Hybridization of epoxy resin with a layered titanate and UV light durability and controlled refractive index of the resulting nanocomposite
Yasufumi
Fuse
a,
Yusuke
Ide
b and
Makoto
Ogawa
*ab
aGraduate School of Creative Science and Engineering, Waseda University, 1-6-1 Shinjyuku-ku, Nishiwaseda, Tokyo, 169-8050, Japan
bDepartment of Earth Sciences, Waseda University, 1-6-1 Shinjyuku-ku, Nishiwaseda, Tokyo, 169-8050, Japan
First published on 24th February 2010
Abstract
A layered titanate-epoxy nanocomposite was synthesized by the reaction of a layered titanate modified with glycidyl and octadecyl groups and an epoxy resin followed by curing. The nanocomposite exhibited durability toward UV light compared with the pristine epoxy resin. Moreover, the refractive index (at 598 nm) of the nanocomposite was controlled in the rage of 1.51–1.53 by the added amount (0.00–0.04 mass%) of the organically modified titanate.
1. Introduction
The hybridization of polymers with a nanoscopic inorganic or organic phase has widely been investigated to improve the properties as well as to impart additional functions.1 To embed fillers in polymers at a nanometre scale, direct mixing of the two phases has often not been applicable, therefore, either surface modification of the nanoparticles or copolymerization of the filler and polymer sources has been conducted.1 Layered solids with swelling ability like smectite clays are hybridized with polymers to form nanocomposites where ultrathin silicate layers are dispersed in polymers.2 Since the pioneering work on smectite clay-polyamide nanocomposite which showed improved mechanical properties if compared with that of the pristine polymer,3 clay-polymer nanocomposites have attracted increasing attention.4 Layered solids with various compositions and particle sizes, which correlate to the physicochemical properties of the filled oxide sheets, are available, however, much fewer studies on nanocomposites with layered solids except for smectite clays have been conducted because of the difficulty in synthesizing the nanocomposites, or limited swelling of layered solids.5In this article, the successful synthesis of a layered titanate-polymer nanocomposite and the two useful properties of the nanocomposite are reported. Epoxy resin, which is one of the most commonly used engineering thermosetting plastics, was chosen as a polymer to be modified by embedding the titanate. The resulting layered titanate-epoxy nanocomposite exhibited UV light-durability, which was due to the effective absorption of UV light by the hybridized layered titanate, since layered titanates are semiconductors with the band gap energy corresponding to UV light.6 Moreover, the refractive index of the nanocomposite was controlled due to the higher refractive index of layered titanates (∼2.0 at 600 nm7) than that of epoxy resin. Although physicochemical properties of clay-polymer nanocomposites, such as improved mechanical and gas barrier ones and reduced flammability, have been reported,4 to our knowledge, the present report is the first example of the UV light durability and controlled refractive index of a layered solid-based nanocomposite. The present results suggest the merits of layered titanates as fillers for functionalizing polymers multiply.
For synthesizing the nanocomposite, a layered titanate, K0.59Ti1.66Li0.34O3.78,8 was modified with silane coupling reagents,9 glycidylpropyltrimethoxysilane and octadecyltrimethoxysilane, to swell in an epoxy resin. It has been reported that the surface modification of interlayer hydroxyl groups of layered alkali silicates with polymerizable silyl groups and subsequent copolymerization of the attached silyl groups with monomers resulted in the swelling of the layered solids in the corresponding polymers.10 We expect, in this study, that the intercalation of an epoxy resin and subsequent copolymerization of the epoxy resin with the attached glycidyl groups are facilitated by the expansion of the interlayer space with the co-attached alkyl groups.
2. Experimental
2.1 Reagents and materials
A layered lepidocrocite-type titanate, K0.59Ti1.66Li0.34O3.78, was synthesized by the reported method.8 Dodecylammine hydrochloride (>97%) was purchased from Tokyo Chemical Industry Co., Ltd. and used as received. Glycidylpropyltrimethoxysilane (GTMS) and octadecyltrimethoxysilane (C18TMS) were purchased from Gelest, Inc. and Tokyo Chemical Industry Co., Ltd., respectively and used as received. Glycerolpolyglycidylether, methyl nadic anhydride, dodecenyl succinic anhydride, and 2,4,6-tris(dimethylaminomethyl)phenol were purchased from Nisshin EM Co., Ltd. and used as received.2.2 Preparation of organically modified titanates
To immobilize the two organosilyl groups in an interlayer space of K0.59Ti1.66Li0.34O3.78, the dodecylammonium-exchanged form of the titanate (abbreviated as C12N+-TLO) was firstly synthesized9b and it was then reacted with GTMS and C18TMS sequentially, as reported for the preparation of a layered titanate modified with phenyl and octadecyl groups.11 The silylation of C12N+-TLO was conducted in a manner similar to that we have developed for the silylation of the dodecyltrimethylammonium-exchanged form of a layered alkali silicate.12 C12N+-TLO (0.20 g), which was synthesized by a reaction between K0.59Ti1.66Li0.34O3.78 and an aqueous solution of dodecylammine hydrogen chloride,8a was dispersed in a solution of GTMS (a molar Si/Ti1.66Li0.34O3.78 ratio = 0.50) in toluene (20 mL) and the mixture was heated at 60 °C for 12 h. After removal of the solvent from the mixture by evaporation, the resulting solid was dispersed in a solution of C18TMS (a molar Si/Ti1.66Li0.34O3.78 ratio = 0.50) in toluene (20 mL) and the mixture was heated at 60 °C for 12 h. The product was separated by removing the solvent from the mixture and washed with hexane. The synthesis of the organic derivative of K0.59Ti1.66Li0.34O3.78 modified only with GTMS was also conducted in a similar way, in which C12N+-TLO was reacted with GTMS (a molar Si/Ti1.66Li0.34O3.78 ratio = 1.0).2.3 Embedment of organic derivatives into epoxy resin
C18TMS-GTMS-TLO or GTMS-TLO (1.0 mg) was added to glycerolpolyglycidylether (a molecular weight of 189, 1.0 mL) and the mixture was stirred at 50 °C for 12 h. Methyl nadic anhydride (0.55 mL), dodecenyl succinic anhydride (0.60 mL), and 2,4,6-tris(dimethylaminomethyl)phenol (0.070 mL) were added to the mixture and the resulting mixture was stirred for 5 min at room temperature. The obtained mixture was degassed by centrifugation (3000 rpm, 15 min) and then casted on a glass substrate or poured into silicone mould followed by curing at 60 °C for 24 h.2.4 Characterization
X-Ray diffraction (XRD) patterns of products were recorded on a Rigaku RAD IB powder diffractometer equipped with monochromatic Cu Kα radiation operated at 20 mA and 40 kV. Infrared spectra of KBr disks were recorded on a Shimadzu FT-8200 Fourier-transform infrared spectrophotometer at a resolution of 2.0 cm−1. CHN elemental analysis was performed on a Perkin Elmer 2400 II instrument. Inductively-coupled plasma atomic emission spectroscopy was performed on a Rigaku SPECTRO CIROS CCD. UV-Vis spectra of liquid samples (an optical pass length of 5 mm) or cast films were recorded on a Shimadzu UV-3100PC spectrometer. Photo-durability of composites was measured for cast films fixed on sample chamber and by 46 cm away from light source (ultra-high pressure mercury lamp (350 W, Ushio Inc.)). Scanning electron microscopy (SEM) was performed on Hitachi S-2380N. Transmission electron microscopy (TEM) was performed on a JEOL JEM-100CX transmission electron microscope. The refractive index was detected on an Abbe refractometer DR-M2 (Atago Co., Ltd.) with the wavelength of 598 nm at 23 °C. The samples were rectangular parallelepiped-shaped, 40 mm long, 8 mm width, and 2 mm thick.3. Results and discussion
Fig. 1 shows the XRD pattern of C18TMS-GTMS-TLO together with those of K0.59Ti1.66Li0.34O3.78 and C12N+-TLO. The basal spacing of C12N+-TLO (2.7 nm) increased to 3.0 nm and 3.2 nm after the reaction with GTMS and C18TMS, respectively. The infrared spectrum of C18TMS-GTMS-TLO (Fig. 2a) showed an absorption band ascribable to Si–O–Ti stretching vibration (954 cm−1),13b in addition to those due to glycidyl (896 cm−1) and alkyl (at around 2900 cm−1) groups. These results show the successful immobilization of glycidylsilyl and octadecylsilyl groups. The XRD pattern is composed of apparently one crystalline compound (Fig. 1d), suggesting that the two organosilyl groups are located in the same interlayer space, as reported previously for the layered titanate organically modified with phenylsilyl and octadecylsilyl groups.11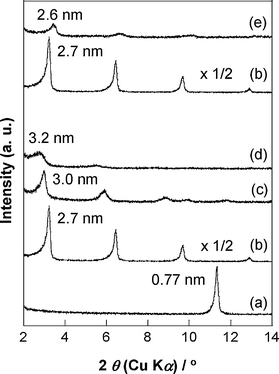 | ||
| Fig. 1 XRD patterns of (a) K0.59Ti1.66Li0.34O3.78, (b) C12N+-TLO, (c) (b) reacted with GTMS, (d) C18TMS-GTMS-TLO, and (e) GTMS-TLO. | ||
 | ||
| Fig. 2 Infrared spectra of (a) C18TMS-GTMS-TLO and (b) GTMS-TLO. | ||
The organic derivative of K0.59Ti1.66Li0.34O3.78 modified only with GTMS (GTMS-TLO) was also prepared, as a reference to discuss the cooperative effect of the two organosilyl groups on the swelling in an epoxy resin. The basal spacing of C12N+-TLO (2.7 nm) decreased to 2.6 nm after the reaction with GTMS (Fig. 1e), which decrease was due to the smaller molecular length of GTMS (1.5 nm × 0.5 nm × 0.5 nm) than that (1.9 × 0.4 nm × 0.4 nm) of C12N+. In addition to the XRD result, the infrared spectrum of GTMS-TLO (Fig. 2b), which shows Si–O–Ti absorption band (952 cm−1), reveals the successful attachment of GTMS onto K0.59Ti1.66Li0.34O3.78. From the basal spacing (3.2 nm and 2.6 nm, respectively) and composition (ca 0.7 groups per Ti1.66Li0.34O3.78) of C18TMS-GTMS-TLO and GTMS-TLO, taking the molecular size of C18TMS (2.6 nm × 0.5 nm × 0.5 nm) and GTMS into consideration, the attached silyl groups are thought to take an identical arrangement (interdigitated monolayer) in the two silylated derivatives. Accordingly, larger basal spacing (3.2 nm) of C18TMS-GTMS-TLO than that (2.6 nm) of GTMS-TLO is explained by the presence of C18TMS.
The composition of the obtained organic derivatives of K0.59Ti1.66Li0.34O3.78 is shown in Table 1. It should be noted here that the surface coverage with organosilyl groups is similar for C18TMS-GTMS-TLO and GTMS-TLO (0.74 and 0.70 groups per Ti1.66Li0.34O3.78, respectively). Based on the composition and the available surface area of K0.59Ti1.66Li0.34O3.78, 0.23 nm2 per Ti1.66Li0.34O3.78 unit cell (= 2ac = 2 × 0.38 nm × 0.30 nm, where a and c are the lattice parameters of the titanate14), the distance between the adjacent organosilyl groups was calculated to be 0.57 nm (= (0.23/0.7)1/2) (equivalent to 3.0 groups per nm2 (= 0.7/0.23)). In the light of the bottom area of the two organosilyl groups (0.5 nm × 0.5 nm) if their molecular shape is rectangular parallelepipeds, the organosilyl groups are thought to cover the interlayer surface of K0.59Ti1.66Li0.34O3.78 almost fully so that an epoxy resin can interact neither with surface titanol groups nor silanol groups formed by the hydrolysis of the remained methoxy groups of the attached C18TMS and GTMS. The surface coverage with organosilyl groups and the amount of interlayer hydroxy groups have been found to affect the adsorptive property of the organosilyl derivatives of layered solids.11,13,15 In the case of the present C18TMS-GTMS-TLO and GTMS-TLO, the surface coverage with organosilyl groups is similar and the presence of titanol and silanol groups is negligible as described above. Therefore, it is possible to discuss the cooperative effect of glycidyl and alkyl groups on the intercalation of an epoxy resin and subsequent copolymerization of the epoxy resin with the attached glycidyl groups.
The reaction of C18TMS-GTMS-TLO with an epoxy resin followed by curing resulted in the formation of the composite with texture homogeneity (Fig. 3 (I) c) and transparency in visible light wavelength region comparable to that of the pristine epoxy resin (Fig. 4 c). On the other hand, particles with several tens of μm, which is the aggregates of GTMS-TLO particles with a diameter of 1–2 μm (Fig. 3 (II) b), were observed in the GTMS-TLO-epoxy composite (Fig. 3 (I) b). The TEM image (Fig. 5) of the composite synthesized from C18TMS-GTMS-TLO showed that the titanate sheets with the thickness of ca. 1–5 nm and the diameter of several hundreds of nm were dispersed in the polymer. These results indicate the cooperative effect of the attached GTMS and C18TMS on the swelling of K0.59Ti1.66Li0.34O3.78 in the epoxy resin; the intercalation of an epoxy resin and copolymerization of the epoxy resin with the attached GTMS are facilitated by the expansion of the interlayer space with the attachment of C18TMS (Fig. 1 d and e).
 | ||
| Fig. 3 (I) Photographs of (a) epoxy resin and the composite with (b) GTMS-TLO and (c) C18TMS-GTMS-TLO; (II) SEM images of (a) GTMS-TLO and (b) C18TMS-GTMS-TLO. | ||
 | ||
| Fig. 4 Transmittance spectra of (a) epoxy resin and the composite with (b) GTMS-TLO and (c) C18TMS-GTMS-TLO and UV-vis absorption spectrum of a suspension of C18TMS-GTMS-TLO in benzene (1 mass%). | ||
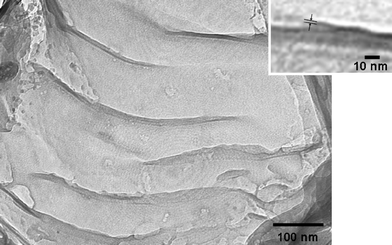 | ||
| Fig. 5 A TEM image of the C18TMS-GTMS-TLO-epoxy nanocomposite. The inset shows the magnified TEM image. | ||
The UV-Vis absorption spectrum of a colloidal suspension of C18TMS-GTMS-TLO (1.0 mass%) was shown in Fig. 4. An absorption peak was observed at around 280 nm, which was shifted to a shorter wavelength region from an absorption edge (350 nm) of K0.59Ti1.66Li0.34O3.78. Similar absorption peaks have been observed for the aqueous16 or organic13 suspensions of layered titanates and was due to the absorption of swelled titanate sheets.16 In the UV-Vis absorption spectra of the layered titanate-epoxy composites, the absorption onset of the epoxy resin was at around 450 nm, so that the location of the absorption due to the titanate sheets is not seen owing to the overlapping. The titanate sheets were shown to be dispersed in C18TMS-GTMS-TLO-epoxy nanocomposite as described above. The integral (2.4) of the absorption band at UV light wavelength region of the C18TMS-GTMS-TLO suspension, which correlates to the oscillator strength of the titanate sheets, is higher than that (0.013) of epoxy resin,17 suggesting that the titanate sheets absorb UV light preferentially to prevent UV light-degrading of the nanocomposite. The UV-light durability of the present materials was investigated using an ultra-high pressure mercury lamp as UV light source.
Fig. 6 depicts the variation in the transmission spectrum of the pristine epoxy resin with UV-light irradiation time. The transmittance at around 400 nm gradually decreased (Fig. 7 ○), as a result of the decomposition of organic groups in the epoxy resin to be yellow-colored (the pristine epoxy resin prepared in this study is originally pale yellow-colored). The GTMS-TLO-epoxy mixture, in which K0.59Ti1.66Li0.34O3.78 did not swell, was degraded similarly (Fig. 7 ◇). On the other hand, the degradation of the C18TMS-GTMS-TLO-epoxy nanocomposite was remarkably suppressed (Fig. 7 □). The titanate sheets were thought to absorb UV light efficiently to prevent the epoxy matrix from degrading. It has been reported that epoxy encapsulant around light emitting diode is UV light-degraded to discolor followed by the decrease in the light output intensity.18 The effect of the photocatalytic decomposition of organic substance by the titanate induced by UV light on the present photo-degradation of the epoxy resin is not clear at present.
 | ||
| Fig. 6 Variation in transmittance spectra of epoxy resin with UV light irradiaiton time; inset shows the expanded verion of the figure at the wavelength region from 430 to 370 nm. | ||
 | ||
| Fig. 7 Variation in transmittance at 400 nm as a function of UV light irradiation time of (○) epoxy resin and the composite of epoxy resin with (◇) GTMS-TLO and (□) C18TMS-GTMS-TLO. | ||
Another noticeable property of the C18TMS-GTMS-TLO-epoxy nanocomposite was the controlled refractive index. The control of refractive indices of organic polymers by hybridizing molecular titania (domain size of several to several tens of nm) has widely been investigated.19,20 In this study, as depicted in Fig. 8, the refractive index of epoxy resin was successfully controlled with the added amount (mass%) of the titanate. There is a linear relationship between the refractive indices of the nanocomposite and the C18TMS-GTMS-TLO concentration, suggesting again that the titanate sheets are homogeneously dispersed in epoxy resin and also implying that the refractive index variation reflects only the change of the amount of the titanate embedded in epoxy resin since the filler size does not vary depending on the loaded amount. Layered titanates with different particle size are available,8,14 therefore, the present result motivates us to investigate the effect of the aspect ratio (diameter/thickness) of the titanate sheets on the refractive index of nanocomposites. The gas barrier properties of clay-polymer nanocomposites have been demonstrated to correlate with the aspect ratio of the silicate layers.21
 | ||
| Fig. 8 Variation in refractive index of C18TMS-GTMS-TLO-epoxy nanocomposite with the weight concentration of the titania. | ||
Fig. 9 shows the variation in the refractive index of the present nanocomposite with the titania volume fraction22 together with that of the reported molecular titania (a domain size of ca. 100 nm)-based epoxy nanocomposite.20 It is worth mentioning that to increase the refractive index of epoxy resin the present system needs a much smaller amount of titania than the previously reported system. We believe that the ultrathin sheet morphology of the present filler plays an important role in the observed effect.
 | ||
| Fig. 9 Refractive index variation of (●) C18TMS-GTMS-TLO-epoxy nanocomposite and (□) the reported titania-based epoxy nanocomposite20 with the volume fraction of titania. Inset: an expanded version of the figure. | ||
4. Conclusion
We have successfully synthesized the layered titanate-epoxy nanocomposite by the reaction of a glycidyl and alkyl groups-modified K0.59Ti1.66Li0.34O3.78 with epoxy resin. The resulting nanocomposite, where the titanate sheets were dispersed in at the nanometre scale, exhibited durability toward UV light compared with the pristine epoxy resin. In addition, the refractive index of the nanocomposite was controlled with the amount of added organically modified titanate.Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (B) (19350103) from Japan Society for the Promotion of Science.References and notes
- P. M. Ajayan, L. S. Schadler and P. V. Braun, Nanocomposite Science and Technology, Wiley, Weinheim, 2003 Search PubMed.
- Y. Fukushima, A. Okada, M. Kawasumi, T. Kurauchi and O. Kamigaito, Clays Clay Miner., 1988, 23, 27 CAS.
- Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, Y. Fukushima, T. Kurauchi and O. Kamigaito, J. Mater. Res., 1993, 8, 1185 CrossRef CAS.
- Polymer-Clay Nanocomposites, ed. T. J. Pinnavaia and G. W. Beall, Wiley, New York, 2000 Search PubMed.
- (a) Z. Wang, T. Lan and T. J. Pinnavaia, Chem. Mater., 1996, 8, 2200 CrossRef CAS; (b) M. Darder, M. López-Blanco, P. Aranda, F. Leroux and E. Ruiz-Hitzky, Chem. Mater., 2005, 17, 1969 CrossRef CAS; (c) K. Tamura, S. Yokoyama, C. S. Pascua and H. Yamada, Chem. Mater., 2008, 20, 2242 CrossRef CAS.
- M. Shibata, A. Kudo, A. Tanaka, K. Domen, K. Maruya and T. Onishi, Chem. Lett., 1987, 1017 CAS.
- T. Sasaki, Y. Ebina, T. Tanaka, M. Harada, M. Watanabe and G. Decher, Chem. Mater., 2001, 13, 4661 CrossRef CAS.
- (a) Y. Fuse, Y. Ide and M. Ogawa, Bull. Chem. Soc. Jpn., 2008, 81, 767 CrossRef CAS; (b) R. Hiroi, S. S. Ray, M. Okamoto and T. Shiroi, Macromol. Rapid Commun., 2004, 25, 1359 CrossRef CAS.
- (a) E. Ruiz-Hitzky and M. Rojo, Nature, 1980, 287, 28 CrossRef CAS; (b) T. Yanagisawa, K. Kuroda and C. Kato, Bull. Chem. Soc. Jpn., 1988, 61, 3743 CAS.
- (a) K. Isoda, K. Kuroda and M. Ogawa, Chem. Mater., 2000, 12, 1702 CrossRef CAS; (b) C.-M. Leu, Z.-W. Wu and K.-H. Wei, Chem. Mater., 2002, 14, 3016 CrossRef CAS.
- Y. Ide and M. Ogawa, Angew. Chem., Int. Ed., 2007, 46, 8449 CrossRef CAS.
- Y. Ide and M. Ogawa, Bull. Chem. Soc. Jpn., 2007, 80, 1624 CrossRef CAS.
- (a) Y. Ide and M. Ogawa, Chem. Commun., 2003, 1262 RSC; (b) Y. Ide and M. Ogawa, Chem. Lett., 2005, 34, 360 CrossRef CAS; (c) Y. Ide and M. Ogawa, J. Colloid Interface Sci., 2006, 296, 141 CrossRef CAS.
- T. Sasaki, F. Kooli, M. Iida, Y. Michiue, S. Takenouchi, Y. Yajima, F. Izumi, B. C. Chakoumakos and M. Watanabe, Chem. Mater., 1998, 10, 4123 CrossRef CAS.
- (a) M. Ogawa, S. Okutomo and K. Kuroda, J. Am. Chem. Soc., 1998, 120, 7361 CrossRef CAS; (b) I. Fujita, K. Kuroda and M. Ogawa, Chem. Mater., 2003, 15, 3134 CrossRef CAS; (c) I. Fujita, K. Kuroda and M. Ogawa, Chem. Mater., 2005, 17, 3717 CrossRef CAS; (d) D. Mochizuki, S. Kowate and K. Kuroda, Chem. Mater., 2006, 18, 5223 CrossRef CAS.
- T. Sasaki and M. Watanabe, J. Phys. Chem. B, 1997, 101, 10159 CrossRef CAS.
- Calculated by the integration of absorption bands raging from 320 nm to 240 nm of a benzene suspension of C18TMS-GTMS-TLO (7.3 × 10−3 mol L−1 as Ti1.66Li0.34O3.78) and a hexane solution of glycerolpolyglycidylether (7.3 × 10−3 mol L−1).
- N. Narendran, Y. Gu, J. P. Freyssinier, H. Yu and L. Deng, J. Cryst. Growth, 2004, 268, 449 CrossRef CAS.
- (a) B. Wang, G. L. Wilkes, J. C. Hedrick, S. C. Liptak and J. E. McGrath, Macromolecules, 1991, 24, 3449 CrossRef CAS; (b) M. Nakade, K. Kameyama and M. Ogawa, J. Mater. Sci., 2004, 39, 4131 CrossRef CAS.
- C. Guan, C.-L. Lü, Y.-F. Liu and B. Yang, J. Appl. Polym. Sci., 2006, 102, 1631 CrossRef CAS . The volume fraction of titania was calculated from the weight ratio using densities of 3.9 g cm−3 and 1.2 g cm−3 for titania and epoxy resin, respectively.
- K. Yano, A. Usuki and A. Okada, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2289 CrossRef CAS.
- The volume fraction of the titanate sheets was calculated using an equation, [Ti1.66Li0.34O3.78] × 2acL/Z × NA,23 where [Ti1.66Li0.34O3.78], ac, L, Z, and NA are the molar concentration of the titanate (1.1 × 10−3), the basal area of the titanate unit cell (0.23 nm2)14, the thickness of the indivisual titanate sheets (0.4 nm),24 and the Avogadro constant, respectively.
- N. Miyamoto and T. Nakato, J. Phys. Chem. B, 2004, 108, 6152 CrossRef CAS.
- T. Sasaki, Y. Ebina, Y. Kitami, M. Watanabe and T. Oikawa, J. Phys. Chem. B, 2001, 105, 6116 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
