Well-defined PCL-graft-PDMAEMA prepared by ring-opening polymerisation and click chemistry†
Vincent
Darcos
*,
Sarah
El Habnouni
,
Benjamin
Nottelet
,
Abdeslam
El Ghzaoui
and
Jean
Coudane
Max Mousseron Institute of Biomolecules, UMR CNRS 5247, University of Montpellier 1, University of Montpellier 2, Faculty of Pharmacy, 15 avenue Charles Flahault, BP 14491, 34093, Montpellier, France. E-mail: vincent.darcos@univ-montp1.fr; Fax: +33 467548552; Tel: +33 467548552
First published on 25th January 2010
Abstract
Amphiphilic and cationic PCL-based degradable polyester was synthesized by copper-catalyzed azide-alkyne cycloaddition (CuAAC).
The use of biodegradable micelles that are usually self-assembled from biodegradable amphiphilic block copolymers in water represents one of the most promising nanocarrier systems for drug and gene delivery.1–3 During the past decades, much research effort has been focused on the development of stimuli-responsive micelles in that release of water-insoluble drug could be controlled by exerting an appropriate stimulus (pH, temperature, redox potential, etc.).
Due to their demonstrated biocompatibility and biodegradability, aliphatic polyesters such as poly(glycolide), poly(lactide) or poly(ε-caprolactone) have been increasingly used in recent years for biomedical applications including nanocarriers in drug delivery. However, because of their hydrophobic and semicrystalline nature, and the lack of functional groups along the polyester backbone, their biomedical applications are limited. For example, they are often too hydrophobic for applications in aqueous environments. The introduction of pendent functional groups along these polyester chains is highly desirable to tailor and modulate physico-chemical properties, such as hydrophilicity, biodegradation rate, bioadhesion, crystallinity, and biological activity. Pendent functionalisation of aliphatic polyesters can be achieved by polymerisation of functionalised lactones,4 post-polymerisation modification, or a combination of these two approaches. The second approach was developed by Coudane and co-workers to graft functional groups onto preformed polyester.5–8 Poly(ε-caprolactone) (PCL) was reacted with lithium diisopropyl amide leading to the formation of polycarbanion, which was then added to various electrophiles. For example, an amphiphilic partially degradable graft copolymer, poly(ε-caprolactone)-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) (PCL-g-PDMAEMA), was prepared by anionic polymerization of DMAEMA monomers using a PCL-based macropolycarbanion as initiator.9 However, the macromolecular architecture of the copolymer was not controlled due to the high reactivity of the anionic species toward the ester groups.
We report here the synthesis of a well-defined poly(ε-caprolactone)-graft-poly(N,N-dimethylamino-2-ethyl methacrylate) (PCL-g-PDMAEMA) using click chemistry. Because of its high selectivity, tolerance to a broad range of functional groups, and mild reaction conditions, “click chemistry”, especially the copper-catalyzed Huisgen 1,3-dipolar cycloaddition of azides and terminal alkynes, is a powerful strategy for post-polymerisation reaction modification of aliphatic polyesters. Copper-catalyzed azide-alkyne cycloaddition (CuAAC) has been previously used for the preparation of block copolymers or polyesters with various pendent functional groups.10–13
Our synthetic approach to graft PDMAEMA on PCL is based on a three-step sequence combining (1) the synthesis of poly(α-propargyl-ε-caprolactone-co-ε-caprolactone), (2) the synthesis of α-azide terminated PDMAEMA by copper-mediated atom transfer radical polymerisation (ATRP), followed by (3) the CuAAC of both precursors. The reaction pathway for the synthesis of the functional polyester, poly(α-propargyl-ε-caprolactone-co-ε-caprolactone) 2 is shown in Scheme 1.
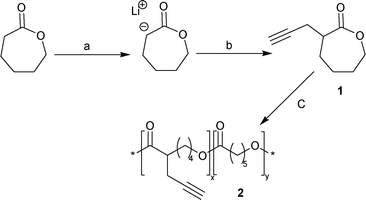 | ||
| Scheme 1 Reagents and conditions: (a) LDA/THF, −78 °C, (b) propargyl bromide, −78 °C to −30 °C, (c) ε-caprolactone, Sn(OTf)2, isopropanol, toluene, room temperature, 48 h. | ||
Lactone 1, α-propargyl-ε-caprolactone, was prepared from ε-caprolactone by reaction with lithium diisopropyl amide (LDA), followed by quenching with propargyl bromine according to a previous procedure.† Note that this strategy is a well-known method in organic chemistry to prepare functional lactones. In polymer chemistry, different substituted monomers were previously prepared such as α-allyl-δ-valerolactone,14 α-propargyl-δ-valerolactone,15 α-benzylcarboxylate-ε-caprolactone,16 α-iodo-ε-caprolactone.17
Copolymerization of εCL and 1 was carried out by ring opening polymerisation initiated with isopropanol and catalyzed with Sn(OTf)2 in toluene at room temperature ([εCL]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [1]
[1]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [Sn(OTf)2]
[Sn(OTf)2]![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [iPrOH] = 90
[iPrOH] = 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Copolymerisation was carried out with more than 90% conversion after 48 h. The SEC trace of the copolymer displayed a monomodal signal with an Mn,SEC and Mw/Mn of 6000 g mol−1 (relative to polystyrene standard) and 1.2 respectively. The molar ratio of 1 in the copolyester (F1= 0.08) was calculated by 1H NMR integration using the ratio of the propargyl signal (–C≡C–H from monomer 1) at δ = 2.01 ppm versus the backbone signal at δ = 4.03 ppm (CH2O of the polymer backbone from 1 and εCL).
1). Copolymerisation was carried out with more than 90% conversion after 48 h. The SEC trace of the copolymer displayed a monomodal signal with an Mn,SEC and Mw/Mn of 6000 g mol−1 (relative to polystyrene standard) and 1.2 respectively. The molar ratio of 1 in the copolyester (F1= 0.08) was calculated by 1H NMR integration using the ratio of the propargyl signal (–C≡C–H from monomer 1) at δ = 2.01 ppm versus the backbone signal at δ = 4.03 ppm (CH2O of the polymer backbone from 1 and εCL).
Azide-containing PDMAEMA (3) precursor was prepared following a similar procedure to that reported by Agut et al.18 Well defined α-azido PDMAEMA was synthesized by ATRP at 60 °C in toluene in the presence of CuBr/PMDETA as the catalytic system using a specific azide-functionalized initiator, the (3-azidopropyl)bromoisobutyrate. The controlled polymerisation of DMAEMA via ATRP has been well documented to obtain well-defined pH-sensitive PDMAEMA.19 SEC showed monomodal trace with polydispersity less than 1.2. Molecular weights (Mn) determined by SEC were relative to polystyrene standards, so more accurate values were provided by 1H NMR. Accordingly, Mn,NMR of 3 was determined to be 6500 g mol−1, in good agreement with the theoretical value 7000 g mol−1. Moreover, the presence of azide groups was characterized by the presence of azide absorbance peak at ∼2100 cm−1 in FT-IR spectrum.
Finally, the click reaction between 2 and 3 led to the formation of a graft copolymer 4 as shown in Scheme 2. CuAAC was performed in THF with CuBr/PMDETA as the catalytic system at room temperature for 24 h. SEC trace of PCL-g-PDMAEMA 4 showed a clear shift toward the high molecular weight in comparison with the starting homopolymers 2 and 3 (Fig. 1). A decrease of polydispersity was also observed. SEC traces strongly suggest that the PCL backbone does not degrade under the “click reaction” protocol. Indeed, compared to many other reactions, CuAAC has the advantage of being highly efficient under very mild conditions, leading to limited degradation of aliphatic polyester chains.12 The content of grafted PDMAEMA was estimated by 1H NMR integration using the ratio between εCL units and DMAEMA units. The experimental PDMAEMA-graft ratio was 0.09, in good agreement with the targeted ratio (0.08), corresponding to the molar ratio of 1 in the copolymer 2. The graft using CuAAC was highly efficient with a yield close to 100%.
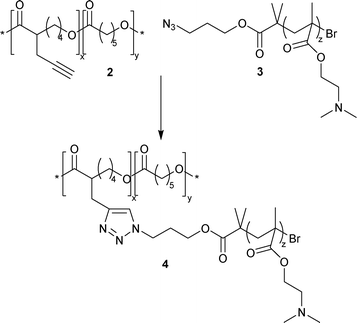 | ||
| Scheme 2 Reagents and conditions: CuBr/PMDETA, room temperature, 24 h. | ||
 | ||
Fig. 1 SEC traces of poly(α-propargyl-ε-caprolactone-co-ε-caprolactone) 2 (![[thick line, graph caption]](https://www.rsc.org/images/entities/char_e117.gif) ), α-azide PDMAEMA 3 ( ), α-azide PDMAEMA 3 (![[dash dash, graph caption]](https://www.rsc.org/images/entities/char_e091.gif) ), and PCL-g-PDMAEMA 4 (⋯). ), and PCL-g-PDMAEMA 4 (⋯). | ||
Amino groups of graft copolymer 4 were quaternized with methyl iodide to obtain water-soluble PCL-based copolymer. The aggregation properties of this graft copolymer were studied in aqueous media at pH 6. The copolymer was expected to aggregate in the form of micelles or larger aggregates with hydrophobic PCL cores and cationic PDMAEMA coronas. The aggregation behaviour was investigated by dynamic and static light scattering (DLS-SLS). The critical micellar concentration (CMC) for graft copolymer 4 was estimated in pure water, and was found to be ∼0.03 mg mL−1. This value is in good agreement with the CMC determined previously by Bougard et al. for PDMAEMA-g-PCL copolymers prepared by ATRP.20 These authors have found CMC values between 0.01 and 0.1 mg mL−1 depending on the effective weight fraction of PDMAEMA in the copolymer.
Then, the distribution of the micelles size was calculated from the autocorrelation functions using CONTIN algorithms. The micelles size distribution, measured at 0.3 mg mL−1, is shown in Fig. 2. Two populations were observed at 25 and 200 nm. Nevertheless, the population at 25 nm is dominant. The radius of the micelles is in the reasonable range for core-shell architecture where the condensed core of PCL is surrounded by a shell of expanded charged PDMAEMA chains.
 | ||
| Fig. 2 Size distribution of quaternized PCL-g-PDMAEMA micelles at 0.3 mg mL−1. | ||
To get more insight into the aggregation of the graft copolymer, the formation of aggregates by the quaternized precursor PDMAEMA (3) was also investigated. In aqueous solutions, quaternized PDMAEMA forms aggregates but at the higher concentration than the corresponding quaternized graft copolymer. The CMC, determined by light scattering, was found to be equal to 8 mg mL−1. This outstanding difference is explained by the hydrophobic nature of the PCL block in the graft copolymer.
When used as drug carriers in aqueous media, polymeric micelles are capable of encapsulating hydrophobic drugs in their core, thus improving the drug water solubility. The quaternized graft copolymer micelles were used to solubilise an anti-mycobacterial drug, the clofazimine (Fig. 3). This drug has the disadvantage of being highly hydrophobic with inherent water solubility below 0.3 μg mL−1 and Log P = 7.5. The aqueous graft copolymer solution where the concentration is above the CMC present a different behaviour in comparison with water and copolymer solution below the CMC. A deep red colour appears in copolymer solution where the concentration is above the CMC, which means that clofazimine was well solubilised. But, as the copolymer concentration decreases and becomes slightly lower than the CMC, the red colour becomes less lively as a consequence of the strong decrease in the clofazimine solubilisation.
 | ||
| Fig. 3 Clofazimine with (A) an aqueous graft copolymer solution where the concentration in graft copolymer is above the CMC (left), (B) water (center), (C) an aqueous graft copolymer solution where the concentration in graft copolymer is below the CMC (right). | ||
In summary, well-defined graft copolymers composed of a degradable poly(ε-caprolactone) backbone and poly[N,N-dimethylamino-2-ethyl methacrylate] (PDMAEMA) grafts were synthesized by Huisgen's 1,3-dipolar cycloaddition (click chemistry) from homopolymers containing azide and alkyne functionalities. The aggregation behaviour of the quaternized graft copolymer (4) was investigated. From the results for the micellar solutions herein reported, it was observed that the quaternized graft copolymer systems form micelles. Moreover, solubilisation of hydrophobic drugs such as clofazimine using graft copolymer micelles was observed.
The authors would like to thank the CNRS and the French Ministry of Education for the grant of S. El Habnouni.
Notes and references
- K. Kataoka, A. Harada and Y. Nagasaki, Adv. Drug Delivery Rev., 2001, 47, 113–131 CrossRef CAS.
- N. Nishiyama and K. Kataoka, Pharmacol. Ther., 2006, 112, 630–648 CrossRef CAS.
- V. P. Torchilin, Expert Opin. Ther. Pat., 2005, 15, 63–75 Search PubMed.
- X. D. Lou, C. Detrembleur and R. Jerome, Macromol. Rapid Commun., 2003, 24, 161–172 CrossRef CAS.
- B. Nottelet, A. El Ghzaoui, J. Coudane and M. Vert, Biomacromolecules, 2007, 8, 2594–2601 CrossRef CAS.
- M.-H. Huang, J. Coudane, S. Li and M. Vert, J. Polym. Sci., Part A: Polym. Chem., 2005, 43, 4196–4205 CrossRef CAS.
- B. Saulnier, S. Ponsart, J. Coudane, H. Garreau and M. Vert, Macromol. Biosci., 2004, 4, 232–237 CrossRef CAS.
- S. Ponsart, J. Coudane and M. Vert, Biomacromolecules, 2000, 1, 275–281 CrossRef CAS.
- B. Nottelet, M. Vert and J. Coudane, Macromol. Rapid Commun., 2008, 29, 743–750 CrossRef CAS.
- J.-F. Lutz, Angew. Chem., Int. Ed., 2008, 47, 2182–2184 CrossRef CAS.
- W. H. Binder and R. Sachsenhofer, Macromol. Rapid Commun., 2007, 28, 15–54 CrossRef CAS.
- P. Lecomte, R. Riva, C. Jerome and R. Jerome, Macromol. Rapid Commun., 2008, 29, 982–997 CrossRef CAS.
- L. P. Yang, X. H. Dong and C. Y. Pan, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 7757–7772 CrossRef CAS.
- B. Parrish, J. K. Quansah and T. Emrick, J. Polym. Sci., Part A: Polym. Chem., 2002, 40, 1983–1990 CrossRef CAS.
- B. Parrish, R. B. Breitenkamp and T. Emrick, J. Am. Chem. Soc., 2005, 127, 7404–7410 CrossRef CAS.
- A. Mahmud, X.-B. Xiong and A. Lavasanifar, Macromolecules, 2006, 39, 9419–9428 CrossRef CAS.
- S. El Habnouni, V. Darcos and J. Coudane, Macromol. Rapid Commun., 2009, 30, 165–169 CrossRef CAS.
- W. Agut, D. Taton and S. Lecommandoux, Macromolecules, 2007, 40, 5653–5661 CrossRef CAS.
- A. M. Funhoff, S. Monge, R. Teeuwen, G. A. Koning, N. M. E. Schuurmans-Nieuwenbroek, D. J. A. Crommelin, D. M. Haddleton, W. E. Hennink and C. F. van Nostrum, J. Controlled Release, 2005, 102, 711–724 CrossRef CAS.
- F. Bougard, C. Giacomelli, L. Mespouille, R. Borsali, P. Dubois and R. Lazzaroni, Langmuir, 2008, 24, 8272–8279 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures. See DOI: 10.1039/c0py00004c |
| This journal is © The Royal Society of Chemistry 2010 |
