Impregnated copper on magnetite: an efficient and green catalyst for the multicomponent preparation of propargylamines under solvent free conditions
María J. Aliaga, Diego J. Ramón* and Miguel Yus
Instituto de Síntesis Orgánica (ISO), and Departamento de Química Orgánica, Facultad de Ciencias, Universidad de Alicante Apdo. 99, E-03080-, Alicante, Spain. E-mail: djramon@ua.es; Fax: +34-965903549; Tel: +34-965903986
First published on 16th November 2009
Abstract
Impregnated copper on magnetite is a versatile, inexpensive and simple catalyst for the selective multicomponent reaction of terminal alkynes, aldehydes and secondary amines to give the corresponding propargylamines with excellent yields. The catalyst can be easily recovered and reused by using a simple magnet. The process could be repeated up to ten times without losing its activity.
It is widely acknowledged that there is a growing need for more environmentally acceptable processes in the chemical industry, the new paradigms focusing more on the elimination (no formation) of wastes, as well as on avoiding the use of toxic and/or hazardous reagents and solvents.1 Multicomponent reactions2 have great potential in this area, since in just one step several reagents are added and create a new molecule with at least two more bonds, decreasing the whole environmental impact for the synthesis of the same product by a sequential pathway.
Among all known multicomponent reactions, the acetylene-Mannich reaction3,4 is an interesting approach to the synthesis of propargylamines, which have found broad application as precursors of different nitrogen-containing compounds, such as allylamines, pyrrolidines, oxazoles, pyrroles, as well as intermediates in the synthesis of different natural products including dynemicins, pharmaceuticals, herbicides and fungicides. Some of them have even been tested in the treatment of Parkinson's5 and Alzheimer's6 diseases.
Different complexes and salts of transition metals, such as iron,7 zinc,8 ruthenium–copper,9 silver,10 indium,11 iridium,12 gold,13 and mercury,14 have been employed for the multicomponent acetylene-Mannich reaction. However, the copper derivatives are most often employed. Although the use of homogenous copper catalysts15 have shown their potential activity, their loss at the end of the reaction decreased their utility, at least for industrial purposes. Different strategies have been followed using copper catalysts, including the use of ionic liquids,16 immobilization on organic–inorganic hybrid materials,17 the use of nano-particles18 and impregnation in inorganic materials.19 Very recently, a magnetically separable copper ferrite nanoparticle20 has been proposed as an alternative to previous catalysts. These results prompted us to present our independent results using impregnated copper on magnetite.
Magnetite is an old material with interesting new catalytic properties,21 which has been used generally as an inert support for other catalysts. One important way to prepare these catalysts is by impregnation, which could be easily performed by basic precipitation–adsorption of aqueous solutions of different salts, such as cobalt, rhodium, palladium and platinum22 as well as ruthenium23 on the surface of either pre-formed or commercially available magnetite.
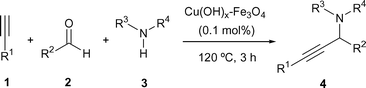
In this communication we would like to present the use for the first time of a new impregnated copper on magnetite catalyst as an efficient and green catalyst for the multicomponent acetylene-Mannich reaction. Initially, the reaction outlined in Table 1 was used as the standard one for the optimization of the reaction conditions, using nearly equimolecular amounts of all three reagents.
| |||||
|---|---|---|---|---|---|
| Entry | Catalyst (mol%) | Solvent | T/°C | t/h | Yield 4a (%)a |
| a Isolated yields after column chromatography (silica gel: hexane–ethyl acetate).b Magnetite size <5 μm.c Magnetite size 20–30 nm. | |||||
| 1 | Fe3O4b(42) | PhMe | 90 | 96 | 0 |
| 2 | — | PhMe | 150 | 72 | 40 |
| 3 | Fe3O4b(42) | PhMe | 150 | 72 | 45 |
| 4 | Fe3O4c(42) | PhMe | 150 | 72 | 71 |
| 5 | Ru(OH)x–Fe3O4 (3) | PhMe | 150 | 72 | 42 |
| 6 | Cu(OH)x–Fe3O4 (2.1) | PhMe | 150 | 16 | >99 |
| 7 | Cu(OH)x–Fe3O4 (2.1) | PhMe | 120 | 16 | >99 |
| 8 | Cu(OH)x–Fe3O4 (2.1) | PhMe | 80 | 19 | 10 |
| 9 | Cu(OH)x–Fe3O4 (0.1) | PhMe | 120 | 16 | >99 |
| 10 | Cu(OH)x–Fe3O4 (0.05) | PhMe | 120 | 72 | 61 |
| 11 | Cu(OH)x–Fe3O4 (0.1) | Dioxane | 120 | 16 | 63 |
| 12 | Cu(OH)x–Fe3O4 (0.1) | Et2O | 120 | 16 | 64 |
| 13 | Cu(OH)x–Fe3O4 (0.1) | AcOEt | 120 | 16 | 63 |
| 14 | Cu(OH)x–Fe3O4 (0.1) | MeCN | 120 | 16 | 72 |
| 15 | Cu(OH)x–Fe3O4 (0.1) | H2O | 120 | 16 | 29 |
| 16 | Cu(OH)x–Fe3O4 (0.1) | — | 120 | 0.45 | >99 |
The reaction using commercial magnetite did not produce the expected compound at 90 °C after several days. However, the reaction gave the expected product 4a in a moderated yield at 150 °C after three days, without catalyst,3 with the presence of commercial magnetite not improving the result (Table 1, entries 2 and 3). The use of commercial nano-powder magnetite improved the result slightly. Once the possible activity of the support was tested, we prepared two impregnated metal catalysts. The result with ruthenium (3.0 mol%) was clearly disappointing. However, the same reaction using the related copper catalyst† (2.1 mol%) gave compound 4a in practically quantitative yield (Table 1, entry 6). After finding the best catalyst other parameters for the reaction were optimized, such as temperature (entries 6–8), amount of catalyst (entries 9 and 10), and solvent (entries 11–16), with the optimal conditions being the use of 0.1 mol% of copper catalyst under solvent free conditions at 120 °C (Table 1, entry 16).
Once the catalytic activity of catalyst was proven, the scope of the reaction was tested (Table 2), finding excellent results for the reaction independently of the nature of substituents of the alkyne 1, the secondary amine 3, and nature of the aldehyde. The only exception was the reaction with 2-hydroxybenzaldehyde, in which the result was a modest 39% (Table 2, entry 5) and it should be pointed out that the reaction failed using primary amines.
| ||||||
|---|---|---|---|---|---|---|
| Entry | R1 | R2 | R3 | R4 | 4 | Yield (%)a |
a Isolated yields after column chromatography (silica gel: hexane–ethyl acetate.b 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 diastereomeric ratio 5 diastereomeric ratio | ||||||
| 1 | Ph | Ph | (CH2)5 | 4a | >99 | |
| 2 | Me(CH2)3 | Ph | (CH2)5 | 4b | >99 | |
| 3 | Ph(CH2)2 | Ph | (CH2)5 | 4c | >99 | |
| 4 | Ph | 4-ClC6H4 | (CH2)5 | 4f | >99 | |
| 5 | Ph | 2-HOC6H4 | (CH2)5 | 4g | 39 | |
| 6 | Ph | 3,4,5-(MeO)3C6H2 | (CH2)5 | 4h | >99 | |
| 7 | Ph | (CH2)5CH | (CH2)5 | 4i | >99 | |
| 8 | Ph | Ph | (CH2)2O(CH2)2 | 4j | >99 | |
| 9 | Ph | Ph | (CH2)4 | 4k | 92 | |
| 10 | Ph | Ph | (S)-(CH2)3CHCH2OH | 4l | 81b | |
| 11 | Ph | Ph | H | nBu | 4m | 0 |
| 12 | Ph | Ph | nBu | nBu | 4n | 93 |
When the reaction was performed using ketones (Scheme 1), the yields were significantly lower with the reaction times being longer.
 | ||
| Scheme 1 Acetylene-Mannich reaction using ketones. | ||
The former results imply that the catalyst is selective for the reaction with aldehydes in the presence of ketones. In fact the reaction of equimolecular amounts of compounds 1a, 3a, benzaldehyde (2a) and acetophenone (5b) only rendered the compound 4a in 85% yield after two days of reaction time.
Once the catalytic activity of the impregnated copper on magnetite was demonstrated, we approached the problem of the reuse, finding that the chemical yields were practically constant in a range between 94 and >99%, after 10 cycles of reaction, for the preparation of compound 4a (Fig. 1), with the catalyst being maintained inside the flask by the help of a magnet.
 | ||
| Fig. 1 Yields of compound 4a after different numbers of cycles. | ||
In order to explore the possible degradation of the catalyst under the reaction conditions, the BET surface area was determined at the beginning of the process and after 10 cycles (8.76 m2g−1), being similar in both cases, which could be interpreted as showing that there is no significant sintering process under the assayed reaction conditions. A similar result could be extracted from the observation of TEM microscopic images, which did not show any difference between the initial catalyst and the 10-fold reused one (Fig. 2).
 | ||
| Fig. 2 TEM microscopy images of catalyst after (left) and before using 10 times (right). | ||
To finish with the study on the stability of the catalyst, we analysed the liquid phase obtained after one cycle of the reaction for the preparation of compound 4a. The catalyst was isolated by using a magnet and washed with ethyl acetate; the resulting mixture without filtration was concentrated and re-dissolved in methanol. The flame atomic absorption spectroscopy (FAAS) showed the presence of around 0.0058 mg of iron in the methanolic solution (less than 0.006% of the initial magnetite added) as well as copper 0.0083 mg (0.6% of the initial amount). However, if prior to the concentration, a simple filtration is done, the FAAS did not show the presence of iron atoms and only 0.2% of the initial copper, excluding the presence of high concentrations of homogeneous species, with the FAAS results showing the capacity of the magnet used to trap the nanoparticles.
In conclusion, impregnated copper on magnetite is an excellent catalyst for the multicomponent acetylene-Mannich reaction between terminal alkynes, secondary amines, and aldehydes. The reaction has a very high atom efficiency,24 since the only by-product of the reaction is water. The absence of solvent implies both a very low energetic cost and very low environmental impact. All these facts, together with the simplicity of the protocol, the wide scope of substrates and their simple recycling permitted us to anticipate a good future for this process hereby documented not only in academia but also in industry.
Acknowledgements
This work was supported by the Spanish Ministerio de Ciencia y Tecnología (Consolider Ingenio 2010 CSD2007-00006, CTQ2007-65218/BQU). M. J. A. thanks the Consolider Ingenio program for a fellowship.Notes and references
- For recent reviews, see: (a) R. A. Sheldon, Green Chem., 2005, 7, 267–278 RSC; (b) R. Noyori, Chem. Commun., 2005, 1807–1811 RSC; (c) M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Ind. Ecol., 2008, 12, 316–328 CrossRef CAS; (d) R. A. Sheldon, Chem. Commun., 2008, 3352–3365 RSC.
- For recent reviews, see: (a) Multicomponent Reactions, ed. J. Zhu, H. Bienaymé, Wiley-VCH, Weinheim, 2005 Search PubMed; (b) D. J. Ramón and M. Yus, Angew. Chem., Int. Ed., 2005, 44, 1602–1634 CrossRef CAS; (c) A. Dömling, Chem. Rev., 2006, 106, 17–89 CrossRef; (d) F. Liéby-Muller, C. Simon, T. Constantieux and J. Rodriguez, QSAR Comb. Sci., 2006, 25, 432–438 Search PubMed; (e) G. Guillena, D. J. Ramón and M. Yus, Tetrahedron: Asymmetry, 2007, 18, 693–700 CrossRef CAS; (f) D. M. D'Souza and T. J. J. Müller, Chem. Soc. Rev., 2007, 36, 1095–1108 RSC; (g) L. F. Tietze, T. Kinzel and C. C. Brazel, Acc. Chem. Res., 2009, 42, 367–378 CrossRef CAS.
- For the first multicomponent reaction between aldehydes, amines and 1-alkynes, see: C. Mannich and F. T. Fu, Ber. Dtsch. Chem. Ges., 1933, 66b, 418–420 Search PubMed.
- For reviews, see: (a) C. Wei, Z. Li and C.-J. Li, Synlett, 2004, 1472–1483 CAS; (b) L. Zani and C. Bolm, Chem. Commun., 2006, 4263–4275 RSC; (c) V. V. Kouznetsov and L. Y. Vargas Méndez, Synthesis, 2008, 491–506 CrossRef CAS.
- J. J. Chen, D. M. Swope and K. Dashtipour, Clin. Ther., 2007, 29, 1825–1849 CrossRef CAS.
- M. Naoi, W. Maruyama, M. Shamoto-Nagai, H. Yi, Y. Akao and M. Tanaka, Mol. Neurobiol., 2005, 31, 81–93 Search PubMed.
- W.-W. Chen, R. V. Nguyen and C.-L. Li, Tetrahedron Lett., 2009, 50, 2895–2898 CrossRef CAS.
- (a) L. Zani, T. Eichhorn and C. Bolm, Chem.–Eur. J., 2007, 13, 2587–2600 CrossRef CAS; (b) E. Ramu, R. Varala, N. Sreelatha and S. R. Adapa, Tetrahedron Lett., 2007, 48, 7184–7190 CrossRef CAS.
- C. J. Li and C. Wei, Chem. Commun., 2002, 268–269 RSC.
- (a) C. Wei, Z. Li and C. J. Li, Org. Lett., 2003, 5, 4473–4475 CrossRef CAS; (b) L. Zhang, C. Wei, R. S. Varma and C.-J. Li, Tetrahedron Lett., 2004, 45, 2443–2446 CrossRef CAS; (c) Y. Zhang, A. M. Santos, E. Herdtweck, J. Mink and F. E. Kuhn, New J. Chem., 2005, 29, 366–370 RSC; (d) K. M. Reddy, N. S. Babu, I. Suryanarayana, P. S. S. Prasad and N. Lingaiah, Tetrahedron Lett., 2006, 47, 7563–7566 CrossRef CAS; (e) R. Maggi, A. Bello, C. Oro, G. Sartori and L. Soldi, Tetrahedron, 2008, 64, 1435–1439 CrossRef CAS; (f) P. Li, L. Wang, Y. Zhang and M. Wang, Tetrahedron Lett., 2008, 49, 6650–6654 CrossRef CAS.
- Y. Zhang, P. Li, M. Wang and L. Wang, J. Org. Chem., 2009, 74, 4364–4367 CrossRef CAS.
- (a) S. Sakaguchi, T. Kubo and Y. Ishii, Angew. Chem., Int. Ed., 2001, 40, 2534–2536 CrossRef CAS; (b) S. Sakaguchi, T. Mizuta, M. Furuwan, T. Kubo and Y. Ishii, Chem. Commun., 2004, 1638–1639 RSC.
- (a) C. Wei and C. J. Li, J. Am. Chem. Soc., 2003, 125, 9584–9585 CrossRef CAS; (b) M. L. Kantam, B. V. Prakash, C. R. V. Reddy and B. Sreedhar, Synlett, 2005, 2329–2332 CrossRef CAS; (c) V. K.-Y. Lo, Y. Liu, M.-K. Wong and C.-M. Che, Org. Lett., 2006, 8, 1529–1532 CrossRef CAS; (d) B. Huang, X. Yao and C.-J. Li, Adv. Synth. Catal., 2006, 348, 1528–1532 CrossRef CAS; (e) M. Kidwai, V. Bansal, A. Kumar and S. Mozumdar, Green Chem., 2007, 9, 742–745 RSC; (f) X. Zhang and A. Corma, Angew. Chem., Int. Ed., 2008, 47, 4358–4361 CrossRef CAS; (g) P. Oña-Burgos, I. Fernández, L. Roces, L. Torre Fernández, S. García-Granda and F. López Ortiz, Organometallics, 2009, 28, 1739–1747 CrossRef CAS; (h) V. K.-Y. Lo, K. K.-Y. Kung, M.-K. Wong and C.-M. Che, J. Organomet. Chem., 2009, 694, 583–591 CrossRef CAS.
- L. P. Hua and W. Lei, Chin. J. Chem., 2005, 23, 1646–1080 CrossRef.
- For selected examples, see: (a) H. Z. S. Huma, R. Halder, S. S. Kalra, J. Das and J. Iqbal, Tetrahedron Lett., 2002, 43, 6485–6488 CrossRef CAS; (b) N. Gommermann, C. Koradin, K. Polborn and P. Knochel, Angew. Chem., Int. Ed., 2003, 42, 5763–5766 CrossRef CAS; (c) Y. Ju, C.-J. Li and R. S. Varma, QSAR Comb. Sci., 2004, 23, 891–894 Search PubMed (erratum: QSAR Comb. Sci., 2005, 24, 319); (d) L. Shi, Y.-Q. Wu, M. Wang, F.-M. Zhang and C.-A. Fan, Org. Lett., 2004, 6, 1001–1003 CrossRef CAS; (e) B. Sreedhar, P. S. Reddy, B. V. Prakash and A. Ravindra, Tetrahedron Lett., 2005, 46, 7019–7022 CrossRef CAS; (f) N. Gommermann and P. Knochel, Chem.–Eur. J., 2006, 12, 4380–4392 CrossRef CAS; (g) F. Colombo, M. Benaglia, S. Orlandi and F. Usuelli, J. Mol. Catal. A: Chem., 2006, 260, 128–134 CrossRef CAS; (h) A. Bisai and V. K. Singh, Org. Lett., 2006, 8, 2405–2408 CrossRef CAS; (i) J. V. Madhav, B. S. Kuarm, P. Someshwar, B. Rajitha, Y. T. Reddy and P. A. Crooks, Synth. Commun., 2008, 38, 3215–3223 CrossRef CAS; (j) Z. Shao, X. Pu, X. Li, B. Fan and A. S. C. Chan, Tetrahedron: Asymmetry, 2009, 20, 225–229 CrossRef CAS.
- S. B. Park and H. Alper, Chem. Commun., 2005, 1315–1317 RSC.
- (a) B. Sreedhar, P. S. Reddy, C. S. V. Krishna and P. V. Babu, Tetrahedron Lett., 2007, 48, 7882–7886 CrossRef CAS; (b) P. Li and L. Wang, Tetrahedron, 2007, 63, 5455–5459 CrossRef CAS; (c) P. R. Likhar, S. Roy, M. Roy, M. S. Subhas, M. L. Kantam and R. L. De, Synlett, 2007, 2301–2303 CrossRef CAS; (d) M. Wang, P. Li and L. Wang, Eur. J. Org. Chem., 2008, 2255–2261 CrossRef CAS.
- (a) M. Kidwai, V. Bansal, N. K. Mishra, A. Kumar and S. Mozumdar, Synlett, 2007, 1581–1584 CrossRef CAS; (b) M. L. Kantam, S. Laha, J. Yadav and S. Bhargava, Tetrahedron Lett., 2008, 49, 3083–3086 CrossRef CAS.
- (a) G. W. Kabalka, L. Wang and R. M. Pagni, Synlett, 2001, 676–678 CAS; (b) B. M. Choudary, C. Sridhar, M. L. Kantam and B. Sreedhar, Tetrahedron Lett., 2004, 45, 7319–7321 CrossRef CAS; M. K. Patil, M. Keller, B. M. Reddy, P. Pale and J. Sommer, Eur. J. Org. Chem., 2008, 4440–4445 CrossRef CAS.
- M. L. Kantam, J. Yadav, S. Laha and S. Jha, Synlett, 2009, 1791–1794 CrossRef CAS.
- (a) R. Martínez, D. J. Ramón and M. Yus, Adv. Synth. Catal., 2008, 350, 1235–1240 CrossRef CAS; (b) R. Martínez, D. J. Ramón and M. Yus, Org. Biomol. Chem., 2009, 7, 2176–2181 RSC.
- (a) Y. Usami, K. Kagawa, M. Kawazoe, Y. Matsumura, H. Sakurai and M. Haruta, Appl. Catal., A, 1998, 171, 123–130 CrossRef CAS; (b) Z. Wang, B. Shen, Z. Aihura and N. He, Chem. Eng. J., 2005, 113, 27–34 CrossRef CAS; (c) A. Uheida, G. Salazar-Alvarez, E. Björkman, Z. Yu and M. Muhammed, J. Colloid Interface Sci., 2006, 298, 501–507 CrossRef CAS; (d) A. Uheida, M. Iglesias, C. Fontàs, M. Hidalgo, V. Salvadó, Y. Zhang and M. Muhammed, J. Colloid Interface Sci., 2006, 301, 402–408 CrossRef CAS.
- (a) M. Kotani, T. Koike, K. Yamaguchi and N. Mizuno, Green Chem., 2006, 8, 735–741 RSC; (b) F. Shi, M. K. Tse, S. Zhou, M.-M. Pohl, J. Radnik, S. Hübner, K. Jähnisch, A. Brückner and M. Beller, J. Am. Chem. Soc., 2009, 131, 1775–1779 CrossRef CAS.
- (a) R. Martínez, G. J. Brand, D. J. Ramón and M. Yus, Tetrahedron Lett., 2005, 46, 3683–3686 CrossRef CAS; (b) R. Martínez, D. J. Ramón and M. Yus, Tetrahedron, 2006, 62, 8982–8987 CrossRef CAS; (c) R. Martínez, D. J. Ramón and M. Yus, Tetrahedron, 2006, 62, 8988–9001 CrossRef CAS.
Footnote |
| † Procedure for the preparation of copper catalyst: To a stirred solution of CuCl2 (0.13 g, 1 mmol) in deionised water (120 mL) was added Fe3O4 (4 g, 17 mmol, powder < 5 μm, BET area: 9.86 m2g−1). After 10 min at room temperature, the mixture was slowly basified with NaOH 1 M until pH around 13. The resulting basified mixture was stirred during approximately 24 h and then filtrated under vacuum. The solid catalyst was washed several times with deionised water (3 × 50 mL) and dried during three days at room temperature (BET area: 9.15 m2g−1). Incorporation of 1.37% of Cu according to X-ray fluorescence. |
| This journal is © The Royal Society of Chemistry 2010 |