Photoelectrocatalytic degradation of methyl orange over mesoporous film electrodes
Received 20th August 2009, Accepted 24th November 2009
First published on 11th December 2009
Abstract
Mesoporous TiO2 films and ion-doped photocatalytic films, displaying a worm-like pattern, have been synthesized by dip-coating of ITO glass into an organic–inorganic sol followed by aging and calcination of the coating at different temperature. The prepared films were investigated by X-ray diffraction (XRD), UV-Vis reflectance spectra, scanning electron microscope (SEM), transmission electron microscopy (TEM) and photoelectrochemical measurement, and were confirmed to be of mesoporous characteristic. Degradation of methyl orange (MO) has been performed using the new film electrodes under UV light and artificial solar light illumination. The influence of variables, such as applied bias, pH, supporting electrolyte, MO concentration and the load of the films, on the degradation of the dye was investigated. More than 97% degradation of MO was achieved under the feasible experimental conditions in 2 h photoelectrocatalytic reaction with UV light illumination and mesoporous film TiO2/ITO as electrode. The activity of the mesoporous film V-TiO2 was the highest of the newly synthesized films V-TiO2, Ce-TiO2, F-TiO2 and pure TiO2 under artificial solar light. The degradation ratio of MO was about 43% over 2 h reaction using V-TiO2/ITO as the electrode. The activity of the mesoporous film under artificial solar light needs to be increased further.
1. Introduction
Textile dyeing is crucial for the commercial success of textile products, particularly in such industries as garments, furnishings and upholstery. Around 700![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t of about 10
000 t of about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different types of dyes and pigments are produced each year for the dye industry.1,2 The dyeing processes, including subsequent washing procedures, require a great amount of water. Unfortunately, a significant fraction of residual textile dyes and several other types of chemicals are discharged with effluents from dyeing mills into natural and domestic water systems.3,4,5 As a result, important freshwater sources of modern people can become highly contaminated. Most of the dyes are difficult to biodegrade under an aerobic environment because of their synthetic origin and mainly complex aromatic molecular structures. As a matter of fact, elimination of the harmful dye pollutants has drawn the attention of both scientists and government. Laws and government regulations regarding the removal of dyes from wastewater treatment plants become more and more stringent.
000 different types of dyes and pigments are produced each year for the dye industry.1,2 The dyeing processes, including subsequent washing procedures, require a great amount of water. Unfortunately, a significant fraction of residual textile dyes and several other types of chemicals are discharged with effluents from dyeing mills into natural and domestic water systems.3,4,5 As a result, important freshwater sources of modern people can become highly contaminated. Most of the dyes are difficult to biodegrade under an aerobic environment because of their synthetic origin and mainly complex aromatic molecular structures. As a matter of fact, elimination of the harmful dye pollutants has drawn the attention of both scientists and government. Laws and government regulations regarding the removal of dyes from wastewater treatment plants become more and more stringent.Conventional sequential anaerobic/aerobic digestion has been used to treat textile wastewater, but proved to be unsatisfactory due to the fact that many of the dyes are xenobiotic and non-biodegradable.1,6 Also, textile dyes can not be readily removed by many other chemical and physical methods, such as flocculation, precipitation, reverse osmosis, adsorption and chlorination. In fact, these treatment processes usually promote only phase transfer.
The technology of semiconductor-based photocatalysis has been used for degradation of organic and inorganic contaminants in wastewater.7,8 In recent years, the technology of photocatalysis has progressed rapidly. Many photocatalytic materials have been developed, including TiO2, WO3, Fe2O3, SnO2, ZnO, CdS and so on. The attractiveness of the heterogeneous photocatalytic oxidation processes lies in the fact that it bears the ability to completely mineralize organic dye contaminants, and the resulting water can be recycled or re-used. Out of the semiconductor materials, TiO2 has received considerable attention for its non-toxic, efficiently photocatalytic, chemically stable and relatively inexpensive merits under most environmental conditions. Despite the preferable qualities, the wide band gap and low activity delay its wide use. Activation of pure semiconductor TiO2 requires ultraviolet light irradiation, which means that only a small fraction of the sun's energy can be used. It is necessary to shift the optical response of TiO2 from the UV to the visible spectral range to gain a favorable effect on the photocatalytic efficiency of the material. Many efforts have been devoted to lowering the band-gap energy and improving photocatalytic activity of TiO2, such as the doping of cations of transition metals9 and anions.10
Ion-doped TiO2 has been studied extensively,11,13 and visible-light response of the material has been improved. However, the doped semiconductor particles suffer from separation and reusing. Owing to the fast recombination of the photo-generated electron–hole pair, the light-quantum efficiency of the doped TiO2 must be increased greatly. The low quantum efficiency of light of the materials constitutes their major drawback, and hinders their application. Introduction of a positive voltage bias to TiO2 film electrodes could give significant results in driving away the photogenerated electrons. The biasing potential across the photoanode coated with photoactive material can prevent electron–hole recombination and result in an extension in the lifetime of the holes. The process is referred to photoelectrocatalysis (PEC). PEC oxidation technology was confirmed to be more efficient than photocatalytic (PC) oxidation technology for degradation of organic pollutants.1,4,8,12,14–16 For the PEC/PC heterogeneous process, organic pollutants are firstly adsorbed on the surface of the semiconductor, and then degraded. It is an advantage to have a large surface area in the process.
Mesoporous materials have attracted a great deal of attention since the discovery of the M41.17 Mesoporous silica has been studied extensively up to now. In recent years, mesoporous transition-metal oxides have also been explored owing to the potential applications in separation, catalysis and energy conversion.18–20 Titania-based mesoporous materials are one of the attractive transition-metal oxides. Several groups have successfully synthesized mesoporous titania with high surface area.19–22 Mesoporous thin films were likewise found to be able to sensitize a host matrix for luminescent rare-earth ions.23 In spite of the advantages of the mesoporous materials, few studies have been conducted on the application of mesoporous semiconductors for the remediation of textile dyes. It is necessary to explore PEC/PC processes based on mesoporous semiconductor materials, especially on TiO2-based mesoporous materials for the treatment of dye-containing wastewater.
Azo compounds, characterized by the presence of an azo group bound to aromatic rings, represent an important class of textile dyes, and are commonly used in the dye industry. In this paper, pure titania and ion-doped titania mesoporous film electrodes were prepared using the sol–gel method. The resulting film electrodes were investigated through a series of characterizations. The efficiency and the feasibility of photoeletrocatalysis and photocatalysis of the prepared films were tested through degrading methyl orange (MO), an azo dye, under UV light and artificial solar light respectively for different experimental conditions.
2. Experimental
2.1 Preparation
Tetrabutyl titanate was used as a precursor for preparing TiO2 colloidal suspensions. Cetyltrimethyl-ammonium bromide (CTAB) was used as the template. Acetylacetone (Acac) and concentrated hydrochloric acid (HCl) were used as the inhibitors of the condensation reaction of tetrabutyl titanate. The sources of the doping ions (cerium, vanadium, fluoride) were Ce(NO3)3·6H2O, NH4VO3 and NH4F, respectively. Chemicals mentioned above and methyl orange (MO) were purchased from Shanghai Chemical Reagent Co., China. All reagents used were of analytical reagent grade.Typical steps for the synthesis of a mesoporous titania film are as follows. A solution X was formed by dropwise addition of tetrabutyl titanate and Acac to an ethanolic solution of the surfactant with stirring at room temperature for 1 h. A solution Y was formed by mixing absolute ethanol, HCl, and deionized water. The ratio of absolute ethanol in the solution X and Y was 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. TiO2 colloidal suspension was prepared by dropwise addition of the solution Y to X with vigorous stirring. After addition of Y to X, the mixture was stirred for 1 h at room temperature. The molar composition of the matrix gel is: n[Ti] : n[CTAB] : n[EtOH] : n[H2O] : n[Acac] = 1 : 0.17 : 20 : 5 : 0.3. The ion-doping TiO2 colloidal suspensions were prepared with the same procedure as the pure TiO2 colloidal suspension, except that Ce(NO3)3·6H2O, NH4VO3 or NH4F was added in the solution X with a molar radio of n[Ti] : n[doped ion] = 60, 60, and 30, respectively.
1. TiO2 colloidal suspension was prepared by dropwise addition of the solution Y to X with vigorous stirring. After addition of Y to X, the mixture was stirred for 1 h at room temperature. The molar composition of the matrix gel is: n[Ti] : n[CTAB] : n[EtOH] : n[H2O] : n[Acac] = 1 : 0.17 : 20 : 5 : 0.3. The ion-doping TiO2 colloidal suspensions were prepared with the same procedure as the pure TiO2 colloidal suspension, except that Ce(NO3)3·6H2O, NH4VO3 or NH4F was added in the solution X with a molar radio of n[Ti] : n[doped ion] = 60, 60, and 30, respectively.
ITO glass (3 cm × 3 cm) was cleaned with absolute ethanol and then rinsed with distilled water. The matrix gel was dip-coated on the clear ITO substrate, the thin film was left to age for 1 h at room temperature under a relative humidity of about 70%, and then aged for 72 h at 70 °C. Finally, the ion-doped TiO2 films were calcined at 450 °C and the pure TiO2 film was calcined at 350 °C. Films of different thicknesses, corresponding to different loads of the mesoporous films, were achieved by repeating the above operation. The pure TiO2 mesoporous film electrode and the ion-doped mesoporous film electrodes are expressed as TiO2/ITO, V-TiO2/ITO, Ce-TiO2/ITO and F-TiO2/ITO, respectively.
2.2 Characterization
The mesostructures of the as-synthesized thin films were confirmed by small-angle X-ray diffraction measurements (Rigaku, D/max-2500, Cu-Kα radiation, λ = 1.5406 Å). The diffuse reflectance UV-vis spectra (DRS) were recorded on a Varian Cary 100 spectrometer with an integration sphere in the region 800–200 nm at split width of 1.5 nm and a scan speed of 400 nm min−1, in which a baseline was corrected by using a calibrated sample of barium sulfate. TEM images were obtained by JEM-2100 transmission electron microscope. The powders, which were obtained by removing the thin films from the ITO substrate using a razor blade, were ground and dispersed in ethanol, and the resultant suspensions were dropped on a copper grid and allowed to dry. After that, TEM measurement was conducted. SEM measurements were taken on a scanister (BRUKERS-3400N). BET surface areas and pore diameter distribution of the samples were measured according to the N2 adsorption isotherms measured by a Micrometrics ASAP 2010. Energy dispersive spectroscopy (EDS) was also obtained through a SEM (BRUKERS-3400N) equipped with a link analyzer (Oxford, ISIS-300) to determine the surface composition of the films.Linear sweep voltammetry measurements were carried out by a CHI660C electrochemical workstation system (Shanghai Chenhua Instrument Co., China). The electrochemical system was composed of a Ag/AgCl electrode as the reference electrode, a platinum wire as the auxiliary electrode and the newly synthesized mesoporous thin film electrode as the working electrode.
2.3 Activity tests
The photoelectrocatalytic degradation of MO was performed in a 350 ml reactor with three electrodes as described above. Air was continually bubbled into the compartment of the reactor. The photoactive area of the synthesized mesoporous film electrode is 7 cm2. 350 W high-pressure mercury lamp and 350 W Xe lamp were used as the UV light and artificial solar light sources respectively. The working electrode was illuminated by the light from the light source through a quartz window of one of the sides of the reactor cell. The quartz window was 16 cm apart from the light source. The bias voltage applied to the thin film electrode was supplied by the electrochemical workstation.The concentration of methyl orange in the solution was measured by UV-vis spectrophotometer (756MC, Shanghai Exact Instrument Co., China) at 464 nm after agitation for 30 min. The degradation ratio of MO was defined as:
| | | Degradation ratio = (C0−Ct)/C0× 100% | (1) |
where
C0 is the initial concentration of MO in the solution;
Ct denotes the concentration of MO at the time
t starting from the moment the
electrodes were put into the solution while stirring.
3. Results and discussion
3.1 Structure of the thin films
3.1.1 X-Ray diffraction patterns and DRS spectra. The low-angle powder XRD patterns of the mesoporous materials Co-MTiO224,25 and Co-TiO2-SiO211 showed broad diffractions positioned at around 0.65° and 0.5° in 2θ respectively, which matches the character of the mesoporous structure. The low-angle powder XRD patterns of the newly synthesized thin film, pure TiO2 and ion-doped TiO2 exhibit well-resolved patterns with the diffraction peaks at 2θ of about 0.4°–0.6°, as shown in Fig. 1, which indicates the presence of a mesoporous structure of titania.11 But this also suggests the lack of a long-range ordered structure.26 With the incorporation of the doping ions, the intensity of the refraction peaks becomes greater compared to the pure mesoporous TiO2, although the latter was calcined at a lower temperature. This indicates that incorporation of the doping ions favors the formation of the mesoporous structure.The wide-angle XRD pattern of the prepared films is also shown in the Fig. 1 (inset), which can supply useful information about the crystallinity of the prepared materials. Obviously, there are visible lines corresponding to anatase titania for pure TiO2, V-TiO2, F-TiO2 and Ce-TiO2. This indicates that titania in the prepared samples coexists as anatase and amorphous tetrahedrally coordinated Ti oxide species.27,28 Moreover, no diffraction peaks corresponding to the doped metal oxides are observed. This implies that the doped elements are well dispersed in the titania framework.
The diffuse reflectance (DR) UV-vis spectra of the films are displayed in Fig. 2. Significant absorption in the visible light range was observed for the doped films. These results confirm that the doping ions (vanadium, fluoride and cerium) improved the visible-light absorption property of titanium dioxide. Similar results can be found elsewhere.19,29,30 The shift of the optical absorption edge to visible light for the V-TiO2 film may be ascribed to the lowering of the energy level of the conduction band owing to the doping of vanadium ion to TiO2. The band gap of the titanium dioxide could be narrowed down through the doping of fluoride ions and cerium ions, and thus resulted in the enhanced visible light absorption.
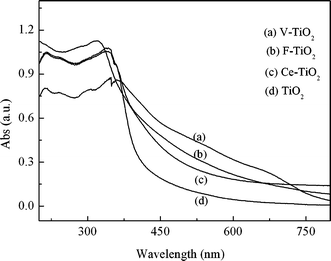 |
| | Fig. 2 DRS spectra of the films TiO2/ITO, V-TiO2/ITO, Ce-TiO2/ITO, and F-TiO2/ITO. | |
3.1.2 TEM and SEM images. It has been reported that the platinised TiO2 film, directly dip-coated on ITO glass, is not stable enough and easily broke and peeled off. This constitutes an obstacle in the photoelectrocatalytic research of the platinised TiO2.31 The SEM film of the newly synthesized TiO2/ITO is uniform to the nanometre scale. No crack appears on the film except a white spot as shown in Fig. 3, which is assigned to an agglomerate of the semiconductor particles. TEM image of the prepared mesoporous film is shown on Fig. 3. The TEM pattern of the TiO2 sample reveals a less ordered, wormhole mesopore structure. The similar pattern of mesoporous TiO2 particles was reported by Sanchez and co-workers.18 As shown in Fig. 3, a network of channels is regular in diameter, although long-range packing order is absent.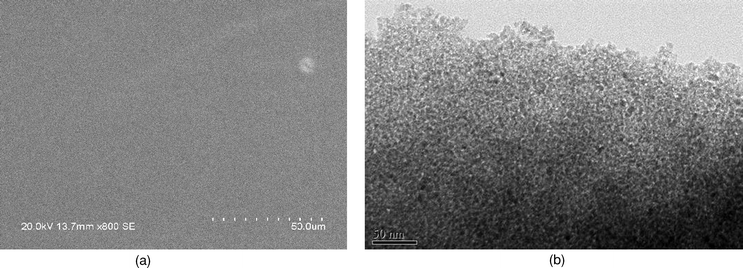 |
| | Fig. 3 SEM (a) and TEM (b) images of the mesoporous TiO2 film. | |
3.1.3 Adsorption/desorption isotherms and EDS analysis. The N2 adsorption/desorption isotherms and corresponding BJH pore size distributions of the prepared samples are shown in Fig. 4a and b, and the structural parameters are summarized in Table 1. The samples show the type-IV isotherms, according to the IUPAC convention, indicating the films' mesoporous character. The steep changes in volume adsorbed for P/P0 = 0.45–0.8 indicate that the samples possess framework-confined mesopores.32 H1 hysteresis loops also appear in the isotherms, that is to say the samples are of mesoporous structure.33 Compared with the pure TiO2 calcined at a lower temperature, the BET specific areas of the samples increases with the incorporation of the doping ions. Obviously, the incorporation of ions into the titania framework improves the surface, which is in agreement with the report where mesoporous Co–TiO2–SiO2 powders were synthesized with cetyltrimethyl-ammonium bromide as the structure director.11 The BET surface area of the sample TiO2 calcined at 450 °C is only 61 m2 g−1, much lower than the area 139 m2 g−1 of the sample TiO2 calcined at 350 °C, indicating a collapse of the mesoporous structure. For this reason, the mesoporous TiO2 film used in the following experiment is the sample calcined at 350 °C, while the ion-doped films used are all calcined at 450 °C. The higher BET surface areas of the doped mesoporous films suggest that the samples keep the mesoporous structure at 450 °C, this result also confirms that the ion-doped films are stable than the pure TiO2 film.
Table 1 Structural parameters and doping-ion content in the synthesized samples
| Sample | Calcination T/°C | Calcination time/h | BET surface area/m2 g−1 | Vp/cm3 g−1 | Pore diameter/nm | ndoped ion/nTi (%) |
|---|
| TiO2 | 450 | 3 | 61 | 0.05 | 2.8 | 0 |
| TiO2 | 350 | 4 | 139 | 0.24 | 5.4 | 0 |
| TiO2-V | 450 | 4 | 189 | 0.15 | 3.5 | 1.60 |
| TiO2-Ce | 450 | 4 | 166 | 0.25 | 4.6 | 1.62 |
| TiO2-F | 450 | 4 | 148 | 0.26 | 5.7 | 1.12 |
The surfaces of the films were analyzed by EDS. The results presented in Table 1 indicated that the content of the doped ions were 1.60%, 1.62% and 1.12% (ndoped ion/nTi) for the films V-TiO2/ITO, Ce-TiO2/ITO and F-TiO2/ITO respectively. There was no obvious change in ion content of cerium and vanadium between the original sols and the resultant films, showing the ion loss may be neglected for the two elements. A marked loss of fluoride ion was found in the preparation process of the film F-TiO2/ITO.
3.1.4 Electrochemical behavior of the prepared electrodes. Anodic potential was the important factor in affecting the photocurrent, which was the force for photoelectrons to transport across film electrode, and photoelectrons was as current carrier.4 It is useful to have a voltammetry for selecting appropriate potential in degradation of organics. The linear sweep voltammograms of the mesoporous film electrodes are exhibited in Fig. 5 with and without UV illumination. The plots clearly show the production of photocurrent under UV illumination. Moreover, the current increases with the increase of applied potential, especially when the anodic potential is higher than 0.6 V with UV light on. The result implies that the MO in the solution may be degraded effectively by photoelectrocatalysis at an anodic potential higher than 0.6 V. This is further confirmed by the following results of the MO degradation under photoelectrocatalysis. |
| | Fig. 5 Linear voltammograms of the mesoporous film electrodes at a scan rate of 10 mV s−1 in 0.1 M Na2SO4 solution containing 20 mg L−1 MO at pH 2.0 with and without UV illumination. (a) V-TiO2/ITO; (b) F-TiO2/ITO; (c) TiO2/ITO; (d) Ce-TiO2/ITO. | |
3.2 Photocatalytic and photoelectrocatalytic degradation of MO
3.2.1 Effect of differing loads of the films. Films with a different load of the mesoporous material were fabricated by repeating the above operation, and are denoted as 0-layer, 1-layer, 2-layer, 3-layer and 4-layer films, respectively, and corresponding to the film quantity of 0, 1.8, 2.5, 3.6, 4.5 mg mesoporous TiO2. The 0-layer film means the bare ITO.The activities of the as-made films were evaluated in terms of degradation ratio of MO in the photoelectric system. The results are illustrated in Fig. 6. The degradation ratio increased with the layer number of the mesoporous TiO2 up to three layers. Further increase in the layer number of the mesoporous films did not accelerate the degradation. In contrast, the degradation rate decreased slightly with 4 layers. This indicated that the photocatalytic reactions mainly occurred on the outer layers as reported in ref. 31. Moreover, the increase in the layer number may increase the transfer resistance of the photogenerated charges across the semiconductor; therefore, the separation of the photoelectrons from the photoholes becomes difficult, and the efficiency of the photoelectric catalysis is low. For this reason, the 3-layer films were selected for all the following photoelectrocatalytic experiments.
 |
| | Fig. 6 Influence of the layer number on the degradation of MO in 0.1 M Na2SO4 solution at applied potential 0.2 V with UV illumination, MO initial concentration of 20 mg L−1 and pH 2.0. | |
3.2.2 Effect of applied potential. The applied potential changed not only the recombination rate constant but also the charge-transfer rate constant.34 The appropriate potential applied to the semiconductor anode could effectively minimize recombination of the photogenerated charges. But for different reaction systems, especially with photoactive anodes of diverse structures or composition, there are different optimal applied potentials. The results of the photoelectrocatalytic degradation of MO at different applied potentials are shown in Fig. 7. The applied potential of 0 V displayed in the Fig. 6 means that the reaction took place only under UV illumination without applied potential. It is obvious that the applied potential can promote the reaction significantly for the potential between 0.2 and 1.0 V. Moreover, the degradation ratios were nearly equal in 150 min for the potentials of 0.2, 0.6 and 1.0 V, While the reaction result at 2.0 V was always inferior to the results under the other three applied potentials. The electric field formed over the film by the potential from 0.2 to 1.0 V was enough to keep the photogenerated charges apart. Therefore, the applied potential between 0.2 and 1.0 V is reasonable for the degradation of MO in the solution with UV illumination. |
| | Fig. 7 Influence of the applied potential on the degradation of MO in 0.1 M Na2SO4 solution at pH 2.0 with UV illumination, MO initial concentration of 20 mg L−1, TiO2/ITO electrode. | |
3.2.3 Effect of initial pH. The degradation results for MO under photoelectric catalysis at three different initial pH are shown in Fig. 8. It is obvious that the degradation ratio at pH 5.2 is much lower than that at the other two pH, while the best result was obtained at pH 2.0. These results may originate from the different transfer resistance of the photogenerated charges across the interface between the semiconductor and solution. The lower pH corresponds to the smaller transfer resistance.35 This is beneficial to the use of photoactive species on the mesoporous film electrode. Therefore, the degradation rate of the pollutant is higher. In addition, there are the following reactions for the one chamber photoelectrocatalytic reactor:| | | MO + HO˙/H2O2/O2−→ degradation products | (5) |
 |
| | Fig. 8 Influence of the pH on the degradation of MO in 0.1 M Na2SO4 solution at applied potential 0.2 V with UV illumination, MO initial concentration of 20 mg L−1, TiO2/ITO electrode. | |
The H2O2 produced at the cathode according to reaction (3)36 may transform to HO˙ through the above reaction (4). Both H2O2 and HO˙ are active in degradation of MO. The higher reaction efficiency at lower pH is mostly ascribed to the higher concentration of H+, which is advantageous to electrogeneration of H2O2 in the photoelectric catalysis system.
3.2.4 Effect of MO concentration. The relationship between the degradation ratios and the initial concentrations of the dye was investigated in the photoelectrocatalytic system. The results are illustrated in Fig. 9. It is clear that the degradation rate for the system with the initial concentration of 40 mg L−1 MO is much lower than that of the other two. That is to say the lower initial concentration of MO, no more than 20 mg L−1, is advantageous to its degradation in the photoelectrocatalytic system. Similar reports were also given by other researchers.1,35 Water and hydroxyl groups adsorbed on the mesoporous film may be transformed to active species, hydroxyl radicals, according to eqn (6)–(8). The competitive adsorption of dyes molecules at higher concentration may restrict reaction (7) and (8), and lead to a decrease in the degradation of MO. In addition, the higher concentration of MO is unfavorable for UV light reaching to the film electrode.| | | TiO2 + hv→ TiO2-h++ TiO2-e− | (6) |
| | | TiO2-h+ + H2O → TiO2-HO˙ + H+ | (7) |
| | | TiO2-h+ + OH−→ TiO2-HO˙ | (8) |
 |
| | Fig. 9 Influence of the initial concentration of MO on the degradation of MO in 0.1 M Na2SO4 solution at applied potential 0.6 V with UV illumination and pH 2.0, TiO2/ITO electrode. | |
3.2.5 Effect of electrolyte. Electrolytes are generally contained in the dye bath to improve the coloration effect. The influence of electrolytes on the degradation of dye under photoelectric catalysis were investigated with 0.1 mol KCl, NaNO3 and Na2SO4. The results are shown in Fig. 10 and 11. Addition of the above three electrolytes all can accelerate the degradation of MO under UV illumination. The degradation ratios of MO for the system containing the above electrolytes are obviously higher than that detected in the electrolyte free system (without KCl, NaNO3 and Na2SO4). The lower transfer resistance of the photogenerated charges in the electrolyte-containing solution between the semiconductor and solution may contribute to the higher degradation rate. It was almost reasonable to ignore the effect of the charge-transfer resistance in the solution when the concentration of Na2SO4 or KCl was up to 0.1 M while other conditions were pH 2.0, applied bias 0.2 V, 20 mg L−1 MO and 3-layer-film TiO2/ITO electrode.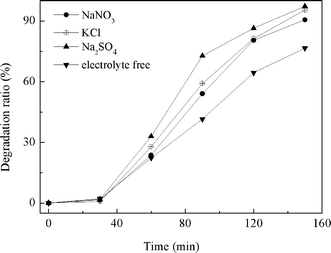 |
| | Fig. 10 Relationship between degradation ratio and reaction time on different kinds of electrolyte solutions at pH 2.0 with UV illumination, MO initial concentration of 20 mg L−1, applied potential 0.2 V, TiO2/ITO electrode. | |
 |
| | Fig. 11 Influence of the concentration of Na2SO4 on the degradation of MO at pH 2.0 with UV illumination, MO initial concentration of 20 mg L−1, applied potential 0.2 V, TiO2/ITO electrode. | |
For the acidic solution containing KCl electrolyte, the following reactions may take place in the system:
| | | TiO2 + hv→ TiO2-h+ + TiO2-e− | (6) |
| | | TiO2-h+ + H2O → TiO2-HO˙ + H+ | (7) |
| | | Cl2 + H2O → HOCl + Cl− + H+ | (10) |
| | | HO˙ + MO → degradation products | (12) |
| | | Cl˙ + MO → degradation products | (13) |
These reactions work together in the processes with photoelectric catalysis. So, addition of KCl to the solution is advantageous for the
degradation of MO.
As for the system containing Na2SO4, there may be the characteristic reactions under photoelectric catalysis:
| | | 2SO42−− 2e + hv→ S2O82− | (14) |
| | | S2O82− + 2H2O → 2HSO4− + H2O2 | (15) |
The species H
2O
2 and HO˙ are active for the
degradation of MO according to reaction
(5). Synergistic action through reactions
(4),
(6),
(7),
(14) and
(15) can effectively increase the
degradation reaction. This is why the
degradation ratios were higher for the Na
2SO
4-containing system.
The presence of NO3− in the system of the photoelectric catalysis was likely to increase the concentration of hydroxyl radicals, as denoted by eqn (16),35 and thereby increasing the degradation rate of MO.
| | | NO3− + H2O + hv→ NO2˙ + HO˙ + HO− | (16) |
3.2.6 Effect of doping ion. The activities of the ion-doping mesoporous film electrodes and the pure mesoporous film electrode TiO2/ITO were investigated. The results are presented in Fig. 12. No obvious difference of the activity for the degradation of MO was found for the ion-doped film electrodes and the film electrode TiO2/ITO, although the TiO2 film was calcined at a lower temperature and its specific area is about 36% smaller than that of the ion-doped film V-TiO2, as shown in the Table 1. The calcining temperature may have an important effect on the film properties and the high surface area may play an important role in the process of photocatalysis, which facilitates the adsorption of organics and allows transfer of the adsorbed compounds to the active sites. However, the situation must be more complicated than the surface area effect.11,37–39 The dispersion of the active sites and the local structure of the film all worked on the process of photocatalysis. Moreover, the energy of UV-light is powerful enough to excite the mesoporous TiO2 and ion-doped TiO2 films for effective degradation of MO under the applied potential, and the degradation was nearly complete. So, it was difficult to distinguish the activity difference for the above newly synthesized films in the photoelectrocatalytic system with UV light illumination. |
| | Fig. 12 Influence of the doping ions on the activity of the mesoporous film in 0.1 M Na2SO4 solution at applied potential 0.2 V with UV illumination, 20 mg L−1 MO and pH 2.0. | |
3.2.7 Degradation of MO under artificial solar light. A Xe lamp was used to provided artificial solar light. It has been reported that V-containing TiO2 exhibited substantial absorption of visible light.40 Artificial solar light absorption of the mesoporous films is shown in Fig. 2. The activities of the newly fabricated V-TiO2/ITO electrode and other electrodes were tested under artificial solar light illumination, and compared with that of photolysis. The results are presented in Fig. 13. The photolysis experiment was conducted under artificial solar light illumination without any electrode. The degradation ratio on the V-TiO2/ITO electrode was a little higher than that on the Ce-TiO2/ITO and F-TiO2/ITO electrodes, but significantly higher than that on TiO2/ITO electrode, while they were all much higher than the result of photolysis under artificial solar light illumination. The experimental results indicate, to some degree, that the doping of vanadium, cerium or fluoride into the TiO2 mesoporous film may improve the activity of the film under artificial solar light illumination and applied bias, although the activity for degrading MO needs to be increased further. The best performance of the V-TiO2 film for the degradation of MO under artificial solar light and bias potential may be plausibly ascribed to its large specific area and lower band-gap energy.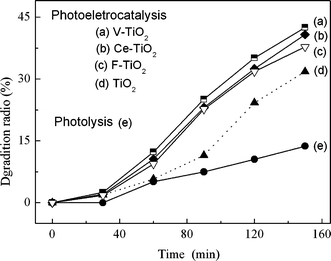 |
| | Fig. 13 Degradation of MO in 0.1 M Na2SO4 solution with visible light illumination, 20 mg L−1 MO and pH 2.0, and applied potential of 0.6 V for the lines (a), (b), (c) and (d). | |
As described above, the pure TiO2 film tested here was calcined at 350 °C and the other three were calcined at 450 °C. It is known that the calcination temperature is related to the crystallinity and microstructure of the materials, and therefore may affect the photoelectrocatalytic property of the films. Unfortunately, the mesoporous structure of the pure TiO2 film collapsed at 450 °C. The comparison between TiO2/ITO and other ion-doped mesoporous films calcined at the same temperature, such as 350 °C, may give more useful information, and is worth studying in the future.
4. Conclusion
Mesoporous film electrodes TiO2/ITO, V-TiO2/ITO, Ce-TiO2/ITO and F-TiO2/ITO can be fabricated through dip-coating of ITO glass into an organic/inorganic sol containing cationic surfactant CTAB. The resultant films were of the characteristics of mesoporous material. The newly synthesized films all were active for the degradation of MO. More than 90% degradation ratio could be obtained by using any of the above films in 2 h reaction in the conditions of 0.1 M Na2SO4, pH 2.0–3.5, applied bias 0.2–1.0 V, 3 layer films and no more than 20 mg L−1 MO under UV light illumination. The photoelectrocatalytic performance of the V-TiO2/ITO was the best of the synthesized films for degrading MO under artificial solar light illumination. But the activity of the films under artificial solar light needs to be improved greatly before being used in large scale systems.
Acknowledgements
The authors acknowledge financial support from the Education Committee of Shanghai (06-OZ-003) and the Committee of Science and Technology of Shanghai (06-JC-14095), China.References
- P. A. Carneiro, M. E. Osugi, J. J. Sene, M. A. Anderson and M. V. B. Zanoni, Electrochim. Acta, 2004, 49, 3807–3820 CrossRef CAS.
- H. Zollinger, Color Chemistry: Synthesis, Properties and Applications of Organic Dyes and Pigments, V. C. H. Publisher, New York, 2nd edn, 1991 Search PubMed.
- A. Houas, H. Lachheb, M. Ksibi, E. Elaloui, C. Guillard and J. M. Hermann, Appl. Catal., B, 2001, 31, 145–157 CrossRef CAS.
- J. Li, L. Li, L. Zheng, Y. Xian and Litong Jin, J. Hazard. Mater., 2006, 68, 765–770 CAS.
- C. Galindo, P. Jacques and A. Kalt, J. Photochem. Photobiol., A, 2001, 141, 47–56 CrossRef CAS.
- N. Kirby, R. Marchant and G. MacMullan, FEMS Microbiol. Lett., 2000, 188, 93–96 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69–96 CrossRef CAS.
- Y. Liu, X. Chen, J. Li and C. Burda, Chemosphere, 2005, 61, 11–18 CrossRef CAS.
- D. Dvoranova, V. Brezova, M. Mazur and M. A. Malati, Appl. Catal., B, 2002, 37, 91–105 CrossRef CAS.
- S. Z. Chen, P. Y. Zhang, D. M. Zhuang and W. P. Zhu, Catal. Commun., 2004, 5, 677–680 CrossRef CAS.
- J. Li, S. Liu, Y. He and J. Wang, Microporous Mesoporous Mater., 2008, 115, 416–425 CrossRef CAS.
- T. A. Egerton, M. Janus and A. W. Morawski, Chemosphere, 2006, 63, 1203–1208 CrossRef CAS.
- R. Asahi, T. Morikawa, T. Ohwaki, K. Aoki and Y. Taga, Science, 2001, 293, 269–271 CrossRef CAS.
- P. A. Carneiro, M. E. Osugi, C. S. Fugivara, N. Boralle, M. Furlan and M. V. B. Zanoni, Chemosphere, 2005, 59, 431–439 CrossRef CAS.
- V. B. Zanoni, J. J. Sene and M. A. Anderson, J. Photochem. Photobiol., A, 2003, 157, 55–62 CrossRef.
- H Selcuk, H. Z. Sarikaya, M Bekbolet and M. A. Anderson, Chemosphere, 2006, 62, 715–721 CrossRef CAS.
- C. T. Kresge, M. E. Leonowicz, W. J. Roth, J. C. Vartuli and J. S. Beck, Nature, 1992, 359, 710–712 CrossRef CAS.
- G. J. de A. A. Soler-Illia, A. Louis and C. Sanchez, Chem. Mater., 2002, 14, 750–759 CrossRef.
- K. L. Frindell, J. Tang, J. H. Harreld and G. D. Stucky, Chem. Mater., 2004, 16, 3524–3532 CrossRef CAS.
- U.-H. Lee, H. Lee, S. Wen, S. Mho and Young-Uk Kwon, Microporous Mesoporous Mater., 2006, 88, 48–55 CrossRef CAS.
- Y. Yue and Z. Gao, Chem. Commun., 2000, 1755–1756 RSC.
- H. S. Yun, K. Miyazawa, H. S. Zhou, I. Honma and M. Kuwabara, Adv. Mater., 2001, 13, 1377–1380 CrossRef CAS.
- K. L. Frindell, M. H. Bartl, M. R. Robinson, G. C. Bazan, A. Popitsch and G. D. Stucky, J. Solid State Chem., 2003, 172, 81–88 CrossRef CAS.
- W. Yao, H. Fang, E. Ou, J. Wang and Z. Yan, Catal. Commun., 2006, 7, 387–390 CrossRef CAS.
- W. S. Chae, S. W. Lee and Y. R. Kim, Chem. Mater., 2005, 17, 3072–3074 CrossRef CAS.
- W. Deng, P. Bodart, M. Pruski and B. H. Shanks, Microporous Mesoporous Mater., 2002, 52, 169–177 CrossRef CAS.
- X. Zhang, F. Zhang and K.-Y. Chan, Appl. Catal., A, 2005, 284, 193–198 CrossRef CAS.
- M. Matsuoka and M. Anpo, J. Photochem. Photobiol., C, 2003, 3, 225–252 CrossRef CAS.
- Jimmy C. Yu, Jiaguo Yu, Wingkei Ho, Zitao Jiang and Lizhi Zhang, Chem. Mater., 2002, 14, 3808–3816 CrossRef CAS.
- X. Zhang, M. Chao, E. Liang, F. Hu and B. Yuan, J. Inorg. Mater., 2009, 1, 34–38 Search PubMed.
- C. He, Ya Xiong, D. Shu, X. Zhu and X. Li, Thin Solid Films, 2006, 503, 1–7 CrossRef CAS.
- P. T. Tanev and T. J. P. innavaia, Science, 1995, 267, 865–867 CrossRef CAS.
- G. Qian, D. Ji, G.-M. Lu, R. Zhao, Y.-X. Qi and J.-S. Suo, J. Catal., 2005, 232, 378–385 CrossRef CAS.
- W. H. Leng, Z. Zhang, J. Q. Zhang and C. N. Cao, J. Phys. Chem. B, 2005, 109, 15008–15023 CrossRef CAS.
- M. H. Habibi, N. Talebian and Jong-Ha Choi, Thin Solid Films, 2006, 515, 1461–1469 CrossRef CAS.
- Y. Xie, Electrochim. Acta, 2006, 51, 3399–3406 CrossRef CAS.
- Y. Xu, Z. Wei and W. Liu, J. Photochem. Photobiol., A, 1999, 122, 57–60 CrossRef CAS.
- David F. Ollis, J. Phys. Chem. B, 2005, 109, 2439–2444 CrossRef.
- S. Mozia, Maria Tomaszewska and Antoni W. Morawski, Appl. Catal., B, 2005, 59, 155–160 CrossRef CAS.
- E. P. Reddy, Lev Davydov and Panagiotis G. Smirniotis, J. Phys. Chem. B, 2002, 106, 3394–3401 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry and Owner Societies 2010 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 t of about 10
000 t of about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 different types of dyes and pigments are produced each year for the dye industry.1,2 The dyeing processes, including subsequent washing procedures, require a great amount of water. Unfortunately, a significant fraction of residual textile dyes and several other types of chemicals are discharged with effluents from dyeing mills into natural and domestic water systems.3,4,5 As a result, important freshwater sources of modern people can become highly contaminated. Most of the dyes are difficult to biodegrade under an aerobic environment because of their synthetic origin and mainly complex aromatic molecular structures. As a matter of fact, elimination of the harmful dye pollutants has drawn the attention of both scientists and government. Laws and government regulations regarding the removal of dyes from wastewater treatment plants become more and more stringent.
000 different types of dyes and pigments are produced each year for the dye industry.1,2 The dyeing processes, including subsequent washing procedures, require a great amount of water. Unfortunately, a significant fraction of residual textile dyes and several other types of chemicals are discharged with effluents from dyeing mills into natural and domestic water systems.3,4,5 As a result, important freshwater sources of modern people can become highly contaminated. Most of the dyes are difficult to biodegrade under an aerobic environment because of their synthetic origin and mainly complex aromatic molecular structures. As a matter of fact, elimination of the harmful dye pollutants has drawn the attention of both scientists and government. Laws and government regulations regarding the removal of dyes from wastewater treatment plants become more and more stringent.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. TiO2 colloidal suspension was prepared by dropwise addition of the solution Y to X with vigorous stirring. After addition of Y to X, the mixture was stirred for 1 h at room temperature. The molar composition of the matrix gel is: n[Ti] : n[CTAB] : n[EtOH] : n[H2O] : n[Acac] = 1 : 0.17 : 20 : 5 : 0.3. The ion-doping TiO2 colloidal suspensions were prepared with the same procedure as the pure TiO2 colloidal suspension, except that Ce(NO3)3·6H2O, NH4VO3 or NH4F was added in the solution X with a molar radio of n[Ti] : n[doped ion] = 60, 60, and 30, respectively.
1. TiO2 colloidal suspension was prepared by dropwise addition of the solution Y to X with vigorous stirring. After addition of Y to X, the mixture was stirred for 1 h at room temperature. The molar composition of the matrix gel is: n[Ti] : n[CTAB] : n[EtOH] : n[H2O] : n[Acac] = 1 : 0.17 : 20 : 5 : 0.3. The ion-doping TiO2 colloidal suspensions were prepared with the same procedure as the pure TiO2 colloidal suspension, except that Ce(NO3)3·6H2O, NH4VO3 or NH4F was added in the solution X with a molar radio of n[Ti] : n[doped ion] = 60, 60, and 30, respectively.












