Chemoenzymatic synthesis of amylose-grafted poly(vinyl alcohol)
Yoshiro
Kaneko
,
Shun-ichi
Matsuda
and
Jun-ichi
Kadokawa
*
Graduate School of Science and Engineering, Kagoshima University, 1-21-40 Korimoto, Kagoshima 890-0065, Japan. E-mail: kadokawa@eng.kagoshima-u.ac.jp; Fax: +81 99 285 3253; Tel: +81 99 285 7743
First published on 11th December 2009
Abstract
In this study, we synthesized an amylose-grafted poly(vinyl alcohol) (PVA) (4) by a chemoenzymatic method. First, synthesis of an amine-functionalized PVA (2) was carried out by reduction of an azide-functionalized PVA (1) using NaBH4. Then, introduction of maltoheptaose to 2 was performed by reductive amination using NaBH3CN to produce a maltoheptaose-grafted PVA (3). Finally, a phosphorylase-catalyzed enzymatic polymerization of α-D-glucose 1-phosphate from the maltoheptaose-chains in 3 was carried out to obtain 4. A film of 4 was prepared by drying its aqueous solution spread on a flat quartz glass substrate. An iodine-doped film was prepared by soaking the film of 4 in KI/I2ethanol solution. UV-Vis analysis indicated that iodine was retained in this film even after it was left standing for 24 h. This is probably because the iodide ion was within the cavities of the amylose graft chains in this film.
Introduction
Poly(vinyl alcohol) (PVA) film exhibits unique properties such as formation of a complex with iodine, that has attracted much attention in terms of application as a polarizing material for liquid crystal displays (LCDs). The polarized film in LCD is generally prepared based on the PVA/iodine complex.1,2 However, because iodine is easily released from the PVA backbone under relatively high temperature and/or high humidity conditions, this film is susceptible to heat and moisture. Therefore, the PVA-based polarized film is practically used by being sandwiched between protecting films such as triacetylcellulose (TAC) films. Development of a sole film, which has an ability to stably retain iodine without use of the protecting film, is hopefully expected.Amylose is a polysaccharide with helical conformation linked through (1 → 4)-α-glucosidic linkages and a well-known host compound that forms a stable inclusion complex with iodine.3 Therefore, a blend film composed of PVA and amylose is possibly one of the candidates for such a new polarized film stably retaining iodine without use of TAC.4,5 However, the blend film may have heterogeneous distribution of amylose chains in PVA matrix due to their aggregation.
To yield such a film stably retaining iodine, we have been considering the preparation of a PVA derivative covalently bonded to amylose, e.g., an amylose-grafted PVA. In the previous studies on the synthesis of the amylose-grafted polymers, enzymatic polymerization has been employed because this type of polymerization is a powerful tool for obtaining amylose chains.6,7 For example, a phosphorylase-catalyzed enzymatic polymerization using α-D-glucose 1-phosphate (G-1-P) as a monomer proceeds with the regio- and stereoselective construction of a glucosidic bond under mild conditions, leading to the direct formation of (1 → 4)-α-glucan chain, i.e., amylose, in the aqueous media.8 This polymerization is initiated from a maltooligosaccharide primer like maltoheptaose. Then, the propagation proceeds through the following reversible reaction to produce amylose:
| [(α, 1 → 4)-G]n + G-1-P ⇆ [(α, 1 → 4)-G]n+1 + P |
This enzymatic polymerization has been combined with the appropriate chemical reactions (chemoenzymatic method) to produce the polymers having the amylose graft-chains. So far, such amylose-grafted polymers as polystyrene,9,10polyacetylene,11,12polysiloxane,13poly(L-glutamic acid),14chitin/chitosan,15,16 and cellulose17 have been prepared by this chemoenzymatic method, in which the maltooligosaccharide-grafted polymers were first prepared by the chemical reaction, and then the phosphorylase-catalyzed enzymatic polymerization using G-1-P from nonreducing terminus of the maltooligosaccharide side-chains in the polymers was performed.
To obtain the amylose-grafted PVA, in this study, we first prepared an amine-functionalized PVA (2), which has the ability to react with maltoheptaose by reductive amination, giving a maltoheptaose-grafted PVA (3). Then, the phosphorylase-catalyzed enzymatic polymerization from the maltoheptaose graft-chains in 3 was performed to produce the amylose-grafted PVA (4). In addition, the film composed of 4/iodine complexes was prepared and the behavior when retaining iodine in this film was investigated.
Experimental
Materials
PVA (average degree of polymerization (DP) = 2000) of a commercial reagent from Nacalai Tesque, Inc., Japan was used. The azide-functionalized PVA (1) was prepared by coupling of 2-azidoethylamine with PVA activated by 1,1′-carbonyldiimidazole according to a method from literature.18Maltoheptaose was prepared by the selective cleavage of one glycosidic bond of β-cyclodextrin under acidic condition according to the literature procedure.13Phosphorylase (300 unit/mL) was supplied from Ezaki Glico Co. Ltd, Japan.19 Other reagents and solvents were used as received.Synthesis of amine-functionalized PVA (2)
The azide-functionalized PVA (1)18 (functionality of azido group = 1.80 unit%, 99.2 mg = 2.15 mmol unit; on the basis of average molecular weight (MW) of one repeating unit) was added to DMSO (10 mL) and the mixture was stirred at 60 °C under argon atmosphere to obtain a clear solution. After NaBH4 (359.4 mg = 9.50 mmol) was added to this solution and the mixture was stirred at 60 °C for 24 h, the mixture was poured into excess amount of methanol. The resulting precipitate was isolated by filtration, washed with methanol, and then dried under reduced pressure at room temperature to give 90.4 mg of 2.
1H NMR (400 MHz, D2O, 60 °C): δ 5.01 (br, –CH–OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–), δ 4.02 (br, –CH– of main chain), δ 3.34 (br, –OC(
O)NH–), δ 4.02 (br, –CH– of main chain), δ 3.34 (br, –OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–CH2–), δ 2.87 (br, –CH2–NH2), δ 1.74 − 1.61 (br, –CH2– of main chain).
O)NH–CH2–), δ 2.87 (br, –CH2–NH2), δ 1.74 − 1.61 (br, –CH2– of main chain).
Synthesis of maltoheptaose-grafted PVA (3)
To a solution of 2 (functionality of amino group = 1.65 unit%, 90.4 mg = 1.99 mmol unit; on the basis of average MW of one repeating unit) in a mixed solvent of 1.0 wt% aqueous acetic acid and methanol (1/1 (v/v), 10 mL), a solution of maltoheptaose (155.7 mg = 0.135 mmol) in the aforementioned mixed solvent (2.0 mL) and a solution of NaBH3CN (47.1 mg = 0.675 mmol) in the mixed solvent (1.0 mL) were successively added with vigorous stirring at room temperature. After the solution was stirred further for 10 days, the solution was poured into excess amount of methanol to precipitate the powdered product. The aqueous solution of the product was dialyzed in a dialysis bag (molecular weight cut off: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against water and the resulting solution was lyophilized to yield 83.7 mg of 3.
000) against water and the resulting solution was lyophilized to yield 83.7 mg of 3.
1H NMR (400 MHz, D2O, 60 °C): δ 5.36 (br, H-1 of maltoheptaose), δ 5.01 (br, –CH–OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–), δ 4.02 (br, –CH– of main chain), δ 4.21–3.34 (br, H-2–H-6 of maltoheptaose), δ 3.39 (br, –OC(
O)NH–), δ 4.02 (br, –CH– of main chain), δ 4.21–3.34 (br, H-2–H-6 of maltoheptaose), δ 3.39 (br, –OC(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)NH–CH2–), δ 3.03 (br, –CH2–NH2 and –CH2–NH), δ 1.74–1.61 (br, –CH2– of main chain).
O)NH–CH2–), δ 3.03 (br, –CH2–NH2 and –CH2–NH), δ 1.74–1.61 (br, –CH2– of main chain).
Synthesis of amylose-grafted PVA (4)
The maltoheptaose-grafted PVA (3) (functionality of maltoheptaose = 0.06 unit%, 11.5 mg = 0.249 mmol unit; on the basis of average MW of one repeating unit) was dissolved in sodium acetate buffer (0.2 mol/L, pH 6.2, 4 mL) by heating, followed by cooling to room temperature, and then sodium salt of G-1-P (30.4 mg = 0.1 mmol) was added to this solution. After the pH value of the solution was adjusted to 6.2 by addition of 0.2 mol/L aqueous acetic acid, phosphorylase (ca. 30 unit) was added to this solution, and the solution was stirred for 14 h at 40–45 °C. The reaction mixture was dialyzed in a dialysis bag (molecular weight cut off: 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–14
000–14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) against water and the resulting solution was lyophilized to yield 28.6 mg of 4.
000) against water and the resulting solution was lyophilized to yield 28.6 mg of 4.
1H NMR (400 MHz, D2O, 60 °C): δ 5.36 (br, H-1 of amylose), δ 4.02 (br, –CH– of main chain), δ 4.21–3.34 (br, H-2–H-6 of amylose), δ 1.74–1.61 (br, –CH2– of main chain).
Measurements
The 1H NMR spectra were recorded using a JEOL ECX 400 spectrometer. The IR spectra were recorded using a Shimadzu FTIR-8400 spectrometer. The UV-Vis spectra were measured on quartz at room temperature using a Jasco V-650 spectrophotometer.Results and discussion
Synthesis of amylose-grafted PVA
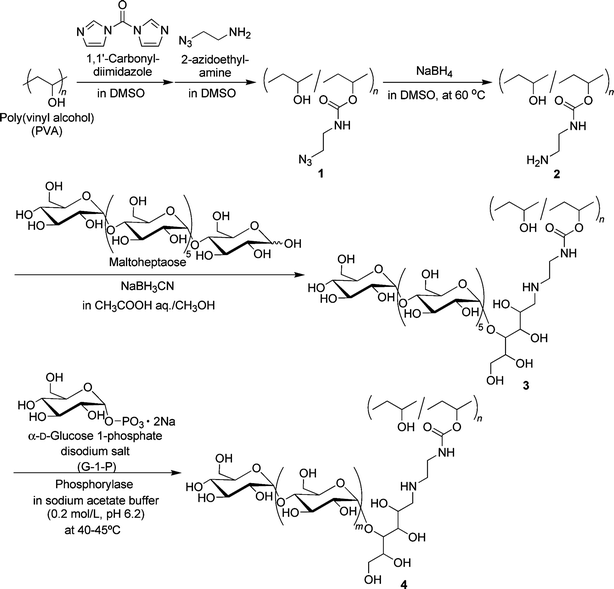
For the preparation of the desired amylose-grafted PVA (4) by the chemoenzymatic method as shown in Scheme 1, the amino group has necessarily been introduced to PVA because the amino group efficiently reacts with maltoheptaose by the reductive amination, giving the maltoheptaose-grafted PVA (3) as a key compound in this study. Therefore, we first performed the synthesis of the amine-functionalized PVA (2) by the reduction of the azido groups of 1† to the amino groups (Scheme 1). The IR spectrum of the product showed the absorption at 1694 cm−1 due to the urethane bond (Fig. 1). In addition, the 1H NMR spectrum of the product showed the signals b and c ascribed to N–CH2–CH2–N, indicating the presence of the aminoethyl groups (Fig. 2). These results indicate that the product had the structure of the amine-functionalized PVA (2) as shown in Scheme 1. In this study, we adjusted the functionality of the amino group to 1.65 unit% (estimated by 1H NMR) in 2, taking into consideration the solubility of the final product 4 obtained by following reactions.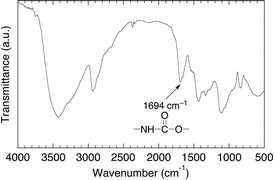 | ||
| Fig. 1 IR spectrum of amine-functionalized PVA (2). | ||
 | ||
| Fig. 2 1H NMR spectrum in D2O at 60 °C of amine-functionalized PVA (2). | ||
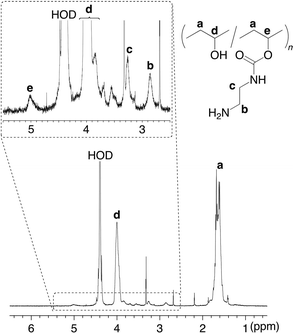
Introduction of maltoheptaose to 2 was carried out by the reductive amination using NaBH3CN in a mixed solvent of 1.0 mol/L aqueous acetic acid and methanol at room temperature to produce the maltoheptaose-grafted PVA (3) (Scheme 1). The 1H NMR spectrum in D2O at 60 °C of the product exhibited both signals due to the PVA main-chain and due to the maltoheptaose graft-chains (Fig. 3), indicating that the product had the structure of 3. The functionality of maltoheptaose was calculated by the 1H NMR spectrum to be 0.06 unit%.
 | ||
| Fig. 3 1H NMR spectrum in D2O at 60 °C of maltoheptaose-grafted PVA (3). | ||
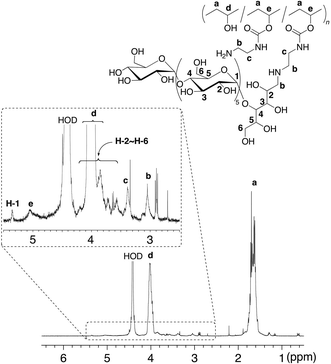
Synthesis of the amylose-grafted PVA (4) was achieved by the phosphorylase-catalyzed enzymatic polymerization of G-1-P from the maltoheptaose primers in 3 (feed molar ratio of G-1-P/maltoheptaose in 3 = 669) in sodium acetate buffer (0.2 mol/L, pH 6.2) at 40–45 °C (Scheme 1). The product was isolated by dialysis against water. The isolated product was soluble in hot water, and thus characterized by the 1H NMR measurement in D2O at 60 °C (Fig. 4). The integrated ratio of the signal due to H-1 of α-glucan (δ 5.36) to the signal due to –CH2– of PVA (δ 1.74 − 1.61) increased compared with that in the 1H NMR spectrum of 3 as shown in Fig. 3. This result indicated that the enzymatic polymerization took place from the maltoheptaose chains in 3 to produce the amylose-grafted PVA (4). On the basis of the functionality of maltoheptaose in 3 (0.06%) and the integrated ratio of the signal due to H-1 of amylose graft-chain to the signal a of PVA main-chain in the 1H NMR spectrum of 4 (Fig. 4), the average DP of the amylose graft chains was estimated to be ca. 215, which corresponded to the MW value of ca. 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800. Because the average DP of the employed PVA was 2000 corresponding to the MW value of 88
800. Because the average DP of the employed PVA was 2000 corresponding to the MW value of 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100, the average MW of 4 was calculated to be ca. 129
100, the average MW of 4 was calculated to be ca. 129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900.
900.
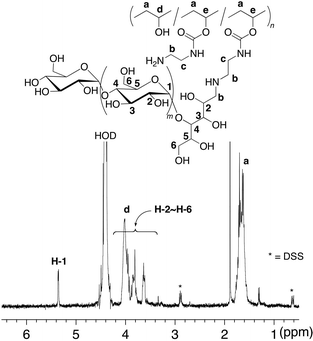 | ||
| Fig. 4 1H NMR spectrum in D2O at 60 °C of amylose-grafted PVA (4). | ||
Preparation of amylose-grafted PVA/iodine complex film and behavior when retaining iodine in the film
Films of 4 and PVA were prepared by drying their aqueous solutions spread on flat quartz glass substrates. Transmittances of the films of 4 and PVA were measured by the UV-Vis spectroscopy. Although the transmittance of the film of 4 (Fig. 5a) was slightly lower than that of PVA film (Fig. 5b), the film of 4 showed sufficiently high transparency in the visible wave length region (transmittance; >95%). We assumed that the slight difference in the transmittances at around 280 nm in both spectra was probably caused by the absorption at that wavelength due to urethane groups in 4.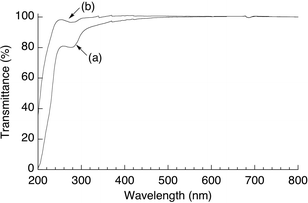 | ||
| Fig. 5 Transmittance curves of the films of (a) 4 and (b) PVA. | ||
The PVA film is generally prepared by drying an aqueous solution of PVA.20 Because it is difficult to completely dissolve amylose, even in hot water, the blend film obtained from the aqueous mixture of PVA and amylose probably has a heterogeneous distribution of amylose chains in PVA matrix. Actually, when we tried to prepare the amylose (DP = 388)/PVA blend film by drying their aqueous mixture, a transparent film was not obtained. Therefore, conjugation of amylose to PVA by covalent linkages in this study is an efficient method for providing the amylose/PVA material, which has the ability to form a transparent film from its aqueous solution.
The complex formation with iodine is a well-known characteristic property of amylose.3 The colorless films of 4 and PVA were soaked in KI/I2ethanol solution to form iodine-doped films. The UV-Vis spectrum of the iodine-doped film of 4, which colored red-purple, showed the absorption at ca. 570 nm due to polyiodine which was assignable to I5− or its linear array (I5−)x, in addition to the absorption at ca. 300 nm attributable to I3− (Fig. 6a).21 Moreover, these absorptions were still observed even after the doped film was left standing for 24 h (Fig. 6a). On the other hand, the absorptions at ca. 300 and 360 nm due to I3− in the UV-Vis spectrum of the iodine-doped PVA were drastically decreased after 24 h (Fig. 6b). The above results indicated that the film of 4 retained iodine for 24 h, whereas most of iodine was released from the PVA film for 24 h. The difference is probably due to the stable complex formation in the film of 4 by inclusion of iodine in the cavities of the amylose graft chains. These results suggest that the present amylose-grafted PVA film has the possibility for utilization as the polarized films.
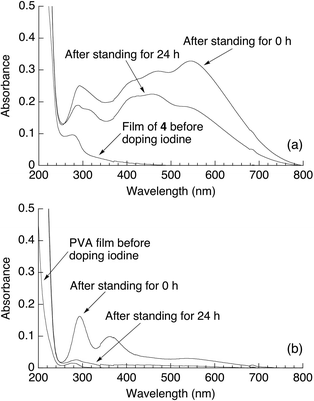 | ||
| Fig. 6 UV-Vis spectra of the iodine-doped films of (a) 4 and (b) PVA. | ||
Conclusions
In this study, we synthesized the amylose-grafted PVA (4) by the chemoenzymatic method from PVA as follows: the reduction of the azido groups of 1 using NaBH4 to convert to the amine-functionalized PVA2, reductive amination using NaBH3CN to produce the maltoheptaose-grafted PVA (3), and the phosphorylase-catalyzed enzymatic polymerization of G-1-P from the maltoheptaose-chains in 3. The film of 4 was soaked in KI/I2ethanol solution to form an iodine-doped film. UV-Vis analysis indicated that iodine was retained in this film even after it was left standing for 24 h. This was probably because the iodide ions were contained in the cavities of the amylose graft chains in this film.Acknowledgements
We acknowledge the gift of phosphorylase from Ezaki Glico Co. Ltd, Osaka, Japan.References
- T. Yosomiya, Y. Suzuki and R. Yosomiya, Angew. Makromol. Chem., 1995, 230, 171 CrossRef CAS.
- H. Yang and F. Horii, Polymer, 2008, 49, 785 CrossRef CAS.
- X. C. Yu, C. Houtman and R. H. Atalla, Carbohydr. Res., 1996, 292, 129 CAS.
- S. Y. Yang and C. Y. Huang, J. Appl. Polym. Sci., 2008, 109, 2452 CrossRef CAS.
- J. Wang, Y. Lu, H. L. Yuan and P. Dou, J. Appl. Polym. Sci., 2008, 110, 523 CrossRef CAS.
- M. Ohmae, S. Fujikawa, H. Ochiai and S. Kobayashi, J. Polym. Sci., Part A: Polym. Chem., 2006, 44, 5014 CrossRef CAS.
- S. Kobayashi and M. Ohmae, Adv. Polym. Sci., 2006, 194, 159 CAS.
- G. Ziegast and B. Pfannemüller, Carbohydr. Res., 1987, 160, 185 CrossRef CAS.
- K. Kobayashi, S. Kamiya and N. Enomoto, Macromolecules, 1996, 29, 8670 CrossRef CAS.
- A. Narumi, K. Kawasaki, H. Kaga, T. Satoh, N. Sugimoto and T. Kakuchi, Polym. Bull., 2003, 49, 405 CrossRef CAS.
- J. Kadokawa, Y. Nakamura, Y. Sasaki, Y. Kaneko and T. Nishikawa, Polym. Bull., 2008, 60, 57 CrossRef CAS.
- Y. Sasaki, Y. Kaneko and J. Kadokawa, Polym. Bull., 2009, 62, 291 CrossRef CAS.
- V. Braunmühl, G. Jonas and R. Stadler, Macromolecules, 1995, 28, 17 CrossRef.
- S. Kamiya and K. Kobayashi, Macromol. Chem. Phys., 1998, 199, 1589 CrossRef CAS.
- S. Matsuda, Y. Kaneko and J. Kadokawa, Macromol. Rapid Commun., 2007, 28, 863 CrossRef CAS.
- Y. Kaneko, S. Matsuda and J. Kadokawa, Biomacromolecules, 2007, 8, 3959 CrossRef CAS.
- Y. Omagari, S. Matsuda, Y. Kaneko and J. Kadokawa, Macromol. Biosci., 2009, 9, 450 CrossRef CAS.
- D. A. Ossipov and J. Hilborn, Macromolecules, 2006, 39, 1709 CrossRef CAS.
- M. Yanase, H. Takata, K. Fujii, T. Takaha and T. Kuriki, Appl. Environ. Microbiol., 2005, 71, 5433 CrossRef CAS.
- E. M. Kim, M. H. Han, Y. J. Lee, D. H. Song, H. K. Lee, O. W. Kwon, D. S. Shin, S. S. Han, S. K. Noh, J. K. Shin, Y.-S. Gal and W. S. Lyoo, J. Appl. Polym. Sci., 2007, 106, 3259 CrossRef CAS.
- A. Cesáro, J. C. Benegas and D. R. Ripoll, J. Phys. Chem., 1986, 90, 2787 CrossRef CAS.
Footnote |
† Although 1 was prepared according to the literature procedure,18 the 1H NMR spectrum of 1 showed an unexpected signal at ca. δ 4.6–4.8, overlapped with those due to OH groups of PVA. We assumed that this signal would be assigned to –CH–O(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)O– obtained by the reaction of 1,1′-carbonyldiimidazole with two OH groups of PVA. However, this signal disappeared by the treatment with NaBH4, probably due to the reduction of the carbonate group to the alcohol group. Therefore, in this study, we used 1 without further purification because the carbonate group in 1 could be degraded at the next reduction step to 2. O)O– obtained by the reaction of 1,1′-carbonyldiimidazole with two OH groups of PVA. However, this signal disappeared by the treatment with NaBH4, probably due to the reduction of the carbonate group to the alcohol group. Therefore, in this study, we used 1 without further purification because the carbonate group in 1 could be degraded at the next reduction step to 2. |
| This journal is © The Royal Society of Chemistry 2010 |