DOI:
10.1039/B9PY00221A
(Paper)
Polym. Chem., 2010,
1, 203-206
Synthesis and host–guest properties of an alternating copolymer containing calix[4]arene and calix[6]arene in its main chain†
Received
20th August 2009
, Accepted 16th October 2009
First published on 16th December 2009
Introduction
Calixarenes are phenolic macrocycles widely used in host–guest chemistry as versatile platforms for synthesis and as building blocks to construct nanometric supramolecules.1 The most common and readily synthesized calixarenes are the cyclic tetramer (calix[4]arene), the hexamer (calix[6]arene) and the octamer (calix[8]arene). By using these calixarenes as a starting material, various calixarene derivatives have been prepared due to their facile synthesis and easy modification by various substituents at the lower and upper rim. Modification of various substituents into calixarenes enhances molecular recognition ability and selectivity compared to pristine calixarenes.2 Furthermore, in order to recognize and capture large molecules, polymers involving calix[4]arene moieties in the side chain3 and main chain,4 and oligomers consisting of calix[4]arene5 have been prepared.
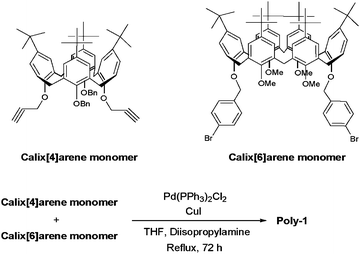
Combinations of different sizes of macrocycles have also attracted a great deal of interest and new challenge because multiple interactions between two or more complex molecules give insight into molecular recognition and self-assembly processes. Molecular recognition and construction of supramolecular architectures using different size macrocycles such as cyclodextrins and cucurbiturils have been reported.6 In this study, we report the first preparation of a polymer containing different size of calixarenes. We synthesized two kinds of difunctional monomers, diethynyl group-substituted calix[4]arene (calix[4]arene monomer) and dibromophenyl group-modified calix[6]arene (calix[6]arene monomer). By Sonogashira coupling of the calix[4]arene monomer with the calix[6]arene monomer, we successfully synthesized a novel alternating copolymer containing calix[4]arene and calix[6]arene in its main chain (Poly-1, Fig. 1). There are few reports on incorporating calix[6]arenes into polymer backbones, while various polymers containing calix[4]arene moieties have been reported.3–5 To the best of our knowledge, this is the first example of the synthesis of a polymer containing both calix[4]arene and calix[6]arene moieties and the alternating copolymer structure of calix[4]arene and calix[6]arene moieties. In this study, we investigated the host–guest properties of Poly-1 compared to that of calix[4]arene and calix[6]arene models.
Experimental
Materials
All solvents and reagents were used as supplied. Anhydrous N,N-dimethylformamide (DMF), tetrahydrofuran (THF), diisopropylamine, copper(I) iodide (CuI), potassium carbonate, sodium sulfate and fluoranthene were purchased from Kanto Chemicals. Dichlorobis(triphenylphosphine)palladium(II) [Pd(PPh3)2Cl2] and 4-bromobenzyl bromide were obtained from Tokyo Chemical Industry Co., Ltd. Methyl iodide, sodium hydride (NaH, 60% dispersion in oil) and triphenylphosphine (PPh3) were purchased from Nakalai Tesque. Potassium trimethylsilanolate was obtained from Aldrich.
Measurements
1H NMR spectra were recorded at 270 MHz and 400 MHz and 13C NMR spectra were recorded at 67.5 MHz and 100 MHz with JEOL-JNM EX270 and EL400 spectrometers, respectively. FT-IR spectra were obtained using a JASCO FT-IR460 plus infrared spectrometer. Fluorescence spectra were recorded on a Hitachi F-2500 fluorescence spectrometer at room temperature. For fluorescence measurements, one centimetre quartz cuvettes were used. Gel permeation chromatography (GPC) analysis was carried out on a Shodex GPC LF804 system using THF as the eluent at 25 °C, at the flow rate of 1 mL min−1 after calibration with standard polystyrene samples. Elemental analyses were performed at the Advanced Science Research Center of Kanazawa University.
5,11,17,23-Tetra-tert-butyl-25,27-bis(prop-2-ynyloxy)-26,28-bis(benzyloxy)calix[4] arene (calix[4]arene monomer), 5,11,17,23-tetra-tert-butyl-25,27-bismethoxy-26,28-bis(benzyloxy)calix[4]arene (calix[4]arene model, 1) and 5,11,17,23,29,35-hexa-tert-butyl-37,38,40,41-tetramethoxy-39,42-bis(benzyloxy)calix[6]arene (calix[6]arene model, 2) were prepared according to the literature.7,8
5,11,17,23,29,35-Hexa-tert-butyl-37,38,40,41-tetramethoxy-39,42-bis[(4-bromo benzyl)oxy]calix[6]arene (calix[6]arene monomer)
For synthesis of the calix[6]arene monomer, we prepared dibromophenyl group-modified calix[6]arene (5,11,17,23,29,35-hexa-tert-butyl-37,38,40,41-tetrahydroxy-39,42-bis[(4-bromobenzyl)oxy]calix[6]arene) according to a previous paper.9 To a solution of the dibromophenyl group-modified calix[6]arene (0.500 g, 0.380 mmol) in THF (7.0 mL), DMF (13 mL), NaH (0.405 g, 10.1 mol) and methyl iodide (0.632 mL, 10.2 mmol) were added. The mixture was refluxed for 4 h. The resulting solution was poured into water. The solution was extracted with dichloromethane and the organic phase was dried over sodium sulfate and concentrated. The residue was washed using ethanol. A white solid was obtained (0.468 g, 0.343 mmol, yield 90.2%). 1H NMR (CDCl3, 270 MHz): δ 1.09 (m, 18H, protons from tert-butyl group), 1.14 (m, 36H, protons from tert-butyl group), 2.66–2.95 (m, 12H, proton from methoxy group), 3.45–4.53 (m, 12H, protons from methylene bridge), 4.79 (s, 4H, O–CH2-bz), 6.87–7.18 (m, 12H, aromatic protons from calix[6]arene), 7.25–7.53 (m, 8H, aromatic protons from 4-bromobenzyl moiety). 13C NMR (CDCl3, 100 MHz): δ 31.3, 34.1 (C of methylene bridge and tert-butyl moiety), 59.5, 60.0 (OCH3), 73.5 (OCH2Ar), 121.3, 125.0, 125.6, 129.5, 133.3, 136.8, 145.5, 151.7, 153.5 (ArC). FAB mass m/z = 1365 (M–H)+. Anal. Calcd. for C84H102Br2O6*C2H5OH: C, 73.07; H, 7.70; N, 0.00. Found: C, 73.14; H, 7.66; N, 0.00.
Scheme 1 shows the synthetic pathway of Poly-1 from calix[4]arene monomer and calix[6]arene monomer. Calix[4]arene monomer (142 mg, 0.144 mmol), calix[6]arene monomer (197 mg, 0.144 mmol), Pd(PPh3)2Cl2 (101 mg, 0.144 mmol), PPh3 (75.4 mg, 0.288 mmol), CuI (36.5 mg, 0.190 mmol), diisopropylamine (2 mL) and THF (2 mL) were placed in a 50 mL of flask equipped with a magnetic stirring bar under nitrogen atmosphere. The reaction was refluxed for 72 h. After cooling, precipitated ammonium salts were filtrated off. The filtrate was poured into methanol to precipitate the polymer. The resulting polymer was collected by filtration, and then dissolved in THF. The mixture was re-precipitated with methanol twice. The solid was collected and dried in a vacuum to yield Poly-1 as a white solid (350 mg, yield 10.3%). 1H NMR (CDCl3, 270 MHz, ppm): δ 0.80–2.00 (m, protons from tert-butyl group), 2.50–3.40 (m, protons from methoxy group), 3.45–5.20 (m, protons from methylene bridge and O–CH2-bz), 6.30–7.20 (m, aromatic protons from calixarene moiety), 7.20–7.60 (m, aromatic protons from benzyl moiety). 13C NMR (CDCl3, 100 MHz, ppm): δ 28.6, 30.0, 32.7, 36.6 (C of methylene bridge and tert-butyl moiety), 58.6 (OCH3), 69.9 (OCH2Ar), 120.8, 124.2, 126.9, 128.3, 129.5, 130.5, 131.9, 132.6, 134.2, 134.7, 135.5, 136.6, 143.2, 143.3, 144.7, 150.2, 152.7 (ArC). GPC analysis (THF, polystyrene standards): Mw = 7100 (Mw/Mn = 1.62).
Results and discussion
The novel difunctional calix[6]arene monomer was synthesized by selective functionalization of calix[6]arene developed by Gutsche and coworkers. According to the previous paper reported by Gutsche and coworkers,9 we synthesized the dibromophenyl group-modified calix[6]arene derivative. The obtained calix[6]arene was reacted with a mixture of methyl iodide and sodium hydride in THF–DMF to yield the methoxy-protected calix[6]arene (Scheme 1, calix[6]arene monomer) as a white powder. The structure of the novel calix[6]arene monomer was completely characterized by 1H and 13C NMR, FAB-MS and elemental analysis. Diethynyl group-modified calix[4]arene (Scheme 1, calix[4]arene monomer) was prepared according to previous papers.7,8 The calix[4]arene monomer is a mixture of the partial cone conformer and cone conformer. The content of the partial cone conformer, from 1H NMR measurements, was found to be 46.5%.
The alternating copolymer (Poly-1), which consists of calix[4]arene and calix[6]arene, was prepared by reacting calix[4]arene monomer with calix[6]arene monomervia a Sonogashira coupling (Scheme 1). Poly-1 was completely characterized by 1H and 13C NMR and gel permeation chromatography (GPC) measurements. Fig. 2 shows the GPC traces of the obtained products in THFeluent using standard polystyrene samples. In calix[4]arene monomer and calix[6]arene monomer, a sharp peak was found at 20.6 mL and 20.8 mL, respectively [Figs. 2(a) and 2(b)]. After the polymerization, the obtained product was mixture of un-reacted monomers, oligomers and polymeric product. After re-precipitation, a unimodal peak was observed around 17–20 mL and peaks from these un-reacted monomers and oligomers were not detected. The data indicates complete isolation of Poly-1 by the re-precipitation technique.
![GPC traces of (a) calix[4]arene monomer, (b) calix[6]arene monomer and (c) Poly-1.](/image/article/2010/PY/b9py00221a/b9py00221a-f2.gif) |
| | Fig. 2
GPC traces of (a) calix[4]arene monomer, (b) calix[6]arene monomer and (c) Poly-1. | |
The 1H NMR spectrum of Poly-1 is shown in the Electronic Supplementary Information (ESI).† Proton resonances from benzyl and calixarene moieties were observed. Broadening of the peaks and the disappearance of the ethynyl proton peaks at 2.47, 2.16 ppm (partial cone conformer) and 2.20 ppm (cone conformer) from calix[4]arene monomer indicated formation of Poly-1. From the peak area of the calix[4]arene and calix[6]arene moieties, content of calix[4]arene unit in Poly-1 was found to be 51.5% and the calculated value from the feed ratio was 50.0%. The data indicate that the polymerization proceeded ideally.
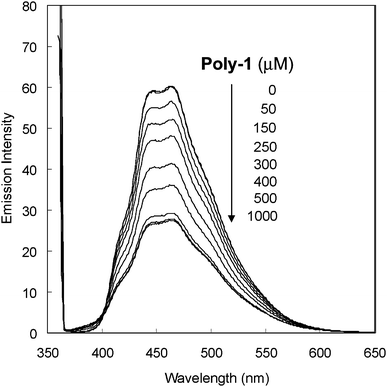
The complexation properties of Poly-1 were investigated by fluorescence measurements. As a guest molecule, fluoranthene was employed [Scheme 2(a)]. For control experiments, we used model compounds of calix[4]arene [1, Scheme 2(b)] and calix[6]arene [2, Scheme 2(c)]. Fluoranthene shows strong emission at 450–470 nm by excitation at 360 nm. Upon addition of Poly-1 to fluoranthene in chloroform solution, the emission from fluoranthene was largely quenched (Fig. 3). In contrast, by adding 1 and/or 2 to fluoranthene solution, little fluorescence quenching was found (see the ESI† ). Fig. 4 shows change in the ratios (I/I0) of the fluorescence intensities at 462 nm from fluoranthene upon addition of 1 (green diamond), 2 (black triangle), both 1 and 2 (1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) :
:![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1, blue square) and Poly-1 (red circle). By adding 1 and/or 2, the values of the fluorescence ratio hardly changed. From these data, it was found that both calix[4]arene and calix[6]arene formed weak host–guest complexes with fluoranthene. This is because the size of fluoranthene is not suited to the size of the cavities of calix[4]arene and calix[6]arene. On the other hand, even with small amounts of Poly-1, large fluorescence quenching was detected. The association constant (K) for the Poly-1-fluoranthene complex was determined to be 2700 ± 900 M−1 by fitting the data to eqn (1).10 From these observations, Poly-1 strongly captured fluoranthene compared to 1 and 2. Poly-1 should participate in multipoint host–guest interactions with fluoranthene, thus Poly-1 was able to form a host–guest complex with fluoranthene. Fluoranthene is a polynuclear aromatic compound and calixarenes are composed of benzene rings, thus the driving force of the complex formation may be π–π interactions. To examine the fluorescence quenching mechanism, Stern–Volmer plots were drawn (see the ESI† ). With the titration of Poly-1, the ratios of I/I0 of the fluorescence intensities increased until reaching a maximum and then remained constant. The nonlinear fluorescence quenching could be mainly assigned to complex formation (static mechanism).
1, blue square) and Poly-1 (red circle). By adding 1 and/or 2, the values of the fluorescence ratio hardly changed. From these data, it was found that both calix[4]arene and calix[6]arene formed weak host–guest complexes with fluoranthene. This is because the size of fluoranthene is not suited to the size of the cavities of calix[4]arene and calix[6]arene. On the other hand, even with small amounts of Poly-1, large fluorescence quenching was detected. The association constant (K) for the Poly-1-fluoranthene complex was determined to be 2700 ± 900 M−1 by fitting the data to eqn (1).10 From these observations, Poly-1 strongly captured fluoranthene compared to 1 and 2. Poly-1 should participate in multipoint host–guest interactions with fluoranthene, thus Poly-1 was able to form a host–guest complex with fluoranthene. Fluoranthene is a polynuclear aromatic compound and calixarenes are composed of benzene rings, thus the driving force of the complex formation may be π–π interactions. To examine the fluorescence quenching mechanism, Stern–Volmer plots were drawn (see the ESI† ). With the titration of Poly-1, the ratios of I/I0 of the fluorescence intensities increased until reaching a maximum and then remained constant. The nonlinear fluorescence quenching could be mainly assigned to complex formation (static mechanism).
| | 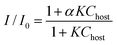 | (1) |
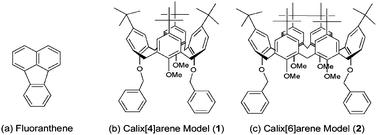 |
| | Scheme 2 Guest and model compounds. | |
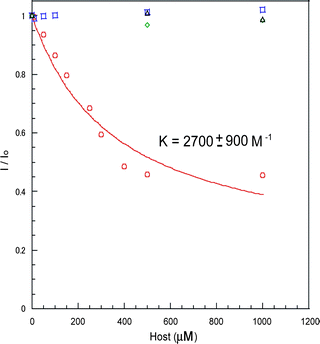 |
| | Fig. 4 Change in the ratios (I/I0) of the emission intensities at 462 nm from fluoranthene (10 µM) upon addition of 1 (green diamond), 2 (black triangle), both 1 and 2 (1![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) : :![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1, blue square) and Poly-1 (red circle) in chloroform. The association constant (K value) between fluoranthene and Poly-1 was determined to be 2700 ± 900 M−1 by fitting data with eqn (1) (red solid line), where α is a constant. 1, blue square) and Poly-1 (red circle) in chloroform. The association constant (K value) between fluoranthene and Poly-1 was determined to be 2700 ± 900 M−1 by fitting data with eqn (1) (red solid line), where α is a constant. | |
Conclusions
A novel alternating copolymer consisting of calix[4]arene and calix[6]arene (Poly-1) was successfully synthesized by Sonogashira coupling. Because the size of fluoranthene is not suited to the cavity size of calix[4]arene and calix[6]arene, calix[4]arene and calix[6]arene barely form host–guest complexes with fluoranthene. On the other hand, Poly-1 was able to strongly capture fluoranthene (K = 2700 ± 900 M−1). The strong binding ability results from multipoint host–guest interactions between the calixarene moieties in Poly-1 and fluoranthene. Future work will focus on the application of Poly-1 to reaction templates to prepare alternating copolymers using both calix[4]arene and calix[6]arene polymerizable guests, because both guests alternate on the main chain of Poly-1.
References
-
C. D. Gutsche, Calixarenes, The Royal Society of Chemistry, Cambridge, 1989 Search PubMed
 ;
Calixarenes: A Versatile Class of Macrocyclic Compounds, ed. J. Vicens and V. Böhmer, Kluwer Academic, Dordrecht, 1991 Search PubMed
;
Calixarenes: A Versatile Class of Macrocyclic Compounds, ed. J. Vicens and V. Böhmer, Kluwer Academic, Dordrecht, 1991 Search PubMed  ; T. Yamagishi, E. Moriyama, G. Konishi and Y. Nakamoto, Macromolecules, 2005, 38, 6871 Search PubMed
; T. Yamagishi, E. Moriyama, G. Konishi and Y. Nakamoto, Macromolecules, 2005, 38, 6871 Search PubMed  ; J. L. Atwood, S. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337 Search PubMed
; J. L. Atwood, S. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337 Search PubMed  ; R. Castro, L. A. Godinez, C. M. Criss and A. E. Kaifer, J. Org. Chem., 1997, 62, 4928 CrossRef CAS
; R. Castro, L. A. Godinez, C. M. Criss and A. E. Kaifer, J. Org. Chem., 1997, 62, 4928 CrossRef CAS  ; L. Zhang, A. Macías, T. Lu, J. I. Gordon, G. W. Gokel and A. E. Kaifer, J. Chem. Soc., Chem. Commun., 1993, 1017 RSC
; L. Zhang, A. Macías, T. Lu, J. I. Gordon, G. W. Gokel and A. E. Kaifer, J. Chem. Soc., Chem. Commun., 1993, 1017 RSC  ; T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2007, 4776 CrossRef CAS
; T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2007, 4776 CrossRef CAS  .
.
- A. Ikeda and S. Shinkai, Chem. Rev., 1997, 97, 1713 CrossRef CAS
 ; D. A. Fulton and J. F. Stoddart, Bioconjugate Chem., 2001, 12, 655 CrossRef CAS
; D. A. Fulton and J. F. Stoddart, Bioconjugate Chem., 2001, 12, 655 CrossRef CAS  .
.
- D. M. Gravett and J. E. Guillet, Macromolecules, 1996, 29, 617 CrossRef CAS
 ; A. I. Costa, L. F. V. Ferreira and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6477 CrossRef CAS
; A. I. Costa, L. F. V. Ferreira and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6477 CrossRef CAS  ; A. I. Costa and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 7054 CrossRef CAS
; A. I. Costa and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 7054 CrossRef CAS  ; J. H. Wosnick and T. M. Swager, Chem. Commun., 2004, 2744 RSC
; J. H. Wosnick and T. M. Swager, Chem. Commun., 2004, 2744 RSC  .
.
- P. J. A. Kenis, O. F. J. Noordman, N. F. van Hulst, J. F. Engbersen, D. N. Reinhoudt, B. H. M. Hams and P. J. M. van der Vorst, Chem. Mater., 1997, 9, 596 CrossRef CAS
 ; H. H. Yu, B. Xu and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 1142 CrossRef CAS
; H. H. Yu, B. Xu and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 1142 CrossRef CAS  ; Y. Yang and T. M. Swager, Macromolecules, 2006, 39, 2013 CrossRef CAS
; Y. Yang and T. M. Swager, Macromolecules, 2006, 39, 2013 CrossRef CAS  ; Y. Yang and T. M. Swager, Macromolecules, 2007, 40, 7437 CrossRef CAS
; Y. Yang and T. M. Swager, Macromolecules, 2007, 40, 7437 CrossRef CAS  .
.
- J. M. Liu, Y. S. Zheng, Q. Y. Zheng, J. Xie, M. X. Wang and Z. T. Huang, Tetrahedron, 2002, 58, 3729 CrossRef CAS
 ; N. Cheriaa, R. Abidi and J. Vicens, Tetrahedron Lett., 2005, 46, 1533 CrossRef CAS
; N. Cheriaa, R. Abidi and J. Vicens, Tetrahedron Lett., 2005, 46, 1533 CrossRef CAS  ; G. Gattuso, A. Notti, A. Pappalardo, M. F. Parisi, I. Pisagatti, S. Pappalardo, D. Garozzo, A. Messina, Y. Cohen and S. Slovak, J. Org. Chem., 2008, 73, 7280 CrossRef CAS
; G. Gattuso, A. Notti, A. Pappalardo, M. F. Parisi, I. Pisagatti, S. Pappalardo, D. Garozzo, A. Messina, Y. Cohen and S. Slovak, J. Org. Chem., 2008, 73, 7280 CrossRef CAS  .
.
- C. Yang, T. Mori, Y. Origane, Y. H. Ko, N. Selvapalam, K. Kim and Y. Inoue, J. Am. Chem. Soc., 2008, 130, 8574 CrossRef CAS
 ; C. Yang, Y. H. Ko, N. Selvapalam, Y. Origane, T. Mori, T. Wada, K. Kim and Y. Inoue, Org. Lett., 2007, 9, 4789 CrossRef CAS
; C. Yang, Y. H. Ko, N. Selvapalam, Y. Origane, T. Mori, T. Wada, K. Kim and Y. Inoue, Org. Lett., 2007, 9, 4789 CrossRef CAS  ; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, Chem. Commun., 2007, 4734 RSC
; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, Chem. Commun., 2007, 4734 RSC  ; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, J. Org. Chem., 2008, 73, 3775 CrossRef CAS
; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, J. Org. Chem., 2008, 73, 3775 CrossRef CAS  ; M. Miyauchi and A. Harada, J. Am. Chem. Soc., 2004, 126, 11418 CrossRef CAS
; M. Miyauchi and A. Harada, J. Am. Chem. Soc., 2004, 126, 11418 CrossRef CAS  ; M. Miyauchi, T. Hoshino, H. Yamaguchi, S. Kamitori and A. Harada, J. Am. Chem. Soc., 2005, 127, 2034 CrossRef CAS
; M. Miyauchi, T. Hoshino, H. Yamaguchi, S. Kamitori and A. Harada, J. Am. Chem. Soc., 2005, 127, 2034 CrossRef CAS  .
.
- L. Baklouti, J. Cherif, R. Abidi, F. Arnaid-Neu, J. Harrowfield and J. Vicens, Org. Biomol. Chem., 2004, 2, 2786 RSC
 .
.
- S. Kanamathareddy and C. D. Gutsche, J. Org. Chem., 1994, 59, 3871 CrossRef CAS
 .
.
- S. Kanamathareddy and C. D. Gutsche, J. Org. Chem., 1992, 57, 3160 CrossRef CAS
 .
.
-
K. A. Connors in Cyclodextrins, Comprehensive Supramolecular Chemistry, ed. J. Szejtli and T. Osa, Pergamon, Oxford, 1996 Search PubMed
 .
.
|
| This journal is © The Royal Society of Chemistry 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![An Alternating copolymer containing calix[4]arene and calix[6]arene in its main chain.](/image/article/2010/PY/b9py00221a/b9py00221a-f1.gif)
![GPC traces of (a) calix[4]arene monomer, (b) calix[6]arene monomer and (c) Poly-1.](/image/article/2010/PY/b9py00221a/b9py00221a-f2.gif)
![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) :
:![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1, blue square) and Poly-1 (red circle). By adding 1 and/or 2, the values of the fluorescence ratio hardly changed. From these data, it was found that both calix[4]arene and calix[6]arene formed weak host–guest complexes with fluoranthene. This is because the size of fluoranthene is not suited to the size of the cavities of calix[4]arene and calix[6]arene. On the other hand, even with small amounts of Poly-1, large fluorescence quenching was detected. The association constant (K) for the Poly-1-fluoranthene complex was determined to be 2700 ± 900 M−1 by fitting the data to eqn (1).10 From these observations, Poly-1 strongly captured fluoranthene compared to 1 and 2. Poly-1 should participate in multipoint host–guest interactions with fluoranthene, thus Poly-1 was able to form a host–guest complex with fluoranthene. Fluoranthene is a polynuclear aromatic compound and calixarenes are composed of benzene rings, thus the driving force of the complex formation may be π–π interactions. To examine the fluorescence quenching mechanism, Stern–Volmer plots were drawn (see the ESI† ). With the titration of Poly-1, the ratios of I/I0 of the fluorescence intensities increased until reaching a maximum and then remained constant. The nonlinear fluorescence quenching could be mainly assigned to complex formation (static mechanism).
1, blue square) and Poly-1 (red circle). By adding 1 and/or 2, the values of the fluorescence ratio hardly changed. From these data, it was found that both calix[4]arene and calix[6]arene formed weak host–guest complexes with fluoranthene. This is because the size of fluoranthene is not suited to the size of the cavities of calix[4]arene and calix[6]arene. On the other hand, even with small amounts of Poly-1, large fluorescence quenching was detected. The association constant (K) for the Poly-1-fluoranthene complex was determined to be 2700 ± 900 M−1 by fitting the data to eqn (1).10 From these observations, Poly-1 strongly captured fluoranthene compared to 1 and 2. Poly-1 should participate in multipoint host–guest interactions with fluoranthene, thus Poly-1 was able to form a host–guest complex with fluoranthene. Fluoranthene is a polynuclear aromatic compound and calixarenes are composed of benzene rings, thus the driving force of the complex formation may be π–π interactions. To examine the fluorescence quenching mechanism, Stern–Volmer plots were drawn (see the ESI† ). With the titration of Poly-1, the ratios of I/I0 of the fluorescence intensities increased until reaching a maximum and then remained constant. The nonlinear fluorescence quenching could be mainly assigned to complex formation (static mechanism).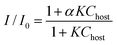


![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) :
:![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 1, blue square) and Poly-1 (red circle) in chloroform. The association constant (K value) between fluoranthene and Poly-1 was determined to be 2700 ± 900 M−1 by fitting data with eqn (1) (red solid line), where α is a constant.
1, blue square) and Poly-1 (red circle) in chloroform. The association constant (K value) between fluoranthene and Poly-1 was determined to be 2700 ± 900 M−1 by fitting data with eqn (1) (red solid line), where α is a constant.; Calixarenes: A Versatile Class of Macrocyclic Compounds, ed. J. Vicens and V. Böhmer, Kluwer Academic, Dordrecht, 1991 Search PubMed
; T. Yamagishi, E. Moriyama, G. Konishi and Y. Nakamoto, Macromolecules, 2005, 38, 6871 Search PubMed
; J. L. Atwood, S. J. Dalgarno, M. J. Hardie and C. L. Raston, Chem. Commun., 2005, 337 Search PubMed
; R. Castro, L. A. Godinez, C. M. Criss and A. E. Kaifer, J. Org. Chem., 1997, 62, 4928 CrossRef CAS
; L. Zhang, A. Macías, T. Lu, J. I. Gordon, G. W. Gokel and A. E. Kaifer, J. Chem. Soc., Chem. Commun., 1993, 1017 RSC
; T. Ogoshi, T. Yamagishi and Y. Nakamoto, Chem. Commun., 2007, 4776 CrossRef CAS
.
; D. A. Fulton and J. F. Stoddart, Bioconjugate Chem., 2001, 12, 655 CrossRef CAS
.
; A. I. Costa, L. F. V. Ferreira and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 6477 CrossRef CAS
; A. I. Costa and J. V. Prata, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 7054 CrossRef CAS
; J. H. Wosnick and T. M. Swager, Chem. Commun., 2004, 2744 RSC
.
; H. H. Yu, B. Xu and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 1142 CrossRef CAS
; Y. Yang and T. M. Swager, Macromolecules, 2006, 39, 2013 CrossRef CAS
; Y. Yang and T. M. Swager, Macromolecules, 2007, 40, 7437 CrossRef CAS
.
; N. Cheriaa, R. Abidi and J. Vicens, Tetrahedron Lett., 2005, 46, 1533 CrossRef CAS
; G. Gattuso, A. Notti, A. Pappalardo, M. F. Parisi, I. Pisagatti, S. Pappalardo, D. Garozzo, A. Messina, Y. Cohen and S. Slovak, J. Org. Chem., 2008, 73, 7280 CrossRef CAS
.
; C. Yang, Y. H. Ko, N. Selvapalam, Y. Origane, T. Mori, T. Wada, K. Kim and Y. Inoue, Org. Lett., 2007, 9, 4789 CrossRef CAS
; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, Chem. Commun., 2007, 4734 RSC
; D. Zou, S. Andersson, R. Zhang, S. Sun, B. Åkermark and L. Sun, J. Org. Chem., 2008, 73, 3775 CrossRef CAS
; M. Miyauchi and A. Harada, J. Am. Chem. Soc., 2004, 126, 11418 CrossRef CAS
; M. Miyauchi, T. Hoshino, H. Yamaguchi, S. Kamitori and A. Harada, J. Am. Chem. Soc., 2005, 127, 2034 CrossRef CAS
.
.
.
.
.
