Highly efficient synthesis of medium-sized lactones via oxidative lactonization: concise total synthesis of isolaurepan†
Makoto Ebine, Yuto Suga, Haruhiko Fuwa* and Makoto Sasaki
Laboratory of Biostructural Chemistry, Graduate School of Life Sciences, Tohoku University, 1-1 Tsutsumidori-amamiya, Aoba-ku, Sendai 981-8555, Japan. E-mail: hfuwa@bios.tohoku.ac.jp
First published on 26th October 2009
Abstract
A catalytic amount of TEMPO in the presence of PhI(OAc)2 effected oxidative lactonization of 1,6- and 1,7-diols, directly affording seven- and eight-membered lactones, respectively, in good yields.
Lactone is a common structural motif widely found in biologically active natural products and pharmaceuticals. In addition, a number of synthetic methods for functionalization of lactones are currently available, making them especially useful synthetic intermediates for the preparation of cyclic ethers.1 Thus, the development of practical synthetic methods for lactones continues to be an important and fundamental research in organic synthesis.2
A variety of methods for the synthesis of medium-sized lactones via the formation of the ester linkage have been reported, which generally involve activation of an ω-hydroxy acid precursor (Scheme 1, strategy A). However, ω-hydroxy acids are often prepared from differentially protected α,ω-diols via multi-step synthesis including oxidations and protective group manipulations. In contrast, the synthesis of lactones via oxidative lactonization of α,ω-diols represents a more direct and step-economical strategy due to the fact that oxidation and lactonization occur in a single flask and that protecting group chemistry is not necessary (Scheme 1, strategy B).3,4 In fact, there has been growing interest in oxidative lactonization of α,ω-diols in recent years. There are a number of precedents that describe oxidative lactonization of 1,4- and 1,5-diols, although most of the reported examples utilized meso-diols. In contrast, there are only a few specific examples for oxidative lactonization of seven- and eight-membered lactones5 presumably because of the increased enthalpic and entropic penalties associated with their formation.6 Thus, the development of a practical and efficient method for oxidative lactonization of α,ω-diols remains a significant challenge for organic chemists.
 | ||
| Scheme 1 Schematic presentation of lactonization strategies for the synthesis of medium-sized lactones | ||
Herein we report that oxidative lactonization of 1,6- and 1,7-diols using a catalytic amount of TEMPO and PhI(OAc)2 as stoichiometric oxidant7 proceeds efficiently to provide synthetically useful seven- and eight-membered lactones, respectively, in good yields.8 The remarkable efficiency of the TEMPO/PhI(OAc)2-mediated oxidative lactonization strategy was highlighted by its successful implementation to a concise total synthesis of (±)-isolaurepan.9,10
Piancatelli, Margarita, and co-workers have reported that TEMPO/PhI(OAc)2 oxidizes alcohols to carbonyl compounds in CH2Cl2 at room temperature.7 Moreover, primary alcohols can be selectively oxidized in the presence of secondary alcohols under these conditions. Forsyth et al. have reported the synthesis of δ-lactones by TEMPO/PhI(OAc)2 oxidation of 1,5-diols.3j Based on these precedents, we investigated the scope of the TEMPO/PhI(OAc)2-mediated oxidative lactonization8 by using various substrates with or without conformational constraint (Table 1). In contrast to the previous synthesis of 211 that relied on Yamaguchi lactonization12 of the corresponding hydroxy acid using a high-dilution technique, the TEMPO/PhI(OAc)2-mediated oxidative lactonization directly afforded 2 from 1,6-diol 1 in 93% yield under non-high-dilution conditions (0.1 M) (entry 1). Even under a higher concentration (0.3 M) and on a large scale, 2 was isolated in 83% yield after single recrystallization, and the formation of dimer or higher oligomers was not observed (entry 2). Hence we were able to synthesize >15 grams of 2 in a single experiment. Importantly, 2 is a versatile intermediate in the synthesis of marine polycyclic ethers.13 A variety of 1,6-diols 3, 5, 7, 9, 11, 13, and 15 could be cleanly oxidized under the TEMPO/PhI(OAc)2 conditions to afford the respective seven-membered lactones 4, 6, 8, 10, 12, 14a,b, and 164n in good to excellent yields (entries 3–10).‡ Oxidative lactonization of 1,6-diol 17 required some optimization. Treatment of 17 with 10 mol% of TEMPO and 2.5 equiv of PhI(OAc)2 in CH2Cl2 (0.1 M, room temperature) gave the desired lactone 18 in 40% yield (entry 11). Increasing both the amount of the reagents and the concentration of the reaction mixture was beneficial, giving 18 in 69% yield (entry 12). Thus, it seems that TEMPO/PhI(OAc)2-mediated oxidative lactonization is generally applicable to the synthesis of seven-membered lactones from 1,6-diols. We were pleased to find that oxidative lactonization of 1,7-diol 19 proceeded to afford eight-membered lactone 20 in good yield (entry 13), which should be useful as an intermediate for the synthesis of eight-membered unsaturated cyclic ether Laurencia metabolites, as exemplified by (+)-laurencin.14 However, 1,7-diol 21 did not give the corresponding eight-membered lactone; instead the hydroxy aldehyde 22 was isolated in 80% yield (entry 14).
| Entry | Diol | Lactone | Yield% |
|---|---|---|---|
| a TEMPO (10 mol%), PhI(OAc)2 (2.5 equiv), CH2Cl2 (0.1 M), room temperature.b TEMPO (20 mol%), PhI(OAc)2 (2.2 equiv), CH2Cl2 (0.3 M), room temperature.c TEMPO (10 mol%), PhI(OAc)2 (2.5 equiv), CH2Cl2 (0.5 M), room temperature.d TEMPO (30 mol%), PhI(OAc)2 (5 equiv), CH2Cl2 (0.5 M), room temperature. | |||
| 1a |  | 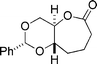 | 93 |
| 2b | 83 | ||
| 1 | 2 | ||
| 3a |  |  | 95 |
| 3 | 4 | ||
| 4a | 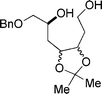 |  | 98 |
| 5 | 6 | ||
| 5a |  |  | 85 |
| 7 | 8 | ||
| 6a |  |  | 62 |
| 7c | 76 | ||
| 9 | 10 | ||
| 8a |  |  | 54 |
| 11 | 12 | ||
| 9a |  |  | 100 (14a:14b = 51![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 49) 49) |
| 13 | 14a | ||
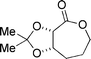 | |||
| 14b | |||
| 10a |  |  | 63 |
| 15 | 16 | ||
| 11a |  |  | 40 |
| 12d | 69 | ||
| 17 | 18 | ||
| 13a |  |  | 67 |
| 19 | 20 | ||
| 14a |  | 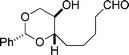 | 80 |
| 21 | 22 |
The effectiveness of our developed TEMPO/PhI(OAc)2-mediated oxidative lactonization strategy was demonstrated in a concise total synthesis of (±)-isolaurepan (23) (Scheme 2). The synthesis commenced with allylation of 1-heptanal to give homoallylic alcohol 24. Olefin cross-metathesis15,16 of 24 with 3-buten-1-ol afforded olefin 25 as a 1.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of E/Z isomers, which was hydrogenated to deliver diol 26 in 53% overall yield.17 Treatment of diol 26 with 10 mol% of TEMPO and 2.5 equiv of PhI(OAc)2 in CH2Cl2 (0.1 M) at room temperature directly afforded seven-membered lactone 27 in 73% yield. Introduction of a propyl side chain was achieved via the intermediacy of a lactone-derived enol phosphate. Thus, enolization of lactone 27 with KHMDS in the presence of (PhO)2P(O)Cl generated the corresponding enol phosphate, which without isolation was alkylated using an organocopper reagent.18 The resulting enol ether 28 was sensitive to hydrolysis during chromatographic purification. Thus, upon isolation, 28 was immediately treated with TMSOTf/Et3SiH to furnish (±)-isolaurepan (23) in 74% overall yield from 27 as a single diastereomer. The 1H, 13C NMR, and HRMS spectra of synthetic 23 matched those reported in the literature.9,10 The present total synthesis proceeded in only six steps from 1-heptanal with an overall yield of 29%, which constitutes the most concise and high-yielding synthesis hitherto reported.
1 mixture of E/Z isomers, which was hydrogenated to deliver diol 26 in 53% overall yield.17 Treatment of diol 26 with 10 mol% of TEMPO and 2.5 equiv of PhI(OAc)2 in CH2Cl2 (0.1 M) at room temperature directly afforded seven-membered lactone 27 in 73% yield. Introduction of a propyl side chain was achieved via the intermediacy of a lactone-derived enol phosphate. Thus, enolization of lactone 27 with KHMDS in the presence of (PhO)2P(O)Cl generated the corresponding enol phosphate, which without isolation was alkylated using an organocopper reagent.18 The resulting enol ether 28 was sensitive to hydrolysis during chromatographic purification. Thus, upon isolation, 28 was immediately treated with TMSOTf/Et3SiH to furnish (±)-isolaurepan (23) in 74% overall yield from 27 as a single diastereomer. The 1H, 13C NMR, and HRMS spectra of synthetic 23 matched those reported in the literature.9,10 The present total synthesis proceeded in only six steps from 1-heptanal with an overall yield of 29%, which constitutes the most concise and high-yielding synthesis hitherto reported.
 | ||
| Scheme 2 Total synthesis of (±)-isolaurepan. Reagents and conditions: (a) allylMgCl, THF, 0 °C; (b) 3-buten-1-ol, Grubbs’ 2nd-generation catalyst, CH2Cl2, 40 °C; (c) H2, Pd/C, EtOAc, room temperature, 53% (three steps); (d) TEMPO (10 mol%), PhI(OAc)2 (2.5 equiv), CH2Cl2 (0.1 M), room temperature, 73%; (e) KHMDS, (PhO)2P(O)Cl, HMPA, THF, −78 °C; then n-PrMgBr, CuI, Me2S, −30 °C; (f) TMSOTf, Et3SiH, CH2Cl2, 0 °C, 74% (two steps). | ||
In summary, we have developed an efficient method for the synthesis of medium-sized lactones based on the TEMPO/PhI(OAc)2-mediated oxidative lactonization of α,ω-diols, which is operationally simple and cost effective and proceeds cleanly even under high concentration conditions without the formation of dimer or higher oligomers. In addition, the TEMPO/PhI(OAc)2-oxidative lactonization strategy alleviates protective group chemistry as well as separate oxidation steps. These features highlight the efficiency and practicality of the oxidative lactonization strategy, being suitable even for multi-gram scale preparation of synthetically useful medium-sized lactones. The remarkable efficiency of the synthesis of (±)-isolaurepan demonstrates the power and usefulness of the oxidative lactonization strategy in the synthesis of medium-sized cyclic ethers.
Acknowledgements
We thank Mr. Kazuya Ishigai for his contributions to the early stage of this work. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan.Notes and references
- For examples, see:
(a) K. C. Nicolaou, G.–Q. Shi, J. L. Gunzner, P. Gartner and Z. Yang, J. Am. Chem. Soc., 1997, 119, 5467 CrossRef CAS
; (b) K. Tsushima, K. Araki and A. Murai, Chem. Lett., 1989, 1313 CAS
; (c) K. C. Nicolaou, D. G. McGarry, P. K. Somers, C. A. Veale and G. T. Furst, J. Am. Chem. Soc., 1987, 109, 2504 CrossRef CAS
; (d) R. W. Carling and A. B. Holmes, J. Chem. Soc., Chem. Commun., 1986, 565 RSC
.
- For recent reviews:
(a) H. M. C. Ferraz, F. I. Bombonato, M. K. Sano and L. S. Longo, Jr, Quim. Nova, 2008, 31, 885 CAS
; (b) I. Shiina, Chem. Rev., 2007, 107, 239 CrossRef CAS
; (c) A. Parenty, X. Moreau and J.-M. Campagne, Chem. Rev., 2006, 106, 911 CrossRef CAS
; (d) G. Rousseau, Tetrahedron, 1995, 51, 2777 CrossRef CAS
.
- Non-metal-catalyzed reactions:
(a) L. P. Kyrides and F. B. Zienty, J. Am. Chem. Soc., 1946, 68, 1385 CrossRef CAS
; (b) M. Fetizon, M. Golfier and J.-M. Louis, Tetrahedron, 1975, 31, 171 CrossRef CAS
; (c) T. Kageyama, S. Kawahara, K. Kitamura, Y. Ueno and M. Okawara, Chem. Lett., 1983, 1097 CAS
; (d) A. M. Horton and S. V. Ley, J. Organomet. Chem., 1985, 285, C17 CrossRef CAS
; (e) T. Miyazawa and T. Endo, J. Org. Chem., 1985, 50, 3930 CrossRef CAS
; (f) C. W. Jefford and Y. Wang, J. Chem. Soc., Chem. Commun., 1988, 634 RSC
; (g) S. Kondo, M. Ohira, S. Kawasoe, H. Kunisada and Y. Yuki, J. Org. Chem., 1993, 58, 5003 CrossRef CAS
; (h) S. Kondo, S. Kawasoe, H. Kunisada and Y. Yuki, Synth. Commun., 1995, 25, 719 CrossRef CAS
; (i) E. J. Corey and A. Palani, Tetrahedron Lett., 1995, 36, 3485 CrossRef CAS
; (j) T. M. Hansen, G. J. Florence, P. Lugo-Mas, J. Chen, J. N. Abrams and C. J. Forsyth, Tetrahedron Lett., 2003, 44, 57 CrossRef CAS
; (k) J. M. Schomaker, B. R. Travis and B. Borhan, Org. Lett., 2003, 5, 3089 CrossRef CAS
and references cited therein.
- Metal-catalyzed reactions:
(a) H. Tomioka, K. Takai, K. Oshima and H. Nozaki, Tetrahedron Lett., 1981, 22, 1605 CrossRef CAS
; (b) Y. Shvo, Y. Blum, D. Reshef and M. Menzin, J. Organomet. Chem., 1982, 226, C21 CrossRef CAS
; (c) Y. Tamaru, Y. Yamada, K. Inoue, Y. Yamamoto and Z. Yoshida, J. Org. Chem., 1983, 48, 1286 CrossRef CAS
; (d) Y. Ishii, K. Suzuki, T. Ikariya, M. Saburi and S. Yoshikawa, J. Org. Chem., 1986, 51, 2822 CrossRef CAS
; (e) S.-I. Murahashi, T. Naota, K. Ito, Y. Maeda and H. Taki, J. Org. Chem., 1987, 52, 4319 CrossRef CAS
; (f) I. Minami and J. Tsuji, Tetrahedron, 1987, 43, 3903 CrossRef CAS
; (g) Y. Ishii, T. Yoshida, K. Yamawaki and M. Ogawa, J. Org. Chem., 1988, 53, 5549 CrossRef CAS
; (h) R. Bloch and C. Brillet, Synlett, 1991, 829 CrossRef CAS
; (i) K. Nozaki, M. Yoshida and H. Takaya, J. Organomet. Chem., 1994, 473, 253 CrossRef CAS
; (j) I. Isaac, G. Aizel, I. Stasik, A. Wadouachi and D. Beaupère, Synlett, 1998, 475 CrossRef CAS
; (k) T. Nishimura, T. Onoue, K. Ohe and S. Uemura, J. Org. Chem., 1999, 64, 6750 CrossRef CAS
; (l) T. Suzuki, K. Morita, M. Tsuchida and K. Hiroi, Org. Lett., 2002, 4, 2361 CrossRef CAS
; (m) J. Zhao and J. F. Hartwig, Organometallics, 2005, 24, 2441 CrossRef CAS
; (n) M. Ito, A. Osaku, A. Shiibashi and T. Ikariya, Org. Lett., 2007, 9, 1821 CrossRef CAS
and references cited therein.
-
(a) S. Kajigaeshi, T. Nakagawa, N. Nagasaki, H. Yamasaki and S. Fujisaki, Bull. Chem. Soc. Jpn., 1986, 59, 747 CAS
; (b) Y. H. Hu, L. G. Ou, X. L. Wang and D. L. Bai, Chin. Chem. Lett., 1999, 10, 281 CAS
; (c) F. F. Bamoharram, M. M. Heravi, M. Roshani, A. Gharib and M. Jahangir, J. Mol. Catal. A: Chem., 2006, 252, 90 CrossRef CAS
.
- G. Illuminati and L. Mandolini, Acc. Chem. Res., 1981, 14, 95 CrossRef CAS
.
- A. D. Mico, R. Margarita, L. Parlanti, A. Vescovi and G. Piancatelli, J. Org. Chem., 1997, 62, 6974 CrossRef CAS
.
- Synthesis of six-membered lactones via the TEMPO/PhI(OAc)2-mediated oxidative lactonization has been reported by Forsyth et al. See ref. 3j. We have reported an isolated example of TEMPO/PhI(OAc)2-mediated oxidative lactonization. See: M. Ebine, H. Fuwa and M. Sasaki, Org. Lett., 2008, 10, 2275 Search PubMed
.
- A. Fukuzawa and T. Masamune, Tetrahedron Lett., 1981, 22, 4081 CrossRef CAS
.
-
(a) H. Kotsuki, Y. Ushio, I. Kadota and M. Ochi, J. Org. Chem., 1989, 54, 5153 CrossRef CAS
; (b) M. J. Davies and C. J. Moody, J. Chem. Soc., Perkin Trans. 1, 1991, 9 RSC
; (c) R. W. Carling, J. S. Clark and A. B. Holmes, J. Chem. Soc., Perkin Trans. 1, 1992, 83 RSC
; (d) M. C. Carreno, R. D. Mazery, A. Urbano, F. Colobert and G. Solladie, Org. Lett., 2004, 6, 297 CrossRef CAS
; (e) K. R. Prasad and P. Anbarasan, Tetrahedron: Asymmetry, 2007, 18, 1419 CrossRef CAS
; (f) D. Tripathi and P. Kumar, Tetrahedron Lett., 2008, 49, 7012 CrossRef CAS
; (g) D. Tripathi, S. K. Pandey and P. Kumar, Tetrahedron, 2009, 65, 2226 CrossRef CAS
.
- M. Sasaki, H. Fuwa, M. Ishikawa and K. Tachibana, Org. Lett., 1999, 1, 1075 CrossRef CAS
.
- J. Inanaga, K. Hirata, H. Saeki, T. Katsuki and M. Yamaguchi, Bull. Chem. Soc. Jpn., 1979, 52, 1989 CAS
.
-
(a) M. Sasaki, M. Ishikawa, H. Fuwa and K. Tachibana, Tetrahedron, 2002, 58, 1889 CrossRef CAS
; (b) C. Tsukano, M. Ebine and M. Sasaki, J. Am. Chem. Soc., 2005, 127, 4326 CrossRef CAS
; (c) M. Sasaki, S. Honda, T. Noguchi, H. Takakura and K. Tachibana, Synlett, 2000, 838 CAS
; (d) I. Kadota, H. Takamura, K. Sato and Y. Yamamoto, J. Org. Chem., 2002, 67, 3494 CrossRef CAS
.
- A. F. Cameron, K. K. Cheung, G. Ferguson and J. M. Robertson, J. Chem. Soc., Chem. Commun., 1965, 638 RSC
.
- S. J. Connon and S. Blechert, Angew. Chem., Int. Ed., 2003, 42, 1900 CrossRef
.
- M. Scholl, S. Ding, C. W. Lee and R. H. Grubbs, Org. Lett., 1999, 1, 953 CrossRef CAS
.
- N. Chida, N. Sakata, K. Murai, T. Tobe, T. Nagase and S. Ogawa, Bull. Chem. Soc. Jpn., 1998, 71, 259 CAS
.
- Alkylation of lactone-derived enol triflates by organocuprates has been reported: K. Fujiwara, D. Awakura, M. Tsunashima, A. Nakamura, T. Honma and A. Murai, J. Org. Chem., 1999, 64, 2616 Search PubMed
. See also ref. 1(b).
Footnotes |
| † Electronic supplementary information (ESI) available: Representative experimental procedure and spectroscopic data for all newly synthesized products. See DOI: 10.1039/b919673k |
| ‡ For oxidative lactonization of 9, we have also evaluated other oxidation reagents such as Ag2CO3 on Celite, PCC, TPAP/NMO, Dess–Martin periodinane, and IBX and found that TEMPO/PhI(OAc)2 is far superior to these oxidants. |
| This journal is © The Royal Society of Chemistry 2010 |
