly miscible with polyacrylamide in all proportions. There must be densification at the volume ratio of 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, but it is lost at 30
10, but it is lost at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and above. At 50
20 and above. At 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 and above the ultrasonic velocity starts increasing. The increasing portions are therefore attributed to through mixing of both the components. When the fresh sodium meta silicate is mixed with polyacrylamide the ultrasonic velocity decreases. Hence there may be more and more interaction between polyacrylamide and sodium meta silicate leading to densification.
50 and above the ultrasonic velocity starts increasing. The increasing portions are therefore attributed to through mixing of both the components. When the fresh sodium meta silicate is mixed with polyacrylamide the ultrasonic velocity decreases. Hence there may be more and more interaction between polyacrylamide and sodium meta silicate leading to densification.
At 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 00, there may well be dispersion of polyacrylamide. At 40
00, there may well be dispersion of polyacrylamide. At 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, the added sodium meta silicate may enhance the dispersion of polyacrylamide, i.e. it makes the medium less dense. But at 30
10, the added sodium meta silicate may enhance the dispersion of polyacrylamide, i.e. it makes the medium less dense. But at 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, there may be a strong association leading to more densification of the medium. It is similar at volume ratios of 20
20, there may be a strong association leading to more densification of the medium. It is similar at volume ratios of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 and 10
30 and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40.
40.
Table 2 illustrates the gradual increase in velocity with increasing the ratio of sodium meta silicate to polyacrylamide. Hence, there may be more and more segregation of polyacrylamide chains with the increasing concentration of silicate. The sodium silicate with its associated water can go between the polymer chains and disperse them. This only happens when there is a strong interaction between the polyacrylamide and sodium meta silicate. The decrease in intermolecular length with increase in amount of sodium meta silicate clearly supports this view.
Table 3 illustrates the increase in velocity with increase in potassium silicate. Hence potassium silicate may aid suppression of inter chain interactions in polyacrylamide. The increase in density and decrease in free length clearly supports the suppression of inter chain interaction in the polyacrylamide solutions.
Acknowledgements
The authors are grateful to Dr Arumugam, Scientist, Central Leather Research Institute, Chennai, Tamilnadu and Dr V. Rajendran, Director, Centre for Nano Technology, K.S.R College of Engineering & Technology, Namakkal District, for encouragement. The authors are also grateful to Dr P Palanichamy, Professor, Department of Chemistry, Anna University, Chennai, Tamil Nadu for support in this research work.References
- S. Jayakumar, S. Ganesh Ram, N. Karunanidhi and V. Kannappan, J. Pure Appl. Ultrason., 2001, 23, 31 Search PubMed.
- S. N. Sudhanshu, S. Nepal, G. P. Bhattarai and M. P. Adhikari, J. Pure Appl. Ultrason., 2002, 24, 79 Search PubMed.
- A. K. Das and B. L. Jha, Acoustics Lett., 1992, 15, 165 Search PubMed.
- G. Ravichandran and T. K. Nambinarayanan, Acoustics Lett., 1966, 12, 245 Search PubMed.
- K. Ramanathan and S. Ravichandran, J. Pure Appl. Ultrason, 2004, 26, 12 Search PubMed.
- S. Ravichandran and K. Ramanathan, J. Pure Appl. Ultrason., 2006, 28, 40 Search PubMed.
- D. S. Wankhede, N. N. Wankhede, M. K. Lande and B. R. Arbad, Indian J. Pure Appl. Phys., 2006, 44, 909 CAS.
- Piotr mieczik, Acoustics Lett., 1995, 18, 124 Search PubMed.
- V. Kannappan and S. Chidambara Vinayagam, Indian J. Pure Appl. Phys., 2006, 44, 670 CAS.
- K. Rajagopal and S. S. Jayabala Krishnan, J. Pure Appl. Ultrason., 2006, 28, 81 Search PubMed.
| This journal is © The Royal Society of Chemistry 2010 |
