Tacticity effects on glass transition and phase behavior in binary blends of poly(methyl methacrylate)s of three different configurations
Ling
Chang
and
E. M.
Woo
*
Department of Chemical Engineering, National Cheng Kung University, Tainan, 701, Taiwan. E-mail: emwoo@mail.ncku.edu.tw; Fax: +886 6 234 4496; Tel: +886 6 275 7575 Ex. 62670
First published on 16th December 2009
Abstract
Using optical and scanning electron microscopies, dynamic mechanical analysis, and differential scanning calorimetry, this study investigated effects of tacticity in PMMAs on the phase behavior in three pairs of binary blends comprising any two of isotactic-, syndiotactic-, and atactic-PMMAs. Miscibility was confirmed for all three binary blends (i.e., aPMMA/iPMMA, aPMMA/sPMMA, and iPMMA/sPMMA); however, the Tgvs. composition relationships for the aPMMA/iPMMA blend differ significantly. Configurational tacticity may lead to subtle differences in molecular interactions in the tactic polymers; and in turn such difference in the interactions causes variation in the glass transition and phase behavior in these three blends. This study also points out that the miscibility nature in iPMMA/sPMMA may constitute a prerequisite condition (necessary but not sufficient) for its tendency in forming complexes.
Introduction
Over the past decades, numerous reports on miscibility of polymers of different tacticties have been published, but, in many cases, arguments and debates on true phase behavior continue.1–11 It is usually difficult to assess the miscibility of polymer blends whose constituents are identical in chemical structures but differ only in tacticity, owing to the proximity of the constituents' glass transitions. Generally, polymers with the same repeat units but different tacticities (i.e., stereoisomers) are not miscible with each other, such as polypropylenes (PP),1,2polystyrenes (PSs)3–5 and poly(methyl methacrylate)s (PMMAs) of different tacticities.6–11 Although blends of polystyrenes (i-, a-, s-PS) are known to be miscible,3–5 not all blends of different tacticities behave similarly to the PS blends. For examples, Maier et al.2 have concluded that polypropylenes (PPs) of different tacticities (a, i, or s) are not all miscible with each other, with the aPP/iPP blend being miscible but the aPP/sPP system being not miscible.Another example can be given by blend systems comprising poly(methyl methacrylate) (PMMA) of different tacticities.6–11 White and Filisko8 concluded that atactic poly(methyl methacrylate) (aPMMA) and isotactic poly(methyl methacrylate) (iPMMA) could exist in one phase or two phases, as verified using differential scanning calorimetry (DSC), with the results depending on sample preparation methods. Krause and Roman9 reported that a binary blend of iPMMA and sPMMA was miscible using dilatometry analysis. However, on the other hand, Bauer and Bletso10 concluded that the iPMMA/sPMMA blend system was immiscible, which was based on characterization results of dilatometry, mechanical damping, and torsional modulus. Schroeder et al.11 indicated that when iPMMA and sPMMA samples were mixed using comparable molecular weights based on DSC measurements, miscible systems were formed in whole-range compositions. These literature results have shown that phase behavior in blends of PMMAs remains yet unresolved and perhaps may be conflicting among reports by various investigators. Therefore, phase behavior in blends of PMMAs of different tacticities had to be further clarified.
Formation of stereocomplexes between iPMMA and sPMMA is an interesting subject, and was first reported by Liquori et al.12 Watanabe et al.13 have reported that iPMMA and sPMMA mixed in some solvents leads to formation of aggregates in complex forms. Furthermore, stereocomplexes are also possible both in bulk or solution forms.14,15 Schomaker et al.15 have studied the stereocomplexation of PMMA in the bulk form as a function of annealing temperature, annealing time and molecular weight by using DSC and WAXS. Others factors, such as polymer concentration,16 mixing ratio,13,15,17 and molecular weight,14,18 might also influence formation of stereocomplexes between iPMMA and sPMMA. For example, the maximum effect of stereocomplex formation on ratio is reported to be i/s = 1/2, although other ratios such as i/s = 2/1 and 1/1 are also possible. Nevertheless, phase behavior in the amorphous state of blends comprising these tactic PMMAs has not been fully substantiated. This study, using mainly thermal analysis and microscopy techniques, attempted to search for evidence to clarify the true phase state of blends comprising tactic polymers such as PMMA, whose stereocomplex formation has drawn extensive interest. The effect of tacticity on glass transition and phase behavior is discussed.
Experimental
Materials and preparation
Three tactic poly(methyl methacrylate)s (i-, s-, and a-PMMAs) were used in preparing binary blends comprising any two of these three polymers. aPMMA (Mw = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 g mol−1 with Tg = 107 °C) was obtained from Chi-Mei, Inc. (Taiwan). Two tactic PMMAs (iPMMA and sPMMA) were obtained from Scientific Polymer Products (SP2), Inc. (USA), with Mw = 300
500 g mol−1 with Tg = 107 °C) was obtained from Chi-Mei, Inc. (Taiwan). Two tactic PMMAs (iPMMA and sPMMA) were obtained from Scientific Polymer Products (SP2), Inc. (USA), with Mw = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Tg = 52 °C, and Mw = 50
000 g mol−1, Tg = 52 °C, and Mw = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Tg = 124 °C, respectively. Percentages of tacticity diads in these PMMAs were further checked by proton nuclear magnetic resonance spectroscopy1H-NMR) to be 95% for iPMMA and 85% for sPMMA.
000 g mol−1, Tg = 124 °C, respectively. Percentages of tacticity diads in these PMMAs were further checked by proton nuclear magnetic resonance spectroscopy1H-NMR) to be 95% for iPMMA and 85% for sPMMA.
Apparatus and procedures
For characterization of phase morphology in blends, a polarized-light microscope (Nikon Optiphot-2, POL), equipped with a charge-coupled device (CCD) digital camera, was used for characterizing optical homogeneity and/or crystalline morphology of the blends. Morphology (fracture surface) of blends was also examined using a scanning electron microscope (SEM, Model JEOL JXA-840). The blend film samples for scanning electron microscopy were solution-cast to be thick enough so that the fracture surface of the thickness (cross section) could be conveniently examined. The samples were examined after dipping into liquid nitrogen. In order that the fractured surface of blend samples could be conducting and to increase the image resolution, the samples were coated with gold by vapor deposition using vacuum sputtering. Tg transitions of the blend samples were measured using a differential scanning calorimeter (DSC-7, Perkin-Elmer) equipped with an intracooler (to −65 °C). Prior to DSC runs, the temperature and heat of transition of the instrument were calibrated with indium and zinc standards. During thermal annealing or scanning, a continuous nitrogen flow in the DSC sample cell was maintained to ensure minimal sample degradation. For determining the glass transition temperatures (Tg), a heating rate of 20 °C min−1 was used, and the Tg values were taken as the onset of the transitions in the DSC thermograms. For measurements of the melting points, a heating rate of 10 °C min−1 was used.Dynamic mechanical analysis was carried out using a Rheometrics RSA III DMA. The size of test pieces was 0.7 cm in width, 3.5 cm in length, and 0.05 cm in thickness. This experiment was performed in tension mode with 0.02% strain amplitude at a frequency of 1 Hz. The temperature ranged from 40 to 160 °C with a heating rate of 3 °C min−1.
Results and discussion
Morphological evidence for phase behavior of the blends is first discussed; with thermal analysis results to be discussed later. Both OM and SEM morphologies of binary blends of any two of aPMMA, iPMMA, and sPMMA were characterized. Blends of PMMAs of different tacticities were found to be homogenous according, to the OM results. Optical micrograph results for three different blend systems: (a) aPMMA/iPMMA (b) aPMMA/sPMMA, and (c) iPMMA/sPMMA, of same composition of 50/50, all revealed similarly clarity, indicating a lack of phase domains. In addition to OM, for larger magnification, the phase homogeneity of the blends was also examined by using scanning electron microscopy at greater magnifications. The blend film samples for scanning electron microscopy were solution-cast to be thick enough so that the fracture surface could be conveniently examined by SEM. The SEM micrographs revealed homogeneity free of any domains in the aPMMA/iPMMA blend (of two compositions: 70/30 and 50/50). The SEM micrographs also showed homogeneity and the absence of discernible domains in all the three blends investigated. For brevity, OM and SEM micrographs are not shown as separate graphs, but will be shown as insets in the following results discussing the thermal analyses and miscibility criteria of the blend Tg.Glass transitions of blends are usually taken as the main criteria for judging the phase behavior in blends. Fig. 1 shows DSC thermograms for the aPMMA/iPMMA blends of various compositions. Apparently, all blends exhibit a single Tg which is dependent on the composition. However, Tg broadening is increasingly significant for some intermediate blend compositions (e.g., 60/40– 40/60). In addition, Tg dependence on composition does not seem symmetric; that is, the Tgs of blends of iPMMA-rich compositions (iPMMA = 100–60 wt% in blend samples) do not change much from that of neat iPMMA with increase in aPMMA content in the blends. Such asymmetry may require careful analysis as the Tg–composition relationship usually provides an interesting correlation with the interaction strength or scales of phase homogeneity in the blends.
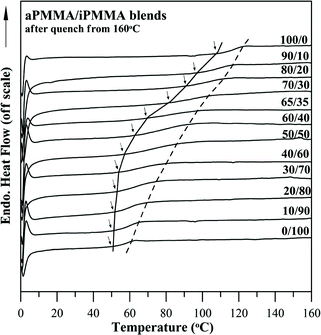 | ||
| Fig. 1 DSC thermograms for aPMMA/iPMMA blends of 12 compositions. | ||
The relationship between Tg (onset positions are indicated with arrows) and blend composition for the blends was analyzed. Fig. 2 shows a plot of the Tgs of the aPMMA/iPMMA blends as a function of composition. As shown in the figure, the relationship between Tg and composition generally shows a negative deviation from linearity. The classical Gordon–Taylor equation19 with a k value of 0.33 only fits the blends in the narrow range of aPMMA-rich compositions. Obviously, the G–T model does not fully fit the Tg–composition relationship for the blend of whole compositions, and the Tg–composition plot is apparently asymmetrical, showing a discontinuity (i.e., cusp) at a blend composition of aPMMA = 70 wt%. As a result, the Kovacs model20 was used to account for the discontinuity in the Tg variation trend. The model was fitted to obtain the g parameter by using known physical constants for the constituents. The densities of both aPMMA and iPMMA were similar and the value used was 1.18 g cm−1. According to the model, if the difference of Tg between the two polymers (Tg2 − Tg1) is more than 50 °C or so, there is a critical temperature (Tc) where the contribution of free volume of the polymer with the higher Tg is zero. The corresponding critical temperature (Tc) and volume fraction (ϕc) were estimated as the following: Tc = Tg2 − (fg2/Δα2), ϕc = fg2/[Δα2(Tg2 − Tg1) + fg2(1 − Δα1/ Δα2), where fg,i is the free volume fraction of component i at the glassy state and Δαi is the thermal expansion coefficient difference of component i between the glassy and liquid states. Depending on two conditions, the blends' Tg is described by the following: above Tc, Tg,blend = (ϕ1Δα1Tg1 + ϕ2Δα2Tg2 + gϕ1ϕ2)/(ϕ1Δα1 + ϕ2Δα2). Below Tc, Tg,blend = Tg1 + [(ϕ2fg2 + gϕ1ϕ2)/ϕ1Δα1]. In this equation, g is the parameter of strength of interactions between the components and can be related to the excess volume. On the basis of the classical value of 0.025 for fg2,27 the critical temperature and volume fraction can be obtained (Tc = 77.8 °C and ϕc = 0.7, respectively). The g value of the blend fitted from the model is −0.042. This negative value suggests that the blend interactions are weak. In addition, it shows an apparent cusp at the composition of 70 wt% aPMMA.
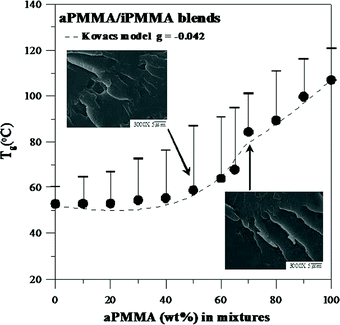 | ||
| Fig. 2 A plot of the Tgvs. composition of aPMMA/iPMMA blends, showing severe asymmetry. Insets: SEM micrographs for two compositions near the cusp point. | ||
For further insight into the phase behavior of the aPMMA/iPMMA blend, DMA analysis was performed. Fig. 3 shows tanδ of aPMMA/iPMMA blend in film form (neat aPMMA, 70/30, 50/50, 30/70, and neat iPMMA). The DMA result further supports miscibility in this blend, as the DMA spectra reveal only a single tanδ peak for each of the samples, which is due to the α-relaxation and its peak is clearly located at a temperature between those of two tanδ peaks for neat aPMMA and iPMMA, respectively. In summary, thermal analysis using DSC and DMA, as well as OM and SEM morphological results, all indicate and support miscibility in the aPMMA/iPMMA blend.
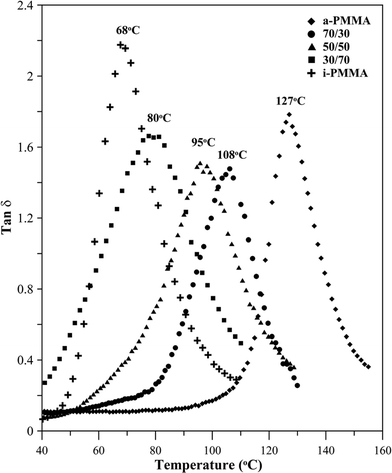 | ||
| Fig. 3 Tanδ of aPMMA/iPMMA blends of various compositions, revealing a single Tg. Heating rate: 3 °C min−1. | ||
Preliminary morphology characterization of the aPMMA/sPMMA blend (rapidly quenched to an amorphous state) exhibited a homogeneous phase in OM and SEM characterization (not shown for brevity). Fig. 4 shows DSC thermograms for seven blends of aPMMA/sPMMA of different compositions. As shown in the figure, the blends exhibit a single, composition-dependent Tg. However, the difference of glass temperature (Tg) between the two constituent polymers (neat sPMMA and aPMMA) is only 17 °C, which may be questioned as being too close to be used as fully valid criteria for confirming the miscibility of the blend. Fig. 5 shows plot of Tgvs. composition for the aPMMA/sPMMA blends. The Tg–composition relationship for the aPMMA/sPMMA blend still exhibits some asymmetry but it is much less than that of the aPMMA/iPMMA blend. Despite the asymmetry, the Tg–composition relationships for the aPMMA/sPMMA blend was fitted with the Gordon–Taylor equation,19Tg,blend = (w1Tg1 + w2kTg2)/(w1 + kw2), where wi and Tg,i are the weight fractions and glass temperature of component i, respectively. The fitted parameters are k = 0.38. The Tg–composition relationship shows an approximate fit to the Gordon–Taylor model (k = 0.38). Again, according to criteria of blend miscibility, the aPMMA/sPMMA blend can be classified as being miscible, although the Tg–composition relationship is widely different from that for the aPMMA/iPMMA system discussed earlier. The difference can be attributed to the tactic configuration variation between sPMMA and iPMMA.
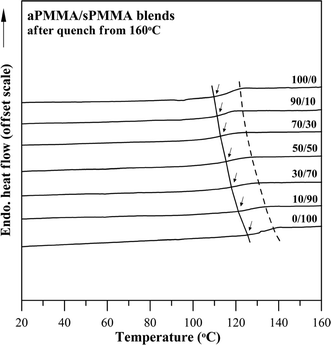 | ||
| Fig. 4 DSC thermograms for aPMMA/sPMMA blends of 7 compositions. | ||
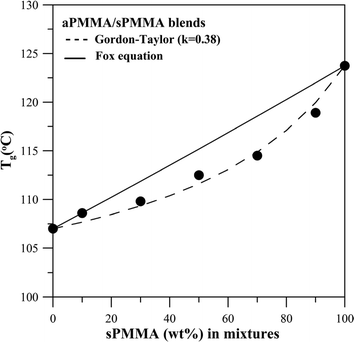 | ||
| Fig. 5 A plot of Tgvs. composition of aPMMA/sPMMA blends, showing less asymmetry than that of the aPMMA/iPMMA blends. | ||
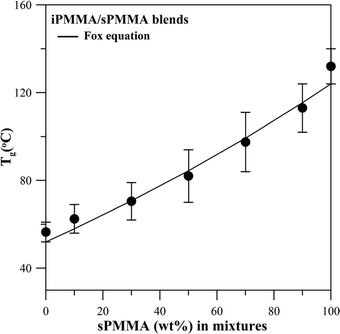
Similar thermal characterization was performed on all three binary pairs of blends comprising tactic PMMAs (i.e., iPMMA/sPMMA and iPMMA/aPMMA, respectively) for comparison. The iPMMA/sPMMA blend also exhibited a homogeneous phase according to the OM and SEM results; subsequently, thermal analysis was performed on the iPMMA/sPMMA blend to reveal its Tg behavior. Fig. 6 shows DSC thermograms for the iPMMA/sPMMA blend of seven compositions. All compositions exhibit one single and composition-dependent Tg. Apparently, the DSC result, along with OM morphology data, indicates miscibility in the iPMMA/sPMMA blend too.
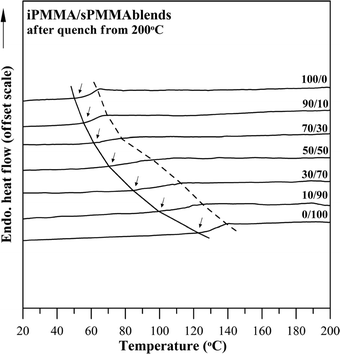 | ||
| Fig. 6 DSC thermograms for aPMMA/sPMMA blends of seven compositions. | ||
T
g–composition relationships, however, may further reveal interactions and mixing scales in mixtures of these two polymers. Fig. 7 shows a plot of Tgvs. composition for the iPMMA/sPMMA blend. The Tg–composition relationship for the iPMMA/sPMMA blend is quite symmetric, which differs from the other two blend systems, whose Tg–composition relationships are asymmetric. Note that it has been known that iPMMA and sPMMA tend to form configurational complexes when blended.12–17,21 This fact infers that these two polymers might have greater interactions than those in the other two binary blends (aPMMA/sPMMA and aPMMA/iPMMA). Compositional dependence of the Tg for the iPMMA/sPMMA blend was plotted using the mid-point Tg data and fitted with the classical Fox equation, shown as a solid curve in this figure. Such a good fit to the Fox Tg model suggests quite good homogeneity to segmental scales in the iPMMA/sPMMA blend. Note that the weight-average molecular weight of iPMMA (Mw = 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) is much larger than that of aPMMA and sPMMA (Mw = 53
000 g mol−1) is much larger than that of aPMMA and sPMMA (Mw = 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 and 50
500 and 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, respectively). However, the phase behavior in these blends is similar and does not alter with the difference in the Mw values.
000 g mol−1, respectively). However, the phase behavior in these blends is similar and does not alter with the difference in the Mw values.
For comparison, results of phase behavior and miscibility in the blends of different tactic PMMAs are summarized. Although miscibility and phase homogeneity is similarly demonstrated for all three binary pairs of blend systems, the strength of interactions leading to miscibility in the blends may vary. For examples, the interaction in the blend of iPMMA with sPMMA seems to be the highest among the three binary blend systems. Interaction strength between sPMMA and iPMMA is closely similar to that between sPMMA and aPMMA, but the former is ranked slightly higher than the latter. The interaction in the blend of iPMMA with aPMMA is the lowest, whose significantly asymmetric Tg–composition relationship suggests that the blends' phase behavior might border on miscibility and immiscibility. Previous literature results have amply shown that semicrystalline or micellar complexes can form in blends of iPMMA with sPMMA;12–18,22–26 however, no prior work has justifiably proven the true phase behavior of iPMMA/sPMMA blends in the amorphous state, from which complexes are packed into complex micelles or crystals. This work, for the first time, has proven that the iPMMA/sPMMA blend, as well as iPMMA/aPMMA and sPMMA/aPMMA, is miscible in the amorphous state, and the miscibility nature in iPMMA/sPMMA may constitute a prerequisite condition (necessary but not sufficient) for forming complexes in this blend.
It is also of interest to compare the effect of tacticity on these blend systems with that on blends of other polymers. Tactic polypropylenes (i- and s-PP) are relatively non-polar but more highly crystalline than tactic PMMAs by nature of their molecular structures. An earlier study26 using the method of equilibrium melting points has shown that the Flory–Huggins interaction parameter for the miscible aPP/iPP blend exhibits a significantly more negative value (χ12 = −0.21, measured near the blend melting temperature range) than the other two pairs showing UCST phenomenon (sPP/iPP and aPP/sPP blends, χ12 = −0.02 and −0.007, respectively, measured in UCST at T = 150–180 °C). Thus, the order of interaction strength is ranked: aPP/iPP ≫ sPP/iPP = aPP/sPP), which is just opposite to the trend of variation of the interaction strength observed for the blends of tactic PMMAs. On the other hand, by comparing the blends of tactic PMMAs to the similarly polar and tactic polystyrenes (i-, a-, s-PS), all three pairs of tactic PSs are known to be miscible, just like the three pairs of tactic PMMAs. Apparently, the weaker effect of configurational tacticity on phase behavior in polymer blends might be offset by other factors in molecular structures, such as crystallinity and polarity, or other specific interactions related to structures in polymers.
Conclusion
This study investigated effects of tacticity in PMMAs on the phase behavior in binary blends comprising any two of i-, s-, and a-PMMA. Miscibility is confirmed unambiguously for all three pairs of binary blends (aPMMA/iPMMA, aPMMA/sPMMA, and iPMMA/sPMMA), which all exhibit a single, composition-dependent glass transition and a homogeneous phase morphology. However, Tgvs. composition relationships differ among the three pairs. By judging from the Tg behavior, the order of interaction in the blends is ranked as: iPMMA/sPMMA > aPMMA/sPMMA ≫ aPMMA/iPMMA. Configuration tacticity in PMMAs may lead to subtle differences in molecular interactions in the tactic polymers; and in turn such difference in the interactions causes variation in the glass transition behavior in these three pairs of blends. Apparently, this study shows that tacticity exerts significant effects on the Tg and phase behavior of blends. However, the tacticity effect on the phase behavior in blends of tactic polymers of lesser polarity may be easily masked or altered by other stronger factors in the molecular structures. An example can be cited by drawing comparison to the case of blends of polypropylenes of different tactic forms. In comparison to the variation trend of phase behavior in tactic but less polar polypropylenes (i-, a-, s-PP) reported earlier in the literature, the weak effect of configurational tacticity on phase behavior in polymer blends might be offset by factors such as crystallinity, polarity, or other specific interactions related to structures in polymers. Finally, this study points out that the miscibility nature in iPMMA/sPMMA may constitute a prerequisite condition (necessary but not sufficient) for forming complexes in this blend.Acknowledgements
This work was financially supported by a basic research grant (NSC-95 2221 E006 183 and NSC-94 2216 E006 003) in three consecutive years funded by National Science Council (NSC) of Taiwan.Referencers
- R. A. Phillips, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 1947 CrossRef CAS
.
- R. D. Maier, R. Thomann, J. Kressler, R. Mülhaupt and B. Rudolf, J. Polym. Sci., Part B: Polym. Phys., 1997, 35, 1135 CrossRef CAS
.
- E. M. Woo, K. Y. Cheng, Y. F. Chen and C. C. Su, Polymer, 2007, 48, 5753 CrossRef CAS
.
- E. M. Woo and F. S. Wu, Macromol. Chem. Phys., 1998, 199, 2041 CrossRef CAS
.
- E. M. Woo, M. L. Lee and Y. S. Sun, Polymer, 2000, 41, 883 CrossRef CAS
.
- E. Schomaker, E. J. Vorenkamp and G. Challa, Polymer, 1986, 27, 256 CrossRef CAS
.
- F. Bosscher, D. Keekstra and G. Challa, Polymer, 1981, 22, 124 CrossRef CAS
.
- A. J. White and F. E. Filisko, J. Appl. Phys., 1982, 53, 6563 CrossRef CAS
.
- S. Krause and N. Roman, J. Polym. Sci., Part A: Polym. Chem., 1965, 3, 1631 Search PubMed
.
- R. G. Bauer and N. C. Bletso, Polym. Prepr., 1969, 10, 632 CAS
.
- J. A. Schroeder, F. E. Karasz and W. J. Macknight, Polymer, 1985, 26, 1795 CrossRef CAS
.
- A. M. Liquori, G. Anzuino, V. M. Coiro, M. D'Alagni, P. de Santis and M. Savino, Nature, 1965, 206, 358 CrossRef CAS
.
- W. H. Watanabe, C. F. Ryan, P. C. Fleischer and B. S. Garrett, J. Phys. Chem., 1961, 65, 896 CrossRef CAS
.
- E. Schomaker and G. Challa, Macromolecules, 1988, 21, 2195 CrossRef CAS
.
- E. Schomaker, H. Hoppen and G. Challa, Macromolecules, 1988, 21, 2203 CrossRef CAS
.
- W. Borchard, M. Pyrlik and G. Rehage, Makromol. Chem., 1971, 145, 169 CrossRef CAS
.
- J. Spěváček and B. Schneider, Makromol. Chem., 1974, 175, 2939 CrossRef CAS
.
- F. Bosscher, G. Ten Brinke and G. Challa, Macromolecules, 1982, 15, 1442 CrossRef CAS
.
- M. Gordon and J. S. Taylor, J. Appl. Chem., 1952, 2, 493 CAS
.
- A. J. Kovacs, Adv. Polym. Sci., 1964, 3, 394 CrossRef CAS
.
- L. Chang and E. M. Woo, Ind. Eng. Chem. Res., 2009, 48, 3432 CrossRef CAS
.
- E. J. Vorenkamp, F. Bosscher and G. Challa, Polymer, 1979, 20, 59 CrossRef CAS
.
- E. L. Feitsma, A. de Boer and G. Challa, Polymer, 1975, 16, 515 CrossRef CAS
.
- T. Mizumoto, N. Sugimura and M. Moritani, Macromolecules, 2000, 33, 6757 CrossRef CAS
.
- S. Asai, T. Kawano, S. Hirota, Y. Tominaga, M. Sumita and T. Mizumoto, Polymer, 2007, 48, 5116 CrossRef CAS
.
- O. N. Tretinnikov, Macromolecules, 2003, 36, 2179 CrossRef CAS
.
- M. L. Williams, R. F. Landel and J. D. Ferry, J. Am. Chem. Soc., 1955, 77, 3701 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2010 |