Synthesis of ellipsoidal hematite/polymer/titania hybrid materials and the corresponding hollow ellipsoidal particles
Longyu
Li
,
Dianbin
Qin
,
Xinlin
Yang
* and
Guangyu
Liu
Key Laboratory of Functional Polymer Materials, the Ministry of Education, Institute of Polymer Chemistry, Nankai University, Tianjin 300071, China. E-mail: xlyang88@nankai.edu.cn; Fax: +86-22-23503510; Tel: +86-22-23502023
First published on 16th December 2009
Abstract
Ellipsoidal tri-layer hematite/poly(N,N′-methylenebisacrylamide-co-methacrylic acid)/titania (Fe2O3/P(MBA-co-MAA)/TiO2) hybrid materials were prepared by a three-stage reaction process, which included the combination of the controlled hydrolysis of inorganic salt to afford ellipsoidal hematite core and titania shell layer and distillation precipitation copolymerization of N,N′-methylenebisacrylamide (MBA) and methacrylic acid (MAA) in acetonitrile for the formation of a P(MBA-co-MAA) sandwiched mid-layer. The corresponding hollow ellipsoidal hematite-sphere-in-titania-spheres were afforded by selective removal of the sandwiched P(MBA-co-MAA) mid-layer viacalcination. Hollow titania ellipsoids were further obtained by selectively etching the hematite core in aqueous hydrofluoric acid (HF) solution from the ellipsoidal hematite-sphere-in-titania-sphere. The resultant ellipsoidal tri-layer Fe2O3/P(MBA-co-MAA)/TiO2 and the corresponding hollow ellipsoidal particles were confirmed by transmission electron microscopy (TEM), Fourier transform infrared spectroscopy, X-ray diffraction (XRD), thermogravimetric (TGA) analysis and vibrating sample magnetometery (VSM).
Introduction
During the past decade, the design and synthesis of inorganic/polymer hybrid materials with well-defined shape and structure has been an important and interesting field for material scientists due to the advantages of the combination of the organic polymer matrix having facile process-ability, various functional groups, and flexibility, together with characteristics of the inorganic components in terms of mechanical, strength, modulus, and thermal stability.1,2 As one of the inorganic–organic hybrid materials, inorganic/organic core-shell hybrid particles have attracted increasing attention because the materials can exhibit novel and excellent properties, such as mechanical, chemical, electrical, rheological, magnetic, optical, and catalytic, by adjusting their composition, size and structure, which have proven, diverse applications such as drug-delivery systems, diagnostics, coatings and catalysts.3–7Titania (TiO2) is an essential functional material because of its peculiar and fascinating physicochemical properties and a wide variety of potential uses in diverse fields, including solar cell energy conversion, environmental purification, and water treatment.8 Very recently, nanometre-size spherical titania particles with narrow-size distribution and diameters in the range of 50–680 nm were produced by the slowly controlled hydrolysis of tetrabutoxy titanium (TBOT) through a titanium glycolate intermediate in ethyleneglycol.9,10 Monodisperse colloidal titania microspheres were successfully synthesized via controlled hydrolysis of the reactivity-reduced glycolated TBOT precursor in acetone in the presence of Tween20surfactant, in which the particle size, ranging from 230 to 650 nm, was finely tuned by altering the concentration of titanium precursor and surfactant.11 Although TiO2 nanoparticles can be routinely prepared, titania with the complex microstructure forms, such as hollow spheres, tubes and porous frameworks, is rarely reported even though these materials may offer additional advantages in technical applications. Particularly, TiO2 hollow structured materials have received more and more attention owing to their low density, high specific areas, good photocatalytic activity as well as larger light-harvesting efficiencies.12–14 Moreover, the hollow structured materials have also been considered as potential candidates for electrode materials in photochemical cells. The hollow titania spheres with unique urchinlike morphology and tunable sphere-in-sphere structure endowed the spheres with greatly enhanced photocatalytic activity possibly owing to multiple reflections of UV light within the interior voids.12 By now, TiO2 hollow spheres have been successively prepared by sol–gel technique in presence of sulfonated polystyrene (PSt) latex particles as templates.15,16 Xia and coworkers have reported the synthesis of amorphous TiO2 mesoscale hollow spheres by templating their sol–gel precursor solution on crystalline arrays of PSt particles and subsequently dissolving the PSt beads in toluene.17 Wang et al. obtained hollow titania spheres with dense, uniform shells of small anatase crystallites after calcination of core–shell particles consisting of PSt cores and titania shells, which were prepared by the hydrolysis of TBOT by catalysis with ammonia ions in the acetonitrile–ethanol mixed solvent.18 However, non-spherical hollow titania particles have rarely been reported in the literature due to the difficulty of getting a suitable non-spherical template,19,20 which would be more attractive for their utilization as ordered arrays due to their lower symmetry compared to the spherical particles. It is still a challenge to obtain non-spherical titania hollow spheres with defined shape and controllable size.
Hematite (α-Fe2O3), which is the most stable iron oxide under ambient conditions with n-type semiconducting properties, low cost and high resistance to corrosion, magnetic recording media, anti-corrosive agent, and lithium-ion batteries with great scientific and technical importance.21–23 Many efforts have been devoted to the incorporation of magnetic particles into the core–shell structures in order to control the shape, size and magnetic properties of the hybrid materials.24–26 For example, the uniform coating of silica onto ultrafine hematite particles has altered most properties of the iron-oxide particles, such as the dispersity in aqueous or non-aqueous media,27 in which the anisometric shape of the hematite cores was preserved with benefits via introduction of other functional materials onto the particles. Furthermore, ellipsoidal hematite is one of the suitable non-spherical templates for the construction of core–shell particles with well-defined shape, such as for the synthesis of hematite/silica/polypyrrole (α-Fe2O3/SiO2/PPy) ellipsoidal tri-layer composite particles and the further development of SiO2, SiO2/PPy, PPy hollow ellipsoidal spheres as well as hollow PPy ellipsoidal particles with movable hematite cores.19
In our previous work, ellipsoidal tri-layer hematite/silica/polydivinylbenzene (α-Fe2O3/SiO2/PDVB) hybrid particles28 and hematite/polymer core–shell hybrid materials29 were successfully prepared by distillation precipitation polymerization in the presence of ellipsoidal inorganic particles as seeds in acetonitrile in absence of any stabilizer or surfactant. Here, we describe the synthesis of tri-layer hematite/poly(N,N′-methylenebisacrylamide-co-methacrylic acid)/titania (α-Fe2O3/P(MBA-co-MAA)/TiO2) hybrid ellipsoids via the encapsulation of inorganichematite cores through distillation precipitation polymerization in acetonitrile to afford α-Fe2O3/P(MBA-co-MAA) core–shell particles with hydrophilic polymer shell layers which have carboxylic acid groups and the further controlled hydrolysis of TBOT in acetonitrile–ethanol mixed solvent to coat the ellipsoidal particles with a titania outer shell layer. Furthermore, hollow ellipsoidal hematite-sphere-in-titania-sphere were obtained viathermal decomposition of polymer by calcination of tri-layer ellipsoids and hollow TiO2 ellipsoidal particles were developed after the removal of α-Fe2O3 cores in aqueous hydrofluoric acid solution (HF) from hollow ellipsoidal hematite-sphere-in-titania-sphere.
Experimental
Chemicals
Ferric chloride (FeCl3·6H2O) was purchased from Tianjin Guangfu Fine Chemical Engineering Institute. Methacrylic acid (MAA, Tianjin Reagent Factory I, China) was purified by vacuum distillation before use. N,N′-Methylenebiacrylamide (MBA, chemical grade, Tianjin Bodi Chemical Engineering Co.) was recrystallized from acetone. 2,2′-Azobisisobutyronitrile (AIBN) was provided by Chemical Factory of Nankai University and recrystallized from methanol. Titanium tetrabutoxide (TBOT) was purchased from Tianjin Chemical Reagent II Co. Hydrofluoric acid (HF, containing 40% of HF) was available from Tianjin Chemical Reagent Institute. Acetonitrile (analytical grade, Tianjin Chemical Reagents II Co.) was dried over calcium hydride and purified by distillation before use. All the other reagents were of analytical grade and used without any further treatment.Preparation of ellipsoidal hematite/P(MBA-co-MAA) core–shell composites by distillation precipitation polymerization
Shuttle-like hematite (α-Fe2O3) particles were synthesized according to the method in the literature.19Hematite ellipsoids were obtained via aging an aqueous solution of 2 × 10−2 M FeCl3 and 4 × 10−4 M NaH2PO4buffer at 95 °C for three days. The formation of hematite particles was observed with the presence of a brick-red color suspension in the hydrolysis system. The resultant hematite particles were centrifugated, decanted and redispersed in acetone three times and then dried at 50 °C under vacuum until a constant weight was reached.The distillation precipitation copolymerization of MBA and MAA to synthesize hematite/poly(N,N′-methylenebisacrylamide-co-methacrylic acid) (α-Fe2O3/P(MBA-co-MAA)) core-shell composites was as following. In a dried 100 mL two-necked flask, 0.05 g of hematite composites were suspended in 80 mL of acetonitrile as a brick-red suspension. Then MBA (0.05 g, 0.32 mmol), MAA (0.1 mL, 0.1 g, 1.2 mmol) and AIBN (0.003 g, 2 wt% relative to the monomer) were dissolved in the suspension. The two-necked flask attaching with a fractionating column, Liebig condenser, and receiver was submerged in a heating mantle. The reaction mixture was heated from ambient temperature till the boiling state within 10 min and the reaction system was kept under refluxing state for 5 min further. Then the polymerization was carried out with distilling the solvent out of the reaction system, and the reaction was ended after 40 mL of acetonitrile was distilled off the reaction mixture within 40 min. After the polymerization, the resultant α-Fe2O3/P(MBA-co-MAA) core–shell composite materials were purified by repeating centrifugation, decantation, and resuspension with ultrasonic irradiation for three cycles. The composite particles were then dried in a vacuum oven at 50 °C until a constant weight was reached. The reproducibility of the copolymerizations was confirmed through several duplicate and triplicate experiments.
Preparation α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids
The coating reaction was performed by controlled hydrolysis of TBOT in the acetonitrile–ethanol (1/3, v/v) mixed solvent with α-Fe2O3/P(MBA-co-MAA) core–shell particles as templates in the presence of ammonia as the catalyst. In a typical experiment, 0.010 g of α-Fe2O3/P(MBA-co-MAA) ellipsoidal templates were dispersed in 40 mL of acetonitrile–ethanol (1/3, v/v) and then mixed with 0.25 mL of 25 wt% ammonia at room temperature. Finally, a solution of 0.25 mL TBOT in 10 mL of acetonitrile–ethanol (1/3, v/v) was added to the above α-Fe2O3/P(MBA-co-MAA) suspension under stirring. After the hydrolysis reaction was performed at room temperature for 1 h, the tri-layer hematite/poly(N,N′-methylenebisacrylamide-co- methacrylic acid)/titania (α-Fe2O3/P(MBA-co-MAA)/TiO2) hybrid ellipsoids were purified by three cycles of centrifugation with ultrasonic dispersion in acetonitrile. The composite particles were then dried in a vacuum oven at 50 °C until constant weight was reached.Preparation of hollow ellipsoids
Ellipsoidal hematite-sphere-in-titania-spheres were afforded by calcination of the α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer hybrid ellipsoids at 500 °C for 2 h.Hollow titania ellipsoidal were obtained by soaking the ellipsoidal hematite-sphere-in-titania-spheres in aqueous HF solution (containing 40% of HF) for 1 h at room temperature.
Characterization
The size and morphology of hematite, hematite/polymer core–shell particles, hematite/polymer/titania tri-layer core–shell particles and the corresponding hollow ellipsoids were determined by transmission electron microscopy (TEM, TechnaiG2 20-S-TWIN).The magnetic properties of hematite particles and hematite/polymer/titania tri-layer core–shell hybrids were studied with a vibrating sample magnetometer (9600 VSM, BDJ Electronic Inc, Troy MI, US) at room temperature.
Fourier transform infrared spectra (FT-IR) were scanned over the range of 400–4000 cm−1 with potassium bromide slice on a Bio-Rad FTS 135 FT-IR spectrometer.
Thermogravimetric (TGA) analysis of the TiO2/PEGDMA core-shell microspheres was performed with a Netzsch TG 209 under a nitrogen atmosphere with a heating rate of 10 °C min−1.
The crystalline structure of the samples was analyzed on a D/max 2500V X-ray diffractometer using Cu Kα (λ = 0.15406 nm) radiation at 40 kV and 100 mA. The crystal size of the TiO2 was estimated by applying the Scherrer equation (φ = kλ/βcosθ), where φ is the crystal size, λ is the wavelength of the X-ray irradiation, k is usually taken to be 0.89 here, β is the peak width at half-maximum height of the (101) peak of anatase after subtracting the instrumental line broadening, and θ is the diffraction angle. The samples for XRD analysis were not thermally annealed according to hematite core and hollow ellipsoidal hematite-sphere-in-titania-sphere. The sample of hollow titania ellipsoids were determined by XRD after thermal annealing during the formation of hollow structure.
Results and discussion
Scheme 1 illustrated the synthesis of α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer hybrid ellipsoids and the further development of various hollow ellipsoids, including ellipsoidal hematite-sphere-in-titania-sphere and hollow titania ellipsoids.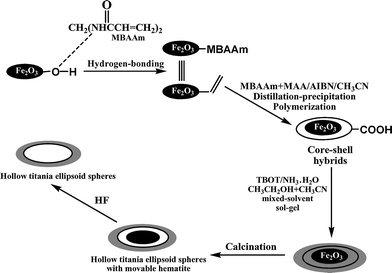 | ||
| Scheme 1 Synthesis of hematite/polymer/titania tri-layer core–shell hybrid particles and the two types of ellipsoidal hollow particles. | ||
Uniform α-Fe2O3 shuttle-like nanoparticles were prepared by homogeneous hydrolysis from a solution of iron salt (FeCl3) in the presence of NaH2PO4buffer.19 The TEM micrograph in Fig. 1A indicated that hematite nanoparticles had an ellipsoidal shape, which had the mean lengths of the major and minor axes of 531 and 113 nm, respectively. The XRD pattern of the product was shown in Fig. 2a, which matched the standard JCPDS data (86-0550) well.
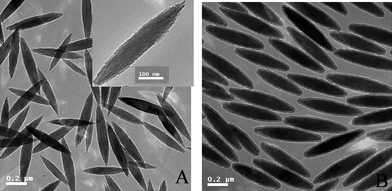 | ||
| Fig. 1 TEM micrographs: (A) hematite; (B) Fe2O3/P(MBA-co-MAA) core–shell particles. | ||
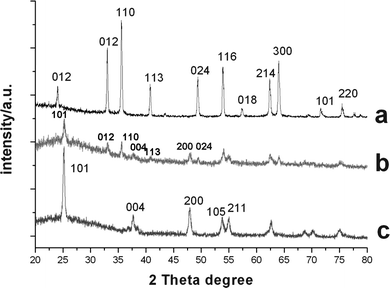 | ||
| Fig. 2 XRD patterns of ellipsoidal particles: (a) hematite; (b) hollow ellipsoidal hematite-sphere-in-titania-spheres; (c) titania ellipsoidal hollow capsules. | ||
In our previous work, α-Fe2O3/P(MBA-co-MAA) core–shell composite ellipsoids with carboxylic acid groups on the shell layer were prepared by distillation precipitation copolymerization of MBA and MAA in acetonitrile in presence of hematite particles as the seeds,29 in which the hydrogen-bonding interaction between the hydroxyl groups on the surface of hematite templates and the amide group as well as the carboxylic acid group of P(MBA-co-MAA) component played a key role for the encapsulation of the inorganic seeds. The typical TEM micrograph of hematite/P(MBA-co-MAA) ellipsoids was shown in Fig. 1B, in which a typical core–shell structure with lighter contrast of P(MBA-co-MAA) shell layer and deeper contrast of inorganichematite core was clearly observed. The major/minor axis of the hematite/P(MBA-co-MAA) ellipsoids increased significantly from 531/113 nm of hematite core to 573/153 nm as summarized in Table 1, which meant that P(MBA-co-MAA) with thickness of 20 nm was uniformly coated onto hematite templates via the polymerization. The TGA analyses indicated that the mass ratio of P(MBA-co-MAA) shell layer was 30.0 wt% with polymer thickness of 20 nm as shown in Table 1 (Entry B). The successful incorporation of P(MBA-co-MAA) component into the shell layer was confirmed further by FT-IR spectrum in Fig. 3b with the strong peaks at 1653 and 1629 cm−1 attributing to the stretching vibration of carbonyl unit of P(MBA-co-MAA) and the bending vibration of the N–H bond of the PMBA component, respectively, while the spectrum in Fig. 3a had only a weak peak at 709 cm−1, attributed to the characteristic adsorption of hematite core.
| Entryb | Particle size (major/minor axis)/nm | Polymer shell thickness (major/minor axis)/nm | Mass ratio of polymer (wt%)a | Titania shell thickness (major/minor axis)/nm | Coercivity (Hc) [Oe] | Saturation magnetization (Ms)/emu g−1 | Remanent magnetization (Mr)/emu g−1 |
|---|---|---|---|---|---|---|---|
|
a The mass ratio of the P(MBA-co-MAA) component in the ellipsoids was determined by thermogravimetric (TGA) analysis as mass loss during the process, heating up to 500 °C.
b
A. Hematite particles.
B. Fe2O3/P(MBA-co-MAA) core-shell ellipsoid particles. C–E. Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids from different volumes of ammonia: (B) 0.20 ml; (C) 0.25 ml; (D) 0.30 ml. F–H. Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids from different TBOT loadings: (A) 0.15 mL; (B) 0.25 mL; (C) 0.35 mL. |
|||||||
| A | 531/113 | 0 | 583.7 | 0.3269 | 0.1243 | ||
| B | 573/153 | 21/20 | 30.0 | 470.2 | 0.2132 | 0.09414 | |
| C | 580/156 | 28.9 | 3/2 | ||||
| D | 622/273 | — | 25/60 | ||||
| E | 619/280 | 23.4 | 24/63 | ||||
| F | 613/235 | — | 20/41 | 744.9 | 0.0864 | 0.05311 | |
| G | 622/273 | — | 25/60 | 419.1 | 0.06903 | 0.047 | |
| H | 660/325 | 19.6 | 43/86 | 371.3 | 0.01546 | 0.01219 | |
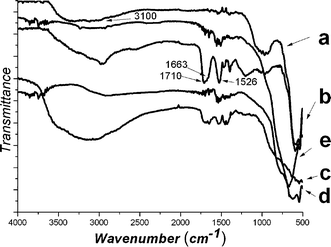 | ||
| Fig. 3 FT-IR spectra: (a) hematite; (b) Fe2O3/P(MBA-co-MAA) core–shell particles; (c) Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids; (d) hollow ellipsoidal hematite-sphere-in-titania-sphere; (e) hollow titania ellipsoidal spheres. | ||
Preparation of α-Fe2O3/P(MBA-co-MAA)/TiO2 ellipsoidal tri-layer spheres
Stable core–shell particles containing polystyrene (PSt) core and uniform titania shells were prepared by the hydrolysis of TBOT with the aid of the catalysis of ammonium cations in acetonitrile–ethanol mixed solvent.19,30 It was supposed that ammonium cations (NH4+) played an essential role during the formation of titania coatings over negative PSt template,19,30 which overcame the repulsive barrier between (C4H9O)3TiO− species and polymer seeds causing the negatively titania species to aggregate together. Fig. 4A–C showed the typical TEM images of α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer particles afforded by the hydrolysis of TBOT in acetonitrile–ethanol (1/3, v/v) mixed solvent with different amounts of ammonium catalyst. The results demonstrated that the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 particles had an ellipsoidal sandwich structure with a smooth surface in the absence of any secondary-initiated particles. In the present work, the NH4+ cations can be held by the counter-charged surface of α-Fe2O3/P(MBA-co-MAA) composites with the carboxylic acid groups on the surface. Consequently, with the accumulation of NH4+ species on the surface of the α-Fe2O3/P(MBA-co-MAA) ellipsoidal seeds, the negatively charged![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) TiO− species were adsorbed by the NH4+ species onto the surface of α-Fe2O3/P(MBA-co-MAA) templates, and the catalytic condensation process by the free NH4+ species could occur facilely on the surface of α-Fe2O3/P(MBA-co-MAA) ellipsoidal seeds. In such a way, α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids with uniform coatings of titania shell layer were formed via the mechanism as described in Scheme 2.
TiO− species were adsorbed by the NH4+ species onto the surface of α-Fe2O3/P(MBA-co-MAA) templates, and the catalytic condensation process by the free NH4+ species could occur facilely on the surface of α-Fe2O3/P(MBA-co-MAA) ellipsoidal seeds. In such a way, α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids with uniform coatings of titania shell layer were formed via the mechanism as described in Scheme 2.
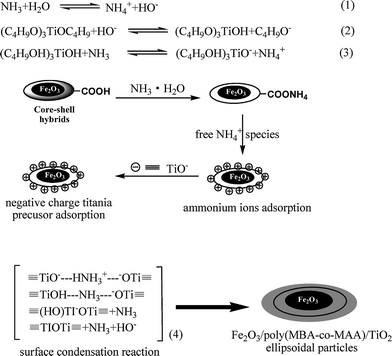 | ||
| Scheme 2 The formation mechanism of the titania coating. | ||
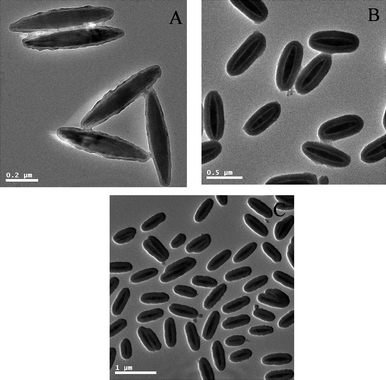 | ||
| Fig. 4 TEM micrographs of Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids formed with the addition of different amounts of ammonia: (A) 0.20 ml; (B) 0.25 ml; (C) 0.30 ml. | ||
A series of experiments were conducted to investigate the effect of ammonium feed on the morphology of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids via the hydrolysis of TBOT in presence of α-Fe2O3/P(MBA-co-MAA) templates. The loading of TBOT, volume of mixed solvent (acetonitrile–ethanol = 1/3, v/v), and amount of α-Fe2O3/P(MBA-co-MAA) seeds were of 0.25 mL, 50 mL and 0.010 g, respectively. The titania coatings (Fig. 4A) on the α-Fe2O3/P(MBA-co-MAA) templates were thin (less than 5 nm) with rough surface for 0.20 mL of ammonium as catalyst. The smooth and homogeneous titania coatings (Fig. 4B) were achieved when the volume of ammonium was increased to 0.25 mL as catalyst. However, when the ammonium catalyst was increased further to 0.30 mL, necked α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids were formed (Fig. 4C), in which hydrolysis reaction of TBOT was too fast to separate the titania species. All these results indicated that the amount of ammonium catalyst had considerable effects on the morphology of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids. The amount of ammonium catalyst was kept at 0.25 mL for the synthesis of α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids in the following work.
An important concern for the present work was to synthesize α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids with well-defined shape and adjustable thickness. A series of experiments were conducted to investigate the effect of the TBOT feed on the morphology of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids via the controlled hydrolysis of TBOT in acetonitrile–ethanol (1/3, v/v). The TEM micrographs of the resultant of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids with different thicknesses of titania shell layer were shown in Fig. 5. The results demonstrated that the final α-Fe2O3/P(MBA-co-MAA)/TiO2 hybrid particles had ellipsoidal shapes with smooth surface in absence of any second-initiated particles with TBOT feed ranging from 0.15 to 0.35 mL, which meant that the electrostatic interaction between the adsorbed NH4+ on α-Fe2O3/P(MBA-co-MAA) templates and the negative titania species efficiently captured the newly formed titania species during the encapsulation of the α-Fe2O3/P(MBA-co-MAA) templates by controlled hydrolysis of TBOT. The TEM micrographs in Fig. 5A–C proved that the structures of the α-Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids were tri-layer, which had a lightest contrast of P(MBA-co-MAA)polymer mid-layer sandwiched between deepest contrast of hematite core and the darker titania outer shell layer.
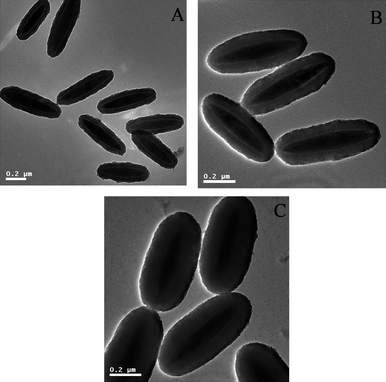 | ||
| Fig. 5 TEM micrographs of Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids from different TBOT loadings: (A) 0.15 mL; (B) 0.25 mL; (C) 0.35 mL. | ||
The experimental conditions for the controlled hydrolysis of TBOT with various TBOT loadings and sizes of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer hybrid ellipsoids are summarized in Table 1. The titania shell thickness was measured by TEM observation, which was calculated as one-half of the difference between the diameters of the major/minor axis directions of the final tri-layer hybrids and those of the corresponding α-Fe2O3/P(MBA-co-MAA) templates. The major/minor axes of the tri-layer ellipsoids were increased from 613/235 nm to 630/325 nm with increasing TBOT feed from 0.15 to 0.35 mL for the controlled hydrolysis. These results demonstrated that the thickness of the titania shell layer was in the range of 20/41 and 28/86 nm in the major/minor axis directions with the TBOT feed enhancing from 0.15 to 0.35 mL for the controlled hydrolysis. This indicated that the growth speed of titania shell layer in the major axis was significantly lower than that in the minor axis for the encapsulation of titania layer onto α-Fe2O3/P(MBA-co-MAA) templates. The TGA analysis of the resultant α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer ellipsoids revealed that the sandwiched P(MBA-co-MAA) mid-layer was significantly decreased from 28.9 wt% with TiO2 thickness of 3 nm (Entry C) to 19.6 wt% with TiO2 thickness of 43 nm (Entry H) as summarized in Table 1. The decrease of mass ratio for the sandwiched P(MBA-co-MAA) mid-layer originated from the gradual increase the thickness of the titania shell layer under different conditions. The coating of titania shell layer was confirmed by the FT-IR spectrum in Fig. 3c with the presence of a strong wide peak ranging from 3000 to 3500 cm−1 due to the stretching vibration of the hydroxyl groups of titania shell layer with simultaneous decrease of the peaks at 1710, 1663 and 1526 cm−1 assigned to the adsorption of the P(MBA-co-MAA) components.
The magenetization property of the Fe2O3/P(MBA-co-MAA)/titania tri-layer hybrids
Bulk α-Fe2O3 is an antiferromagnetic material below the Morin temperature (TM) of 263 K and behaves with weak ferromagnetic property between 263 K and the Neel temperature (TN) of 955 K. The magnetic properties of hematite, Fe2O3/P(MBA-co-MAA) core–shell particles and hematite/P(MBA-co-MAA)/titania hybrid materials were studied by using VSM at room temperature. Fig. 6 shows the magnetization curves of hematite, hematite/P(MBA-co-MAA) core–shell particles and hematite/P(MBA-co-MAA)/titania hybrid materials with various titania shell thicknesses ranging from 41 to 86 nm (minor axis). Obvious magnetic hysterisis loops were observed for the hematite/P(MBA-co-MAA)/titania hybrid from the field-dependent magnetization plots in Fig. 6. In other words, the remanence existed when the magnetic field was removed, indicating that all the resultant hematite/P(MBA-co-MAA)/titania hybrid materials showed hysteresis features and retained weak ferromagnetic properties originating from α-Fe2O3 ellipsoidal cores at room temperature. The magnetic properties of the hematite/P(MBA-co-MAA)/titania trilayer hybrids with different titania shell thicknesses are summarized in Table 1. The coercivity (Hc), saturation magnetization (Ms), and remanent magnetization (Mr) values for hematite ellipsoids were 583.7 Oe, 0.3269 emu g−1, and 0.1243 emu g−1, respectively. In addition, The coercivity (Hc), saturation magnetization (Ms), and remanent magnetization (Mr) values for hematite/poly(MBA-co-MAA) ellipsoids were 470.2 Oe, 0.2132 emu g−1, and 0.09414 emu g−1, respectively. The saturation magnetization of hematite/poly(MBA-co-MAA)/titania trilayer hybrids was slightly decreased from 0.0864 to 0.01546 emu/g with an increase in the titania shell thickness from 41 to 86 nm (minor axis), while the remanent magnetization was simultaneously decreased from 0.05311 to 0.01219 emu g−1. These results indicate that the magnetization of the hematite/P(MBA-co-MAA)/titania tri-layer hybrid materials decreased with the increase in the titania component due to the decrease in the effective mass of the hematite core.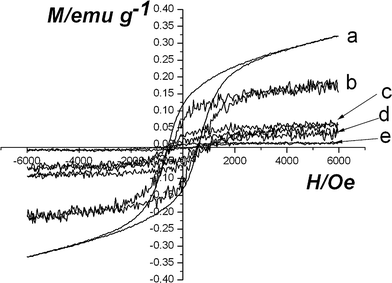 | ||
| Fig. 6 Hysteresis loops for samples at room temperature: (a) hematite; (b) Fe2O3/P(MBA-co-MAA) particles; (c–e) Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids with different titania shell-thicknesses (minor axis): (c) 41 nm; (d) 60 nm; (e) 86 nm. | ||
Hollow ellipsoids
In our previous work, hollow polymer ellipsoids29 and hollow polymer ellipsoids with movable hematite cores28 and hollow polymer microspheres with movable gold (Au) cores31 were prepared by removal of the silica layer from the corresponding multi-layer inorganic/polymer hybrid materials.In the present work, ellipsoidal hematite-sphere-in-titania-spheres were afforded by calcination of the α-Fe2O3/P(MBA-co-MAA)/TiO2 tri-layer hybrid ellipsoids at 500 °C for 2 h, which was shown in Fig. 7A. The results indicated that an ellipsoidal hematite sphere (dark color) was placed in the center of hollow titanium shells, in which the convincing hollow-sphere structures were observed with the presence of circular rings of non-segmented ellipsoidal structure and a cavity in the middle shown by the inserted TEM image in Fig. 7A. These ellipsoidal hematite-sphere-in-titania-spheres may be interesting for the application as micrometre-scale devices due to the multiple reflections of UV light within the sphere interior voids. It is known that sub-micron sized hematite possesses remnant magnetization at zero magnetic field strength. Carrying anisotropic sub-micron hematite cores, titania ellipsoidal capsules would have advantages in photocatalysis. In addition, the XRD pattern in Fig. 2b demonstrated that the titania shell layer was transformed to anstase phase with presence of the diffraction peaks at 2θ values of 25.3, 37.9, 48.15, 54.05 and 55.28°, respectively, which were assigned to the (101), (004), (200), (105), and (211) reflection planes of the body-centered tetragonal phase of TiO2.
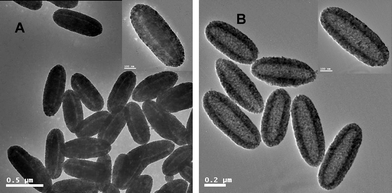 | ||
| Fig. 7 TEM micrographs of the three hollow capsules: (A) hollow ellipsoidal hematite-sphere-in-titania-sphere; (B) hollow titania capsules. | ||
Hollow titantia ellipsoids were obtained by soaking the ellipsoidal hematite-sphere-in titania-spheres in HF to remove the hematite cores as shown in Fig. 7B, in which the convincing hollow-sphere structures were observed with the presence of circular rings of non-segmented ellipsoidal titaniananocrystals and a cavity inside. Further, the XRD pattern in Fig. 2c, in which the typical peaks of (012), (110), (113), (024) reflection planes of the hematite components completely disappeared, proved further the selective removal of hematite cores from ellipsoidal hematite-sphere-in-titania-spheres via soaking in HF.
The successful removal of the P(MBA-co-MAA) sandwiched mid-layer viacalcination was proven by the FT-IR spectra in Fig. 3d–e, in which the peaks at 1710, 1623 and 1526 cm−1 attributed to the characteristic adsorption of the P(MBA-co-MAA) components disappeared. Furthermore, the strong wide peaks ranging from 3000 to 3500 cm−1 in the FT-IR spectra of hollow titania ellipsoids disappeared due to the formation of anstase phase for titania shell layer during the calcination.
Conclusion
Ellipsoidal tri-layer Fe2O3/P(MBA-co-MAA)/TiO2 hybrid materials were prepared by the controlled hydrolysis of TBOT with ammonium as the catalyst in acetonitrile–ethanol (1/3, v/v) mixed solvent in the presence of Fe2O3/P(MBA-co-MAA) core–shell particles as templates, which were prepared by distillation precipitation copolymerization of MBA and MAA with ellipsoidal hematite particles as seeds. The thickness of titania shell layer of the resultant Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids were facilely controlled in the rang of 41–86 nm (minor axis) and 20–28 nm (major axis) by altering the TBOT feed from 0.15 to 0.35 mL during the hydrolysis. The magnetization of the Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids decreased with an increase of the titania component due to a decrease of the effective mass of the magnetic hematite cores. P(MBA-co-MAA)/TiO2 bi-layer hollow ellipsoids were developed with the selective removal of hematite core in aqueous HF solution. Ellipsoidal hematite-sphere-in-titania-sphere were obtained were afforded by calcination of Fe2O3/P(MBA-co-MAA)/TiO2 hybrid ellipsoids at 500 °C in a furniture. Hollow titania ellipsoids were developed further via soaking the ellipsoidal hematite-sphere-in titania-spheres in HF to remove the hematite cores. The application of the ellipsoidal core-shell hybrids and hollow titania ellipsoids with various functionalities is now being studied systematically in our group.Acknowledgements
This work was supported by the National Natural Science Foundation of China with project No.: 20874049.References
- J. Li, X. Hong, Y. Liu, D. Li, Y. Wang, J. Li and Y. Bai, Adv. Mater., 2005, 17, 163 CrossRef CAS.
- F. Hoffmann, M. Connelius, J. Morell and M. Frobe, Angew. Chem., Int. Ed., 2006, 45, 3216 CrossRef.
- F. Caruso, M. Spasova, A. Sucha, M. Giersig and R. A. Caruso, Chem. Mater., 2001, 13, 109 CrossRef CAS.
- M. S. Fleming, T. K. Mandal and D. R. Walt, Chem. Mater., 2001, 13, 2210 CrossRef CAS.
- L. Qi, J. P. Chapel, J. C. Casting, J. Fresnais and J. F. Berret, Langmuir, 2007, 23, 11996 CrossRef CAS.
- F. Fleischhaker and R. Zentel, Chem. Mater., 2005, 17, 1346 CrossRef CAS.
- T. Cui, J. Zhang, J. Wang, F. Liu, W. Chen, F. Xu, Z. Wang, K. Zhang and B. Yang, Adv. Funct. Mater., 2005, 15, 481 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Y. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- X. Jiang, T. Herricks and Y. N. Xia, Adv. Mater., 2003, 15, 1205 CrossRef CAS.
- M. Pal, J. G. Serrano, P. Santiago and U. Pal, J. Phys. Chem. C, 2007, 111, 96 CrossRef CAS.
- H. K. Yu, G. R. Yi, J. H. Kang, Y. S. Cho, V. N. Manoharan, D. J. Pine and S. M. Yang, Chem. Mater., 2008, 20, 2704 CrossRef CAS.
- H. X. Li, Z. Bian, J. Zhu, G. Li, Y. Huo, H. Li and Y. F. Lu, J. Am. Chem. Soc., 2007, 129, 8406 CrossRef.
- A. Syoufian, O. H. Satria and K. Nakashima, Catal. Commun., 2007, 8, 755 CrossRef CAS.
- J. G. Yu, S. W. Liu and H. G. Yu, J. Catal., 2007, 249, 59 CrossRef.
- Z. Z. Yang, Z. W. Liu, Y. F. Lu, Z. B. Hu and C. C. Han, Angew. Chem., Int. Ed., 2003, 42, 1943 CrossRef CAS.
- A. Syoufian, Y. Inoue, M. Yada and K. Nakashima, Mater. Lett., 2007, 61, 1572 CrossRef CAS.
- Z. Y. Zhong, Y. D. Yin, B. Gates and Y. N. Xia, Adv. Mater., 2000, 12, 206 CrossRef CAS.
- P. Wang, D. Chen and F. Q. Tang, Langmuir, 2006, 22, 4832 CrossRef CAS.
- L. Y. Hao, C. L. Zhu, W. Q. Jiang, C. N. Chen, Y. Hu and Z. Y. Chen, J. Mater. Chem., 2004, 14, 2929 RSC.
- L. Gabrielson and M. J. Folkes, J. Mater. Sci., 2001, 36, 1 CrossRef CAS.
- J. Chen, L. N. Xu, W. Y. Li and X. L. Gou, Adv. Mater., 2005, 17, 582 CrossRef CAS.
- A. M. A. Gondal, A. Hameed, Z. H. Yamani and A. Suwayan, Chem. Phys. Lett., 2004, 385, 111 CrossRef.
- D. V. Dimitrov, G. C. Hadjipanyis, V. Papafthymiou and A. Simpoulos, J. Magn. Magn. Mater., 1998, 188, L8 CrossRef.
- L. Levy, Y. Sahoo, K. S. Kim, E. J. Bergey and P. N. Prasad, Chem. Mater., 2002, 14, 3715 CrossRef CAS.
- S. Sacanna, L. Rossi and A. P. Phillipse, Langmuir, 2007, 23, 9974 CrossRef CAS.
- A. Xia, J. H. Hu, C. C. Wang and D. L. Jiang, Small, 2007, 3, 811.
- K. Furusawa, H. Matsumura and T. Majima, J. Colloid Interface Sci., 2003, 264, 95 CrossRef CAS.
- G. Y. Liu, X. L. Yang and Y. M. Wang, Langmuir, 2008, 24, 5485 CrossRef CAS.
- G. Y. Liu, L. Y. Li and X. L. Yang, Polymer, 2008, 49, 4776 CrossRef CAS.
- X. Du and J. H. He, Mater. Res. Bull., 2009, 44, 1238 CrossRef CAS.
- G. Y. Liu, H. F. Ji, X. L. Yang and Y. M. Wang, Langmuir, 2008, 24, 1019 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2010 |
