Interdomain signalling in the blue-light sensing and GTP-binding protein YtvA: A mutagenesis study uncovering the importance of specific protein sites
Yifen
Tang
a,
Zhen
Cao
a,
Elsa
Livoti
b,
Ulrich
Krauss
c,
Karl-Erich
Jaeger
c,
Wolfgang
Gärtner
a and
Aba
Losi
*b
aMax-Planck-Institute for Bioinorganic Chemistry, Stifstrasse 34-36 45470, Mülheim, Germany
bDepartment of Physics, University of Parma, viale G.P. Usberti 7/A, 43100, Parma, Italy. E-mail: aba.losi@fis.unipr.it
cInstitute of Molecular Enzyme Technology, Heinrich-Heine-University Düsseldorf, Research Center Jülich, Stetternicher Forst, 52426, Jülich, Germany
First published on 30th November 2009
Abstract
YtvA from Bacillus subtilis is a blue-light responsive, flavin-binding photoreceptor, built of a light-sensing LOV domain (aa 25-126) and an NTP (nucleoside triphosphate)-binding STAS domain (aa 147-261). The STAS domain is supposed to be the effector part of the protein or a secondary switch. Both domains are connected by a linker polypeptide. The active form of YtvA is generated upon light excitation, causing the formation of a covalent bond between a cysteine residue (Cys62) in the LOV domain and the position 4a of the flavin chromophore. This photoadduct formation within the LOV domain results in a conformational change of the NTP-binding cavity, evidencing intra-protein signal transmission. We have previously shown that Glu105, localized on the beta-scaffold of the LOV-core, is involved in this process. Here, we extend this work by the identification of further residues that upon mutation supress or strongly impair signal transmission by interfering with the communication between the two domains. These comprise L106 and D109 on the LOV domain; K130 and K134 on the linker region; D193, L194 and G196 within the DLSG GTP-binding motif (switch region) and N201 on the STAS domain. Furthermore in the mutated S195A and D193A proteins, GTP affinity is diminished. Other mutations investigated have little or no effect on signal transmission and GTP-binding affinity: R63K that was found to accelerate the thermal recovery of the parent state ca. ten-fold; K128A, Q129A and Y132A within the linker region, and S183A and S212A on the STAS domain. The results show a key role of the LOV domain beta-scaffold and of positively charged residues within the linker for intra-protein signal transmission. Furthermore they evidence the conformational switch function of a structurally conserved strand-loop-helix region (bearing the DLSG GTP-binding motif and N201) within the STAS domain that constitutes a novel GTP-binding fold.
Introduction
The YtvA protein from Bacillus subtilis acts as a positive regulator for the general stress transcription factor σB, specifically in the environmental signalling pathway.1 Like other regulators of the stress responsive network in B. subtilis, YtvA bears a STAS (Sulfate Transporters and Anti-Sigma factor antagonist) domain. Among the σB regulators YtvA has the unique ability to sense and respond to blue light (BL) via its N-terminally located LOV (Light, Oxygen and Voltage) domain, a feature shared with a growing family of BL-sensing proteins.2 This light-sensitivity has recently been shown to up-regulate the σB factor.3-6 A photoactive LOV domain binds a flavin mononucleotide (FMN) as chromophore.7 Photoactivation is achieved by the transient formation of a covalent bond between carbon atom 4a of FMN and a conserved cysteine (Cys62 in YtvA-LOV).8 Structurally, a LOV domain shows a PAS (PerArntSims) fold with an extended, five-stranded (Aβ, Bβ, Gβ, Hβ, Iβ) antiparallel β-scaffold and helical connectors (Cα, Dα, Eα, Fα), such that the N-terminus of the LOV-core (beginning of strand Aβ) is in close vicinity to its C-terminus (end of strand Iβ, Fig. 1) (reviewed in Ref. 9). | ||
| Fig. 1 (top) Structure of YtvA-LOV + linker (PDB entry 2pr5, chain A; (bottom) model of YtvA-STAS. Mutated residues causing a negative effect on signal transmission or GTPTR-binding are shown in black. For the effects of Y132A and R63A see text. | ||
The LOV-based photochemistry has first been discovered in the plant blue-light receptors phototropins (phot), built of two LOV domains in tandem, LOV1 and LOV2, and a C-terminally located S/T-kinase domain.10,11 In phot, the activity of the effector S/T-kinase domain is enhanced after LOV photoactivation with blue-light, resulting in phot auto-phosphorylation,12-14 a key event in signaling.15 LOV proteins other than phot, including the steadily growing bacterial family, bear solely one LOV domain, and the effector module can be represented by several functional motifs, e.g., a histidine kinase, gene-expression regulators, phosphodiesterases, phosphatases).2,16,17 This poses the question whether LOV domains communicate with effector partners through the same or partially overlapping surfaces, such that the structural basis for light-to-signal transduction is basically the same in the different LOV proteins, with only few residues acting as specific signal transmitters within the different systems. There is increasing experimental evidence that this surface largely involves the β-scaffold of the LOV-core (Fig. 1) which on one side hosts residues directly interacting with the isoalloxazine ring of FMN18,19 and on the other side communicates with helical regions flanking the LOV-core.20,21 As an example, a conserved glutamine on strand Iβ (Gln513 in Avena sativa phot1-LOV2) participates in light-driven conformational changes of LOV2,22-26 and of the LOV protein VIVID21 and in the light-activation of phot self-phosphorylation.27 The latter process is triggered by the light-induced unfolding of the so called Jα-linker helix, that interacts in the dark with the β-scaffold of LOV2 and connects LOV2 to the kinase domain.28,29,30 Moreover, the β-scaffold is involved in LOV-LOV dimerization, with or without the assistance/competition of the helical flanking regions.31-33 In VIVID, light-activation induces dimerization through the helical N-cap, but cross-linking data suggest that dissociation or removal of the N-cap induces dimerization via a β-sheet−β-sheet contact.34 In YtvA, the β-scaffold of the LOV-core has been identified as a competitive interface for LOV-LOV dimerization and intraprotein domain interactions,31 and a recently crystallized structure confirms the β-sheet mediated contact within the YtvA-LOV dimer.32
Due to its prokaryotic origin and the relatively small size (261 aa), YtvA is readily expressed as a full length protein and thus offers the possibility to study light induced changes also in the effector/signalling STAS domain. However, no three-dimensional structure of full-length YtvA is yet available, leaving a discussion on residues that potentially enable communication between the LOV- and the STAS domain at a speculative level. A preliminary investigation revealed that glutamic acid 105, localized on strand Hβ, is involved in signal transmission from LOV- into STAS domain (STAS, Sulfate Transporter and Anti-sigma factor antagonist).35 Although its exact function is not yet known, the STAS domain is thought to be the effector module of the protein or a secondary switch. It has been shown to bind ATP (adenosine triphosphate) and GTP (guanosine triphosphate),36 in agreement with a proposed general role of STAS domains as NTP (Nucleotide triphosphate) binding folds.37 E105 should therefore be located at that surface of the LOV domain interacting with the linker region or/and the STAS domain.
Communication between the two domains of YtvA is readily monitored by the blue light-induced conformational changes of the NTP-binding cavity of the STAS domain, i.e. by recording the spectroscopical changes in case a fluorescent, red light absorbing GTP derivative is employed.36 Specifically, a BODIPY® TR GTP (GTPTR) compound was chosen whose absorption and fluorescence maxima (ca. 590 and 620 nm, respectively) do not interfere with those of FMN (ca. 450 and 500 nm).38 Upon binding to YtvA (dissociation constant KDca. 40 μM in the light-activated state), the fluorescence of GTPTR increases with a concomitant, few nanometers red-shift of the absorption maximum.36 The thermally driven recovery reaction results in a further increment of fluorescence and of the absorption red-shift, indicative of conformational changes in the GTPTR binding cavity.36 These light-dark differences are fully reversible in the wild-type protein, but are completely suppressed in the mutated YtvA-E105L protein, although binding of GTPTR is not impaired.35 E105 is conserved in all YtvA-like proteins in the Firmicutes, while in phot-LOV domains this position is invariably occupied by a hydrophobic amino acid (generally leucine).17 In phot1-LOV2 this residue, Leu493, makes van der Waals contact to Ile532 on the Jα-linker,20 and the Ile532E mutation disrupts the LOV2-Jα interaction, rendering the kinase domain constitutively active.29 For YtvA, there is no evidence that light activation implies unfolding of the J-linker,31 neither does the presence of the J-linker prevent YtvA-LOV dimerization.32,39 Dimerization via the LOV-domain is only impaired in the full-length protein, at least in diluted solution.31 It is therefore conceivable that the LOV-core directly interacts with the STAS domain through the β-scaffold, possibly with the participation of the J-linker and by employing specific residues devoted to signal transmission.
We here present an extended investigation on the signalling pathway in YtvA, employing a set of selectively designed mutations, and using the GTPTR-binding assay and the GTPTR-based LOV-STAS signal transmission detection. A similar set of mutations has been recently investigated to study their role in the physiological function of YtvA in B. subtilis, making use of a reporter-protein mediated assay.6
By means of a fast cycling mutated protein, YtvA-R63K, suitable for spectroscopical studies and here fully characterized, we demonstrate the tight coupling between the LOV domain and the GTPTR-binding cavity on the STAS domain, even for the case of a strongly accelerated thermal recovery reaction. We also highlight the functional role of a second acidic amino acid, D109, typical of the YtvA-family and of L106, both localized on strand Hβ. In addition, our data reveal the functional role of charged amino acids (K130 and K134) within the J-linker, and of the DLSG193-196 Walker B- (Switch II) motif40 on the STAS domain. As a whole, the results stress the importance of the LOV-core strand Hβ and of specific residues within the J-linker in inter-domain signal transmission and confirm that YtvA-STAS is a novel NTP-binding fold, showing a conformational change switched by light within its NTP-binding cavity, through its coupling to the LOV domain.
Materials and methods
Site directed mutagenesis of YtvA and generation of recombinant proteins
A total of 16 new mutations had been generated: R63K, L106F, D109L, K128A, Q129A, K130A, Y132A, K134A, S183A, D193A, L194A, S195A, G196A, N201A, S212A. Mutagenesis was performed in two different ways, either by the Quick Change method (QuikChange® II XL, Stratagene) or by the megaprimer approach. The mutated sites in all primers are underlined.Primers used for the Quick Change method were:
R63K. Forward: 5′-GAAATTTTAGGAAAGAACTGT![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) TTCTTACAGGGGAAACACAC-3′
TTCTTACAGGGGAAACACAC-3′
Reverse: 5′-GTGTGTTTCCCCTGTAAGAA![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif) ACAGTTCTTTCCTAAAATTTC-3′
ACAGTTCTTTCCTAAAATTTC-3′
L106F. Forward: 5′-GGAACGATGTTCTGGAATGAATT![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) AATATTGATCCAATGGAAATAGAG-3′
AATATTGATCCAATGGAAATAGAG-3′
Reverse: 5′-CTCTATTTCCATTGGATCAATATT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) AATTCATTCCAGAACATCGTTCC-3′
AATTCATTCCAGAACATCGTTCC-3′
D109L. Forward: 5′-TGGAATGAATTAAATATT![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif) TCCAATGGAAATAGAGGATAAAACG-3′
TCCAATGGAAATAGAGGATAAAACG-3′
Reverse: 5′-CGTTTTATCCTCTATTTCCATTGGA![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif) AATATTTAATTCATTCCA-3′
AATATTTAATTCATTCCA-3′
K128A. Forward: 5′TGATATCACC![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CAAAAAGAATATGAAAAGCTTCTCGAGGATTCCCTCACG-3′
CAAAAAGAATATGAAAAGCTTCTCGAGGATTCCCTCACG-3′
Reverse: 5′-CGTGAGGGAATCCTCGAGAAGCTTTTCATATTCTTTTTG![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) GGTGATATCA-3′
GGTGATATCA-3′
Q129A. Forward: 5′-CAGAATGATATCACCAAG![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) AAAAGAATATGAAAAGC-3′
AAAAGAATATGAAAAGC-3′
Reverse: 5′-GCTTTTCATATTCTTTT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) CTTGGTGATATCATTCTG-3′
CTTGGTGATATCATTCTG-3′
K130A. Forward: 5′- GAATGATATCACCAAGCAA![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) GAATATGAAAAGCTTCTCG-3′
GAATATGAAAAGCTTCTCG-3′
Reverse: 5′-CGAGAAGCTTTTCATATTC![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif) TTGCTTGGTGATATCATTC-3′
TTGCTTGGTGATATCATTC-3′
Y132A. Forward: 5′- CCAAGCAAAAAGAA![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) GAAAAGCTTCTCGAGGATTCCCTCACG-3′
GAAAAGCTTCTCGAGGATTCCCTCACG-3′
Reverse: 5′-CGTGAGGGAATCCTCGAGAAGCTTTTCT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[T with combining low line]](https://www.rsc.org/images/entities/char_0054_0332.gif) TCTTTTTGCTTGG-3′
TCTTTTTGCTTGG-3′
The remaining mutations had been performed by a two step protocol following the megaprimer approach by using a plasmid-specific universal reverse primer and a mutagenic forward primer in a first run and then continuing with the thus generated DNA duplex in a second PCR reaction.
Universal reverse primer: 5′-TTTTGGATCCTTACATAATCGGAAGCACTTTAAC-3′,
Mutagenic forward primers
K134A 5′-CAAAAAGAATATGAA![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CTTCTCGAGGATTC-3′
CTTCTCGAGGATTC-3′
S183A 5′-TTGACGAATATCTTA![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) ACATCCAAAGATG-3′
ACATCCAAAGATG-3′
D193A 5′-GATTATTTGATCATT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) TTATCCGGATTGG-3′
TTATCCGGATTGG-3′
L194A 5′-TATTTGATCATTGAT![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) TCCGGATTGGCCC-3′
TCCGGATTGGCCC-3′
S195A 5′-TTGATCATTGATTTA![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) GGATTGGCCCAAG-3′
GGATTGGCCCAAG-3′
G196A 5′-ATCATTGATTTATCC![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) TTGGCCCAAGTGAAC-3′
TTGGCCCAAGTGAAC-3′
N201A 5′-GATTGGCCCAAGTG![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) GAACAAACGGCC-3′
GAACAAACGGCC-3′
S212A 5′-CAAATTTTCAAGCTG![[G with combining low line]](https://www.rsc.org/images/entities/char_0047_0332.gif)
![[C with combining low line]](https://www.rsc.org/images/entities/char_0043_0332.gif)
![[A with combining low line]](https://www.rsc.org/images/entities/char_0041_0332.gif) CATTTGCTGAAATTG-3′
CATTTGCTGAAATTG-3′
For all mutagenesis experiments, the obtained PCR products were treated with the restriction enzyme DpnI (New England BioLabs). DpnI is specific for methylated and hemimethylated DNA (targeting sequence: 5′-Gm6ATC-3′) and is used to digest the parental DNA template in the PCR products. 0.5 μl DpnI (20000 U/ml) was added to each PCR product and the digestion reaction was carried out at 37 °C for 30 minutes. In all cases, mutations were confirmed by sequencing.
His-tagged, mutated proteins were obtained from expression in E. coli (BL21) (Stratagene, Amsterdam, The Netherlands) using IPTG- (BioMol, Hamburg, Germany) induction and employing the pET28a plasmid (Novagen-Merck, Darmstadt, Germany), as described.8 The proteins were purified by affinity chromatography on a Talon resin (Qiagen, Hilden, Germany) according to the standard given protocol and adding in each step 200 mM PEFABLOC and 0.8 mM mercaptoethanol; the Talon resin loaded with the cell extract, was washed 5 times with the protocol given washing buffer plus 10 mM imidazole. The protein was then eluted in one step with 130 mM imidazole. The protein was concentrated by centrifugation at 10 °C employing centrifuge tubes with 10,000 Da cutoff and a final, storage buffer composed of Na-phosphate 10 mM, NaCl 10 mM, pH = 8. Care was taken not to concentrate the protein above 170 μM during centrifugation, because this leads, in our experience, to irreversible aggregation and inhomogeneous oligomeric composition.
GTP-binding assays and spectroscopy
Guanosine 5′-triphosphate, BODIPY® TR 2′-(or-3′)-O-(N-(2-aminoethyl) urethane), trisodium salt (GTPTR) was purchased from Molecular Probes (Eugene, OR, USA). Absorbance spectra were recorded with a Jasco 7850 UV/Vis spectrophotometer. Steady-state fluorescence measurements were carried out at 20 °C on a Perkin-Elmer LS50 luminescence spectrometer. GTPTR was excited at 590 nm. The output signal was divided by the fraction of absorbed energy (1-10−A, where A = absorbance at the excitation wavelength) in order to obtain a signal, referred to as normalized fluorescence, that is proportional to the fluorescence quantum yield.With the exception of YtvA-R63K, binding experiments for the determination of the dissociation constant, KD, were carried out with YtvA proteins in their blue-light activated state (i.e. FMN covalently bound to Cys 62), achieved by illuminating the sample with a blue-light emitting Led-Lenser ® V8 lamp (470 nm, Zweibrüder Optoelectronics, Solingen, Germany) as previously described.36 The blue-light was switched-off just prior recording of the absorption and fluorescence spectra, ensuring that at least 95% of the sample is present as FMN-Cys adduct, given the long lifetime for the recovery to the dark state (denoted as YtvA450 from the absorption maximum of the bound flavin), τrec > 3500 s at 20 °C.8,41,42 The fluorescence spectra were always recorded upon excitation at 590 nm (where solely GTPTR absorbs) and no energy transfer can occur from or to other fluorophores (e.g. FMN or aromatic amino acids).
The calculation of KD was carried out as previously described.36 We define α = ΔF/ΔFmax as the fraction of bound ligand, where ΔF is the variation of the normalized fluorescence of GTPTR in the presence of the protein with respect to free GTPTR; ΔFmax is the maximum fluorescence enhancement corresponding to αmax, i.e.α =1. The value of ΔFmax was determined by the non-linear fitting for one-site binding (eqn 1):
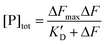 | (1) |
Where [P]tot = total concentration of protein added (bound + free), in our cases ranging between ca. 8 and 80 μM. Assuming a 1:1 complex and with α = ΔF/ΔFmax we can fit αvs. the concentration of free protein, as detailed in (eqn 2), where αmax must extrapolate to 1 if the assumption is correct:
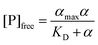 | (2) |
In principle, KD in eqn 2 is different from K′D in eqn 1, but in our case the two values are very similar given that the association is weak and [P]tot≅ [P]free total. Given that binding of GTPTR to YtvA induces a red-shift in the absorption spectrum, we also evaluate KD by employing ΔAbs in place of ΔF in eqn 1.
Results
The residues mutated in this work are shown in Fig. 1, evidenced on a published structure of YtvA-LOV + linker32 and on the modelled structure of the STAS domain.36 Most of the mutated proteins could be expressed as holoproteins (ratio between protein and FMN ca. 1:1), except for D109L, D193A and G196A mutations, for which protein/FMN ratios were 2, 4, and 2, respectively (Table 1). This determination has been carried out as previously described,43 knowing that a 1:1 ratio of protein to chromophore results in a UV/VIS (272/447 nm) absorbance ratio of ca. 4. The presence of chromophore-free apoprotein can cause an overestimation of the affinity for GTPTR,35 possibly by competition with the FMN-binding site on the LOV domain or by conformational modifications occurring when FMN is not bound. To show this “contaminating” effect of the apoprotein, data from an only partially chromophore-loaded YtvA wild type preparation were also included in Table 1. Further, mutated proteins did not show changes in their oligomeric state, i.e. they are present in solution chiefly as monomers as indicated by gel filtration experiments.39| prot/FMN |
K
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b/μM (ΔFluo) a,b/μM (ΔFluo) |
K
D![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a,b/μM (ΔAbs) a,b/μM (ΔAbs) |
Dark-Light GTPTR Spectral Changes | |
|---|---|---|---|---|
| a K D = dissociation constants. b Errors derive from at least 2 sets of measurements for each sample and from errors reported by the fitting algorithm. | ||||
| YtvA-WT | 1 | 38 ± 5 | 26 ± 5 | Yes |
| YtvA-WT | 4 | 15 ± 7 | 8.5 ± 2 | Yes |
| LOV domain mutations | ||||
| R63K | 1 | 28 ± 11 | 16 ± 2 | Yes |
| E105L | 1 | 43 ± 6 | 43 ± 10 | No |
| L106F | 1 | 60 ±13 | 81 ± 23 | Minor |
| D109L | 2 | 13 ± 3 | 11 ± 2 | No |
| Linker | ||||
| K128A | 1 | 26 ± 2 | 36 ± 4 | Yes |
| Q129A | 1 | 20 ± 5 | 42 ± 11 | Yes |
| K130A | 1 | 54 ± 9 | 92 ± 23 | Minor |
| Y132A | 1 | 62 ± 7 | 79 ± 18 | Yes, > ΔF than WT |
| K134A | 1 | 76 ± 20 | 19 ± 5 | No |
| S139A | 1 | 19 ± 2 | 11 ± 2 | Yes |
| STAS domain | ||||
| D193A | 4 | 67 ± 23 | 61 ± 6 | No |
| L194A | 1 | 84 ± 25 | 34 ± 5 | Minor |
| S195A | 1 | 300 ± 70 | 120 ± 50 | Yes |
| G196A | 2 | 10 ± 3 | 2 ± 1 | No |
| N201A | 1 | 23 ± 5 | 52 ± 27 | Minor |
| S183A | 1 | 76 ± 40 | 65 ± 19 | Yes |
| S212A | 1 | 87 ± 10 | 84 ± 15 | Yes |
The effects of mutations were monitored by recording two distinct phenomena: 1) the protein binding capacity for GTPTR (dissociation constant KD, see eqn 1 and 2), determined by the change in its (normalized) fluorescence intensity and by the red-shift in the absorption maximum upon binding; 2) effects on LOV-STAS signal transmission, monitored by following the spectral changes of bound GTPTR after formation of the FMN-cysteine photoadduct. This effect had been described in YtvA-WT as a slight absorption blue-shift and fluorescence decrease that reverses in the dark.35 Throughout the text, α is defined as the fraction of bound ligand, as detailed in the material and methods section.
All the proteins investigated exhibited a photocycle similar to YtvA-WT, with a slow recovery (ca. 4000 ± 1000 s at 20 °C) to the parent state. The sole exception was YtvA- R63K (vide infra).
Ribityl-phosphate binding region
YtvA has a remarkably long photocycle with a recovery lifetime τrec = ca. 3900 s at 20 °C41 showing some variation in dependence of the preparation. This long photocycle may hinder functional and spectroscopical studies, because monitoring light-to-dark state conversion requires lengthy measurements, during which the protein, the fluorescent probe and/or instrumental parameters might change and introduce artefacts, particularly when the observed changes are small as in the case of GTPTR bound to YtvA.35,36 The R63K mutation strongly accelerates the photocycle and the recovery lifetime for FMN, τrec,F, becomes ca. 480 s at 20 °C (Fig. 2), although the photoadduct is formed with the same kinetics as the WT protein (Livoti, E., unpublished data), namely with a lifetime of ca. 2 μs.8 | ||
| Fig. 2 Dark recovery of (a) FMN, (b): W103 (LOV core), and (c) GTPTR (STAS domain) fluorescence for YtvA-R63K. The excitation wavelengths were 305, 280 and 590 nm, respectively. Kinetics traces were recorded at 20 °C. The values of τrec obtained from a mono-exponential fitting were: (a) 485 s; (b) 365 s; (c) 511 s. | ||
The kinetic effects of this mutation are similar to those observed in the LOV1 from Chlamydomonas reinhardtii phot, where the corresponding mutation (R58K) accelerates the recovery time from 204 to 73 s at 20 °C.44 As for the GTP-binding capacity and signal transmission, YtvA-R63K behaves very similar to the WT protein, showing the previously characterized absorption blue-shift and fluorescence decrease of bound GTPTR upon formation of the photoadduct (Table 1).
YtvA-R63K is thus suitable for spectroscopic and functional studies, given its accelerated photocycle and the fact that the dark recovery of three different regions of the protein can be easily followed by monitoring the fluorescence of FMN (LOV core), GTPTR (STAS domain) and of W103 (Hβ strand of the LOV core). Observation of changes of W103 is very supportive, since it was previously demonstrated to be an independent marker for interdomain interactions.45 The resulting recovery lifetimes are τrec,F= 480 s, τrec,TR= 511 s and τrec,W= 365 s, respectively (20 °C), clearly demonstrating the tight coupling of the three protein regions (Fig. 2).
For monitoring the kinetics traces, excitation (λex) and emission (λem) wavelengths were selected as follows: for FMN λex = 305 nm and λem =500, for GTPTRλex = 590 nm and λem = 620 nm, for W103 λex = 280 nm and λem =340 nm. FMN was excited in a “valley” of the absorption spectrum, in the UVB region and not with blue-light, in order to minimize secondary photochemistry leading to formation of the photoproduct during recording of the traces. We have infact verified that this methodology gives identical results as tracing the recovery of absorption, whereas flavin excitation at 450 nm triggers photolysis during emission spectroscopy, leading to artifacts in the kinetics traces.39
Amino acids in the LOV-core β-scaffold
The light-induced absorption and fluorescence changes of bound GTPTR reflecting LOV-STAS signal transmission, are not observed for YtvA-E105L as recently reported35 and the same occurs with YtvA-D109L. The somehow higher affinity of YtvA-D109L for GTPTR (see Table 1) is most probably due to the presence of some apoprotein that could not be avoided with this mutation. These data stress the functional importance of these two negatively charged amino acids, uniquely conserved within the YtvA-like proteins and localized on strand Hβ, on the external side with respect to the FMN cavity (Fig. 1a).Another strategically positioned amino acid of this region is L106. Its side chain points towards the chromophore cavity and comes into close contact with the isoalloxazine ring.32 The L106F mutation diminishes but not completely abolishes the light-induced changes of GTPTR (see Table 1). The affinity for GTP appears to be slightly lower than for YtvA-WT, but remains definitely within the same range; this latter effect might result from a perturbation of the overall protein tertiary structure due to the introduced change and is observed also for other mutations (Table 1). We note nevertheless that these three mutations do not affect either the oligomeric state of the protein, that remains mainly monomeric as shown by gel filtration experiments, or the secondary structure composition, as indicated by circular dichroism data.39,43
Amino acids within the linker region
Other mutations that supress partially or completely the LOV-STAS signal transmission include K130A and K134A, both located within the linker region (Table 1, Fig. 3). On the other hand, K128, Q129 or S139 do not affect appreciably either affinity for GTP or signal-transmission, but behave similar to YtvA-WT.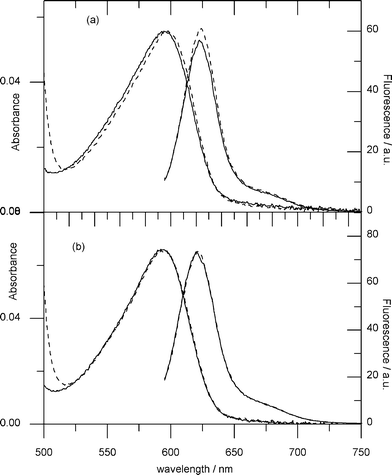 | ||
| Fig. 3 Absorption and fluorescence spectra of GTPTR partially bound to (a) YtvA-K128A and (b)YtvA-K130A, in the dark (dashed lines) and light (solid lines) state. The value of α is ca. 0.5 for both proteins. The two proteins are examples for signal-transmission “innocent” (K128A) and “guilty” (K130A) mutations. | ||
The uncoupling between LOV domain and GTP-binding site on the STAS domain is best appreciated by observing the kinetics traces for the dark-recovery fluorescence of FMN and of GTPTR. For a signal-transmission “guilty” mutation (e.g. K130) the latter fluorescence does not change, whereas the recovery of FMN fluorescence follows the usual pattern (Fig. 4). For a signal-transmission “innocent” change (e.g. K128) the two traces follow instead a similar time-course.
 | ||
| Fig. 4 Time course for the dark-recovery of fluorescence for (a) YtvA-K128A and (b) YtvA-K130A at 20 °C. The GTPTR fluorescence (full lines) has been recorded at 620 nm after 590 nm excitation. FMN (dashed lines) was excited at 305 nm and fluorescence was recorded at 500 nm. Conditions as in Fig. 3. | ||
In the case of mutation Y132A, light induced absorption changes are similar to YtvA-WT (showing a shift of ca. 2.5 nm with α = 0.5), but the light-induced decrease of GTPTR fluorescence is larger (ca. 10% with α = 0.5, compared to 5% in YtvA-WT) (Table 1, Fig. 5), suggesting a larger conformational change in the nucleotide-binding cavity.
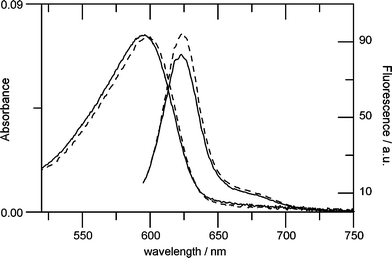 | ||
| Fig. 5 Absorption and fluorescence spectra of GTPTR partially bound to YtvA-Y132A, in the dark (dashed lines) and light (solid lines) state. The value of α is ca. 0.5. The light-induced spectral changes in this protein are larger than in YtvA-WT. | ||
STAS-domain residues
Within the STAS domain all four residues of the supposed GTP-binding/switch DLSG motif (positions 193-196) have been changed and show some effect on the binding of GTP or light-induced changes of bound GTPTR. L194A and G196A impair this signal transmission, whereas S195A strongly diminishes the affinity for GTPTR. The situation is less clear for D193A due to the presence of a large percentage of apoprotein that could not be avoided for this sample, even by changing induction times and expression temperature during three different preparations. It is possible that this mutation, as well as others for which we could not obtain a fully chromophore-loaded protein, somehow interferes with the expression system, given that the chromophore is picked up from the flavins pool of E. coli cells. The fraction of GTPTR bound to apoprotein does not undergo light-induced changes, but obviously interferes with fluorescence of GTPTR bound to the holoprotein. This mutation thus seems to impair GTPTR binding and also the light-switched changes. Among the other STAS domain mutations tested, only N201A has an impairing effect on signal transmission (Table 1), whereas the serine-to-alanine changes at positions 183 and 212 behaved similarly to the WT protein.Discussion
The Hβ strand of the LOV-core β-scaffold
The extended β-scaffold of the LOV-core has previously been discussed as a potential surface that conveys the signal to effector domains (see introduction). Here we have focused on residues within the Hβ strand because this element of secondary structure bears amino acids that characterize a certain class of LOV proteins (see Fig. 6). | ||
| Fig. 6 Partial alignment of LOV proteins forming the YtvA-family in Firmicutes, highlighting the mutations studied in this work (with the exception of R63K, that solely accelerates the photocycle) and their conservation throughout the family (Glu in place of Asp109 has been held as conservative substitution). Accession numbers for the proteins are taken from the UniProt Knowledgebase (Swiss-Prot + TrEMBL) databank.50 Abbreviations: B. = Bacillus, O. = Oceanobacillus, L = Listeria. Residues whose mutation impairs light triggered conformational changes transmission from LOV domain to the GTP-binding cavity on STAS domain and/or GTP affinity, are highlighted in green. Y132, whose mutation in alanine increases the light triggered conformational changes is highlighted in light-blue. Residues whose mutations have negligible effects are in grey. Numbering is from YtvA. Published secondary structures for the LOV domain are shown on top (arrows = strand, cylinders = helices, lines = turns/coils),32 with the nomenclature used in this work. Our modelled secondary structure elements for the STAS domain are shown,36 with nomenclature as in Aravind and Koonin.37 | ||
An acidic amino acid corresponding to E105 is present solely in bacteria and a second acidic residue (aspartate or glutamate) at position 109 is exclusive for YtvA-like proteins in the Firmicutes.17 Both E105 and D109 are oriented towards the exterior of the FMN binding cavity and at the interface of the YtvA-LOV dimer.31,32 Here we have shown that both amino acids are key elements for LOV-to-STAS signal transmission, as detected by the light-induced conformational changes of the GTP-binding cavity. A careful inspection of the GTPTR absorption spectra when bound to YtvA-E105L suggests that this mutated protein is locked in a light-activated conformation,35 whereas the presence of apoprotein in the YtvA-D109L preparation does not allow a similarly strict conclusion. If the NTP-binding properties are linked to a physiological response, the E105L mutation should thus result in constitutive activity of YtvA, e.g. activation of σB, and in any case there should be no light influence for both E105L and D109L. We have now evidence, from comparative in vivo data, that this is indeed the case: in particular, the mutated YtvA-E105L protein constitutively up-regulates σB, whereas the contrary holds for YtvA-D109L.6
It is striking that E105 seems to have a similar role as the corresponding Leu493 in plant phot that makes contact to Ile532 on the Jα-linker.20 The disruption of this contact by the I532E mutation disrupts the LOV2-Jα interaction and renders the kinase domain constitutively active.29 In case of YtvA, nevertheless, we cannot extrapolate with certainty that E105 makes contact with the linker, given that in the dimeric YtvA-LOV this is definitely not the case (vide infra).32
Our results confirm a previous conformational analysis that proposed the β-scaffold as a competitive interface for LOV-LOV dimerization and intraprotein interactions. They further identify the two conserved negatively charged amino acids as essential for the LOV-to-STAS signal transfer.
A leucine or another small apolar amino acid in the position corresponding to L106, is typical for phot-LOV1 and is changed for phenylalanine in LOV2, where it has been suggested to be a key element during phototropin activation.46 In known LOV domain structures, FMN is sandwiched between this residue and the reactive cysteine.18-20,32 In neochrome1-LOV2 (neo1-LOV2),18 a substitution of the corresponding Phe1010 by a leucine renders LOV2 similar to LOV1 in terms of light-induced structural changes, i.e. they become smaller and temperature independent.46 The reported larger light-induced structural changes of LOV2 with respect to LOV1 are in part ascribed to this residue (F1010) due to its larger capacity to activate the kinase domain via perturbations that propagate from FMN to F1010 viaπ-π stacking interaction, and further to the Jα-helix facilitating its unfolding.46 On the contrary, the opposite substitution, L106F in YtvA-LOV impairs signal transmission to the STAS domain, instead of facilitating it (this work). We cannot compare this result with similar data for phot-LOV1 because, to our knowledge, a similar mutation has not been reported so far. It might be that in YtvA-LOV a phenylalanine in this position cannot assume the same conformation as in LOV2 (being stacked with the isoalloxazine ring of FMN), due to other and still undetermined factors. However, our data stress the importance of the amino acid that faces the flavin ring, opposite to the reactive cysteine, in conveying the signal to the partner domain.
We are aware that the oligomeric state of YtvA is subject of controversy. The crystal structure of the extended YtvA-LOV+J-linker domain is dimeric,32 and it was suggested that the J-linker forms a coiled-coil dimer that works as light-activated rotary switch during regulation of a fused kinase domain.47 Nevertheless we showed that the full-length protein is mostly monomeric, at least at μM concentrations (see also Materials and methods) and that the oligomeric state and secondary structure pattern of the protein is not altered by the present mutations of the Hβ-strand.39 This, together with the in vivo data, allows us to conclude that the said mutations are truly affecting light-to-signal-transmission and not the overall conformation of the protein.
The J-linker region
The linker region of YtvA is rich in polar and charged amino acids, of which at least K130 and K134 are involved in signal transmission. Contrary to the LOV2 extended construct28,30 we do not have any indication for a helix unfolding upon light activation of YtvA.31 In the dimeric crystal structure of YtvA-LOV (lacking the STAS domain and the N-cap), the helical linker is not organized underneath the β-scaffold, but points away from the LOV domain, as a clear evidence that it cannot compete with the strong LOV-LOV interaction, largely mediated by this surface (Fig. 3).32 This might not be the case for the full-length protein, for which dimerization is prevented (at least in diluted solution), but solely if the STAS domain is present, i.e. only in the full-length protein.31 We have in fact evidences that both the N-cap31 and the J-linker32,39 are not able to prevent dimerization of YtvA-LOV, although we cannot exclude a different way of dimerization when the flanking regions are present. Unfortunately it is currently not possible to draw with safety the configuration of the linker within the full-length protein, also due to low sequence homology with phot1-LOV2 J-linker. If the protein is functioning as a monomer, it is highly improbable that the J-linker adopts a conformation as in the crystallized YtvA-LOV dimer, because it would be impossible for E105 and D109 to perform their role of “transmitter” to the STAS domain. With the present scenario, we can only state that also in YtvA, as in LOV2, the linker region has a role in signal transmission, involving two positively charged amino acids, K130 and K134. Contrary to E105 and D109, conserved as negatively charged amino acids within the majority of YtvA-like proteins in the Firmicutes,17 K130 is only conserved within the Bacillus species and is substituted for a histidine among Listeria. Position 134, instead, is very variable, being substituted sometimes even with a leucine.The switch region of the STAS domain
It was previously outlined that within STAS domains the loop between strand 3 and helix 2 (Fig. 1) is structurally conserved and does not tolerate inserts.37 In those STAS proteins that are regulated by phosphorylation (e.g. SPOIIAA and RsBr), this loop bears, adjacent to helix 2, the serine or threonine that become phosphorylated,37 corresponding to E202 in Ytva, i.e. directly adjacent to N201. Furthermore, the NTPase activity of SPOIIAA is abolished by phosphorylation or by mutation of Ser58.48 YtvA-STAS lacks a phosphate-binding Ser or Thr residue in the same position as SPOIIAA (Ser58) or Thr205 in RsBr,1,37 and the corresponding E202A mutation in YtvA does have an effect in vivo.6 Still, the conserved loop seems to bear a functional role. In fact the DLSG (193-196) sequence is part of this loop, being localized at the C-terminal end of strand 3 and the beginning of the loop. A DXXG motif is usually referred to as Walker B or Switch II sequence in small GTP-binding proteins,40 and we previously noticed a partial topological and sequence similarity between this protein family and YtvA-STAS.36 Accordingly, from our data it is clear that the DLSG sequence not only is important for GTP-binding to YtvA-STAS (D193A and S195A), but also has a conformational switch activity (D193A, G196A). Quite importantly, and in agreement with our data in vitro, mutation of D193 and S195 abolishes light activation of σBin vivo.6Notwithstanding the similarity, it is quite improbable that YtvA-STAS binds NTPs with the same configuration as GTP-binding proteins, because the required P-loop structure (Walker A)40 is missing. An alternative way of NTP-binding to STAS domains has been suggested, resembling lipid association by Sec14 domains, which have a very similar folding as SPOIIAA but show a poor sequence similarity.37 The conserved loop would thus be involved in binding of the γ-phosphate group, resembling the role of DXXG in small GTP-binding proteins, whereas the β-scaffold and helix 1 and 2 might accommodate the rest of the nucleotide. Structures of Sec14 domains are available in the PDB databank (e.g.3B74),49 but their similarity with STAS domains, as for ligand binding, remains to be demonstrated. This hypothesis is nevertheless supported by the observation that in YtvA the N201A mutation impairs the light-switched conformational changes within the GTP-binding cavity, given that N201 is localized within the conserved loop at the N-terminal end of helix 2. The DXXG sequence is in the vicinity of the phospholipid end l in Sec14, in the same position as YtvA-STAS and GTP-binding proteins, although it is not directly involved in binding.49
Conclusion
In this work we have definitely established the role of two charged amino acids (E105 and D109), localized on the β-scaffold of the LOV core and typical of the bacterial YtvA-like family, in delivering the light-triggered signal to the GTP-binding cavity on the STAS domain. As discussed above, this nicely fit with in vivo novel results.6 Furthermore, it is now clear that also in YtvA the J-linker participates in intraprotein signal-transmission, although not via a light-triggered unfolding as in phot1. Structural features for the full length protein are still needed in order to fully elucidate the pathway of signal-transmission.Finally we have shown that an up-to-now putative GTP-binding/switch motif is indeed strongly involved both in binding and in switching the conformational changes of the nucleotide cavity on the STAS module upon light activation of the LOV domain. Only a few data are available to support a general NTP-binding role for the wide-spread STAS domains, but this functionality is likely to be relevant, as evidenced by the above discussed link with in vivo data.
Abbreviations used:
| ATP | adenosine triphosphate |
| FMN | flavin mononucleotide |
| GTP | guanosine triphosphate |
| GTPTR | Guanosine 5′-triphosphate, BODIPY® TR 2′-(or-3′)-O-(N-(2-aminoethyl) urethane) |
| LOV | Light, Oxygen and Voltage |
| NTP | nucleotide triphosphate |
| phot | phototropin |
| STAS | domain found in Sulfate Transporters and Anti-Sigma factor antagonist |
Acknowledgements
This work has been supported by the Deutsche Forschungsgemeinschaft (FOR526, Z.C., Y.T., A.L.), and by a study-exchange grant of the Socrates-Erasmus program of the European Community (E.L.). Sven Jansen contributed in part of the mutagenesis work during his Diploma Thesis.References
- S. Akbar, T. A. Gaidenko, K. Min, M. O'Reilly, K. M. Devine and C. W. Price, New family of regulators in the environmental signaling pathway which activates the general stress transcription factor of Bacillus subtilis, J. Bacteriol., 2001, 183, 1329–1338 CrossRef CAS.
- A. Losi and W. Gaertner, Bacterial bilin- and flavin-binding photoreceptors, Photochem. Photobiol. Sci., 2008, 7, 1168–1178 RSC.
- T. A. Gaidenko, T. J. Kim, A. L. Weigel, M. S. Brody and C. W. Price, The blue-light receptor YtvA acts in the environmental stress signaling pathway of Bacillus subtilis, J. Bacteriol., 2006, 188, 6387–6395 CrossRef CAS.
- M. Avila-Perez, K. J. Hellingwerf and R. Kort, Blue light activates the sigmaB-dependent stress response of Bacillus subtilis via YtvA, J. Bacteriol., 2006, 188, 6411–6414 CrossRef CAS.
- N. Suzuki, N. Takaya, T. Hoshino and A. Nakamura, Enhancement of a sB-dependent stress response in Bacillus subtilis by light via YtvA photoreceptor, J. Gen. Appl. Microbiol., 2007, 53, 81–88 CrossRef CAS.
- M. Avila-Perez, J. Vreede, Y. Tang, O. Bende, A. Losi and W. Gaertner, In vivo mutational analysis of YTVA from Bacillus subtilis: Mechanism of light-activation of the general stress response, J. Biol. Chem., 2009, 284, 24958–24964 CrossRef CAS.
- W. R. Briggs, The LOV domain: a chromophore module servicing multiple photoreceptors, J. Biomed. Sci., 2007, 14, 499–504 CrossRef CAS.
- A. Losi, E. Polverini, B. Quest and W. Gärtner, First evidence for phototropin-related blue-light receptors in prokaryotes, Biophys. J., 2002, 82, 2627–2634 CrossRef CAS.
- A. Losi and Flavin-based, Blue-light Photosensors: a Photobiophysics Update, Photochem. Photobiol., 2007, 83, 1283–1300 CrossRef CAS.
- W. R. Briggs, T. S. Tseng, H. Y. Cho, T. E. Swartz, S. Sullivan, R. A. Bogomolni, E. Kaiserli and J. M. Christie, Phototropins and their LOV domains: Versatile plant blue-light receptors, J. Integr. Plant Biol., 2007, 49, 4–10 CrossRef CAS.
- J. M. Christie, Phototropin Blue-Light Receptors, Annu. Rev. Plant Biol., 2007, 58, 21–45 CrossRef CAS.
- E. Huala, P. W. Oeller, E. Liscum, I. S. Han, E. Larsen and W. R. Briggs, Arabidopsis NPH1: A Protein Kinase with a Putative Redox-Sensing Domain, Science, 1997, 278, 2120–2123 CrossRef CAS.
- M. Salomon, E. Knieb, T. von Zeppelin and W. Rüdiger, Mapping of Low- and High-Fluence Autophosphorylation Sites in Phototropin 1, Biochemistry, 2003, 42, 4217–4225 CrossRef CAS.
- S. Tokutomi, D. Matsuoka and K. Zikihara, Molecular structure and regulation of phototropin kinase by blue light, Biochim. Biophys. Acta, Proteins Proteomics, 2008, 1784, 133–142 CrossRef CAS.
- S. Inoue, T. Kinoshita, M. Matsumoto, K. I. Nakayama, M. Doi and K. Shimazaki, Blue light-induced autophosphorylation of phototropin is a primary step for signaling, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 5626–5631 CrossRef CAS.
- S. Crosson, S. Rajagopal and K. Moffat, The LOV domain family: photoresponsive signaling modules coupled to diverse output domains, Biochemistry, 2003, 42, 2–10 CrossRef CAS.
- A. Losi, The bacterial counterparts of plants phototropins, Photochem. Photobiol. Sci., 2004, 3, 566–574 RSC.
- S. Crosson and K. Moffat, Structure of a flavin-binding plant photoreceptor domain: insights into light-mediated signal transduction, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 2995–3000 CrossRef CAS.
- R. Fedorov, I. Schlichting, E. Hartmann, T. Domratcheva, M. Fuhrmann and P. Hegemann, Crystal structures and molecular mechanism of a light-induced signaling switch: the Phot-LOV1 domain from Chlamydomonas reinhardtii, Biophys. J., 2003, 84, 2474–2501 CrossRef CAS.
- A. S. Halavaty and K. Moffat, N- and C-Terminal Flanking Regions Modulate Light-Induced Signal Transduction in the LOV2 Domain of the Blue Light Sensor Phototropin 1 from Avena sativa, Biochemistry, 2007, 46, 14001–14009 CrossRef CAS.
- B. D. Zoltowski, C. Schwerdtfeger, J. Widom, J. J. Loros, A. M. Bilwes, J. C. Dunlap and B. R. Crane, Conformational switching in the fungal light sensor Vivid, Science, 2007, 316, 1054–1057 CrossRef CAS.
- D. Nozaki, T. Iwata, T. Ishikawa, T. Todo, S. Tokutomi and H. Kandori, Role of Gln1029 in the photoactivation processes of the LOV2 domain in Adiantum phytochrome3, Biochemistry, 2004, 43, 8373–8379 CrossRef CAS.
- A. I. Nash, W. H. Ko, S. M. Harper and K. H. Gardner, A Conserved Glutamine Plays a Central Role in LOV Domain Signal Transmission and Its DurationGÇá, Biochemistry, 2008, 47, 13842–13849 CrossRef CAS.
- A. Pfeifer, T. Majerus, K. Zikihara, D. Matsuoka, S. Tokutomi, J. Heberle and T. Kottke, Time-Resolved Fourier Transform Infrared Study on Photoadduct Formation and Secondary Structural Changes within the Phototropin LOV Domain, Biophys. J., 2009, 96, 1462–1470 CrossRef CAS.
- M. T. A. Alexandre, R. van Grondelle, K. J. Hellingwerf and J. T. M. Kennis, Conformational Heterogeneity and Propagation of Structural Changes in the LOV2/J alpha Domain from Avena sativa Phototropin 1 as Recorded by Temperature-Dependent FTIR Spectroscopy, Biophys. J., 2009, 97, 238–247 CrossRef CAS.
- T. Koyama, T. Iwata, A. Yamamoto, Y. Sato, D. Matsuoka, S. Tokutomi and H. Kandori, Different Role of the J alpha Helix in the Light-Induced Activation of the LOV2 Domains in Various Phototropins, Biochemistry, 2009, 48, 7621–7628 CrossRef CAS.
- M. A. Jones, K. A. Feeney, S. M. Kelly and J. M. Christie, Mutational analysis of phototropin 1 provides insights into the mechanism underlying LOV2 signal transmission, J. Biol. Chem., 2006, 282, 6405–6414 CrossRef.
- S. M. Harper, L. C. Neil and K. H. Gardner, Structural basis of a phototropin light switch, Science, 2003, 301, 1541–1544 CrossRef CAS.
- S. M. Harper, J. M. Christie and K. H. Gardner, Disruption of the LOV-Jalpha helix interaction activates phototropin kinase activity, Biochemistry, 2004, 43, 16184–16192 CrossRef CAS.
- X. Yao, M. K. Rosen and K. H. Gardner, Estimation of the available free energy in a LOV2-Ja photoswitch, Nat. Chem. Biol., 2008, 4, 491–497 CrossRef CAS.
- V. Buttani, A. Losi, T. Eggert, U. Krauss, K.-E. Jaeger, Z. Cao and W. Gärtner, Conformational analysis of the blue-light sensing protein YtvA reveals a competitive interface for LOV-LOV dimerization and interdomain interactions, Photochem. Photobiol. Sci., 2007, 6, 41–49 RSC.
- A. Möglich and K. Moffat, Structural Basis for Light-dependent Signaling in the Dimeric LOV Domain of the Photosensor YtvA, J. Mol. Biol., 2007, 373, 112–126 CrossRef.
- M. Nakasako, K. Zikihara, D. Matsuoka, H. Katsura and S. Tokutomi, Structural basis of the LOV1 dimerization of Arabidopsis phototropins 1 and 2, J. Mol. Biol., 2008, 381, 718–733 CrossRef CAS.
- B. D. Zoltowski and B. R. Crane, Light activation of the LOV protein Vivid generates a rapidly exchanging dimer, Biochemistry, 2008, 47, 7012–7019 CrossRef CAS.
- V. Buttani, W. Gärtner and A. Losi, NTP-binding properties of the blue-light receptor YtvA and effects of the E105L mutation, Eur. Biophys. J., 2007, 36, 831–839 CrossRef CAS.
- V. Buttani, A. Losi, E. Polverini and W. Gärtner, Blue news: NTP binding properties of the blue-light sensitive YtvA protein from Bacillus subtilis, FEBS Lett., 2006, 580, 3818–3822 CrossRef CAS.
- L. Aravind and E. V. Koonin, The STAS domain a link between anion transporters and antisigma-factor antagonists, Curr. Biol., 2000, 10, R53–R55 CrossRef CAS.
- D. P. McEwen, K. R. Gee, H. C. Kang and R. R. Neubig, Fluorescent BODIPY-GTP Analogs: Real-Time Measurement of Nucleotide Binding to G Proteins, Anal. Biochem., 2001, 291, 109–117 CrossRef CAS.
- V. Buttani, Struttura e funzione di recettori batterici di luce blu contententi i domini proteici LOV (Light, Oxygen and Voltage), Ph.D. Thesis, University of Parma, 2009.
- D. D. Leipe, Y. I. Wolf, E. V. Koonin and L. Aravind, Classification and evolution of P-loop GTPases and related ATPases, J. Mol. Biol., 2002, 317, 41–72 CrossRef CAS.
- A. Losi, B. Quest and W. Gärtner, Listening to the blue: the time-resolved thermodynamics of the bacterial blue-light receptor YtvA and its isolated LOV domain, Photochem. Photobiol. Sci., 2003, 2, 759–766 RSC.
- T. Bednarz, A. Losi, W. Gärtner, P. Hegemann and J. Heberle, Functional variations among LOV domains as revealed by FT-IR difference spectroscopy, Photochem. Photobiol. Sci., 2004, 3, 575–579 RSC.
- A. Losi, E. Ghiraldelli, S. Jansen and W. Gärtner, Mutational effects on protein structural changes and interdomain interactions in the blue-light sensing LOV protein YtvA, Photochem. Photobiol., 2005, 81, 1145–1152 CrossRef CAS.
- A. Losi, T. Kottke and P. Hegemann, Recording of Blue Light-Induced Energy and Volume Changes within the Wild-Type and Mutated Phot-LOV1 Domain from Chlamydomonas reinhardtii, Biophys. J., 2004, 86, 1051–1060 CrossRef CAS.
- A. Losi, E. Ternelli and W. Gärtner, Tryptophan Fluorescence in the Bacillus subtilis Phototropin-related Protein YtvA as a Marker of Interdomain Interaction, Photochem. Photobiol., 2004, 80, 150–153 CrossRef CAS.
- A. Yamamoto, T. Iwata, S. Tokutomi and H. Kandori, Role of Phe1010 in Light-Induced Structural Changes of the neo1-LOV2 Domain of Adiantum, Biochemistry, 2008, 47, 922–928 CrossRef CAS.
- A. Moglich, R. A. Ayers and K. Moffat, Design and Signaling Mechanism of Light-Regulated Histidine Kinases, J. Mol. Biol., 2009, 385, 1433–1444 CrossRef CAS.
- S. M. Najafi, D. A. Harris and M. D. Yudkin, The SpoIIAA protein of Bacillus subtilis has GTP-binding properties, J. Bacteriol., 1996, 178, 6632–6634 CAS.
- G. Schaaf, E. A. Ortlund, K. R. Tyeryar, C. J. Mousley, K. E. Ile, T. A. Garrett, J. Ren, M. J. Woolls, C. R. H. Raetz, M. R. Redinbo and V. A. Bankaitis, Functional anatomy of phospholipid binding and regulation of phosphoinositide homeostasis by proteins of the sec14 superfamily, Mol. Cell, 2008, 29, 191–206 CrossRef CAS.
- C. O'Donovan, M. J. Martin, A. Gattiker, E. Gasteiger, A. Bairoch and R. Apweiler, High-quality protein knowledge resource: SWISS-PROT and TrEMBL, Brief. Bioinform., 2002, 3, 275–284 CrossRef CAS.
| This journal is © The Royal Society of Chemistry and Owner Societies 2010 |
