On the importance of simultaneous infrared/fiber-optic temperature monitoring in the microwave-assisted synthesis of ionic liquids†‡
David Obermayer and C. Oliver Kappe*
Christian Doppler Laboratory for Microwave Chemistry (CDLMC) and Institute of Chemistry, Karl-Franzens-University Graz, Heinrichstrasse 28, A-8010, Graz, Austria. E-mail: oliver.kappe@uni-graz.at; Fax: +43 316 3809840; Tel: +43 316 3805352
First published on 24th November 2009
Abstract
The temperature profiles obtained from both an external infrared and internal fiber-optic sensor were compared for heating and synthesizing the ionic liquid 1-butyl-3-methylimidazolium bromide (bmimBr) under microwave conditions. Utilizing a single-mode microwave reactor that allows simultaneous infrared/fiber-optic temperature measurements, significant differences between the two methods of temperature monitoring were revealed. Due to the strong microwave absorptivity of ionic liquids and the delay experienced in monitoring temperature on the outer surface of a heavy-walled glass vial, external infrared temperature sensors can not be used to accurately control the temperature in the heating of ionic liquids under microwave conditions. The use of internal fiber-optic probes allows the monitoring and control of the heating behavior in a much better way. In order to prevent the strong exotherm in the synthesis of bmimBr under microwave conditions the use of a reaction vessel made out of silicon carbide is the method of choice. Because of the high thermal conductivity and effusivity of silicon carbide, the heat generated during the ionic liquid formation is efficiently exchanged with the comparatively cool air in the microwave cavity via the silicon carbide ceramic.
Introduction
High-speed microwave chemistry has attracted a considerable amount of attention in the past two decades with new and innovative applications in organic/peptide synthesis, polymer chemistry, material sciences, nanotechnology and biochemical processes continually being reported in the literature.1 In many instances, the use of microwave dielectric heating has been shown to dramatically reduce processing times, increase product yields, and to enhance product purities or material properties compared to conventionally processed experiments.1 The initial slow uptake of the technology in the late 1980s and 1990s can be attributed to its lack of controllability and reproducibility, coupled with a general lack of understanding of the basics of microwave dielectric heating. Although many of the early pioneering experiments in microwave chemistry were performed in domestic, sometimes modified, kitchen microwave ovens, the current trend clearly is to use dedicated instruments (both single-mode and multimode reactors) for chemical synthesis which have become available only in the last few years.1,2 Today's commercially available microwave reactors feature built-in magnetic stirrers, direct temperature control of the reaction mixture, and software that enables on-line temperature/pressure control by regulation of microwave power output.2 By coupling the feedback from a suitable temperature probe to the modulation of magnetron output power the reaction mixture can be heated and kept at the pre-selected temperature (“temperature control mode”). This process requires a reliable way of rapidly monitoring the reaction temperature online during the microwave irradiation process. In single-mode microwave reactors the reaction temperature is generally determined by a calibrated external infrared (IR) sensor integrated into the cavity that detects the surface temperature of the reaction vessel from a predefined distance. It is assumed that the measured temperature on the outside of the reaction vessel will correspond to the temperature of the reaction mixture contained inside. Unfortunately, this is not always the case and extreme care must be taken relying on these data.2–5 In particular, it should be emphasized that the IR sensor needs some time until it reflects the genuine internal reaction temperature. This is due to the fact that reaction vessels for use in single-mode microwave reactors are typically made out of borosilicate glass, an excellent thermal insulator. Since the vessel wall needs to be comparatively thick to be able to withstand internal reaction pressures of up to 30 bar, it will take some time for the vessel to be warmed “from the inside” by microwave dielectric heating of its polar contents. Although this delay is typically only in the order of a few seconds, it may be enough to lead to an undetected overshooting of the internal reaction temperature.5A more accurate way of monitoring temperature in a microwave chemistry experiment is to directly determine the temperature of the reaction mixture by an internal fiber-optic (FO) sensor.2–5 FO probes are more accurate and faster responding than IR sensors but are far more expensive and their use is not without complications either. This is in part due to the fact that the mechanically sensitive sensor crystal needs to be protected, requiring the use of appropriate protective immersion wells for the fiber-optic probes. This increases the lifetime of the probe but slows down the response time.5 In addition, recent evidence suggests that the use of one single fiber-optic probe may not suffice to truthfully represent the temperature profile of a microwave heated reaction mixture.5 If efficient stirring/agitation can not be ensured, temperature gradients may develop as a consequence of inherent field inhomogeneities inside a single-mode microwave cavity.5,6
It therefore appears that the simultaneous monitoring of both external IR and internal FO temperature in a microwave chemistry experiment can have significant advantages compared to relying on the output of only one of these sensors. Herein we present a case study highlighting the importance of dual IR–FO temperature measurements in the preparation of imidazolium-based ionic liquids.
Results and discussion
A. Ionic liquids and microwaves
Ionic liquids (ILs) are a new class of solvents that are entirely constituted of ions. They usually consist of an organic cation (mainly a quaternary nitrogen) and an inorganic or organic anion and either are liquids at room temperature or have melting points below 100 °C.7 ILs generally have negligible vapor pressure and are immiscible with common non-polar solvents, meaning that organic products can be easily removed by extraction and the ionic liquid can be recycled. In addition they have a wide accessible temperature range (typically >300 °C), a low toxicity and are non-flammable. Due to these advantages they have attracted much recent attention as environmentally benign solvents.7 From the perspective of microwave chemistry one of the points of key importance is their high polarity which is variable, depending on the cation and anion and hence can effectively be tuned to a particular application. Ionic liquids interact very efficiently with microwaves through the ionic conduction mechanism8 and are rapidly heated at rates easily exceeding 10 °C per second.9–13 Although few reports on the exact measurement of their dielectric properties and loss tangent values (tanδ) exist,11,12 the experimentally attained heating rates of ILs applying microwave irradiation attest to their extremely high microwave absorptivity.4,9,10The standard preparation of ILs by alkylation of nitrogen-containing, e.g., heterocyclic starting materials with an appropriate alkyl halide can easily be performed using microwave technology.13 However, under microwave conditions, these transformations are notoriously difficult to control since these N-alkylations are not only generally exothermic, but in addition the microwave absorptivity during the process changes significantly from moderate (starting materials) to high (ionic liquid).12,14 This combination represents a serious challenge in terms of accurate temperature control for even the most sophisticated microwave reactors available today. Additional complications can arise from phase and viscosity changes during these processes. As a model transformation for our studies on the significance of simultaneous IR–FO temperature control in microwave chemistry, the preparation of 1-butyl-3-methylimidazolium bromide (bmimBr) by alkylation of N-methylimidazole with butyl bromide under solvent free conditions was chosen (Scheme 1).14
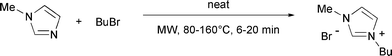 | ||
| Scheme 1 Microwave-assisted preparation of the ionic liquid bmimBr. | ||
B. Microwave heating of bmimBr
Before investigating the actual synthesis of the ionic liquid bmimBr as displayed in Scheme 1, we studied the heating characteristic of this IL under a variety of conditions using different microwave reactors and temperature monitoring methods. Our initial experiments were conducted in a Biotage Initiator EXP 2.5 single-mode reactor using external IR temperature control (400 W maximum magnetron output power).2 A sample of 3 mL of bmimBr was placed in a standard 10 mL Pyrex vial together with an appropriate stir bar and heated in the microwave cavity applying a set temperature of 100 °C (with magnetic stirring). In order to account for differences in the microwave absorptivity of the reaction mixture, four different power level settings (absorption level: very high, high, normal, and low) can be specified by the user on this instrument. The better the absorptivity of the solvent/reaction mixture, the higher the user-selected absorption level and the lower the initial power value chosen by the software algorithm.2 The heating curves of bmimBr obtained on the Initiator using the settings “high” and “very high” are displayed in Fig. 1. As can be seen, the use of the “high” absorption setting leads to a significant IR thermal overshoot of ∼40 °C above the desired set temperature of 100 °C (Fig. 1A). In this case, the magnetron power is carefully raised from an initial ∼44 W setting, to a maximum of ∼140 W. The fact that after ∼37 s of irradiation (after 100 °C has been reached) the power is reduced to 0 W but the temperature measured by the external IR sensor is still rising clearly points to the above mentioned delay problems as a result of the use of heavy-walled high thermal resistance Pyrex vials. Applying the “very high” absorption setting (Fig. 1B), the microwave power is raised slowly from 0 W to ∼90 W leading to an apparent temperature overshoot of only ∼15 °C.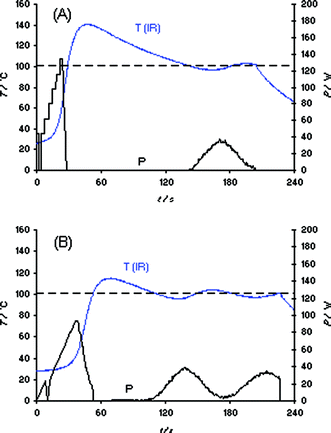 | ||
| Fig. 1 Temperature (T) and power (P) profiles for 3 mL samples of bmimBr heated in a Biotage Initiator EXP 2.5 single-mode reactor using external IR temperature control. Set temperature 100 °C, magnetic stirring (720 rpm), 10 mL Pyrex vial. (A) Absorption level “high”; (B) absorption level “very high”. | ||
In the next phase of our experiments, the same heating runs were repeated in a CEM Discover LabMate single-mode instrument (300 W maximum magnetron output power), again using IR temperature control.2 In addition to the system's built-in external IR sensor, an internal fast-responding FO probe was placed directly into the ionic liquid contained in the Pyrex vial.15 For the CEM Discover instrument, the initial maximum power level is chosen manually to account for the differences in microwave absorptivity of solvents/reaction mixtures with a recommended setting of 50 W for high absorbing, 125 W for medium absorbing and 200 W for poor absorbing media.2 Choosing 30 W of initial maximum microwave power and again a set temperature of 100 °C, temperature monitoring by the external IR sensor appeared to indicate that the software algorithm of the instrument was able to nicely reach and control the desired set temperature after ∼70 s (Fig. 2). The internal FO sensor however revealed a dramatic overshoot in temperature resulting in a peak internal temperature of ∼220 °C. Applying higher power levels (e.g., 50 W) led to an even higher overshoot (also of the IR temperature), whereas even by selecting a microwave power as low as 5 W the significant differences between external IR and internal FO temperatures could not be eliminated (see Fig. S1 in the ESI‡).
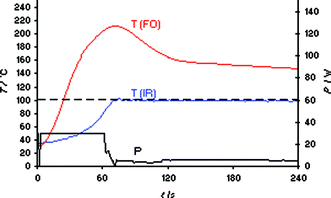 | ||
| Fig. 2 FO/IR temperature (T) and power (P) profiles for a 3 mL sample of bmimBr heated in a CEM Discover LabMate single-mode reactor using external IR temperature control (30 W initial magnetron power). The internal reaction temperature was additionally monitored (slave) by a FO probe (OpSens fiber). Set temperature 100 °C, magnetic stirring (“high”), 10 mL Pyrex vial, flow valve “on” (3.5 bar). | ||
In order to demonstrate that the observed massive temperature overshoots seen for the heating of bmimBr are not limited to imidazolium-based ILs, analogous experiments were performed with an ammonium-derived IL (Ammoeng 100).16 Also in this case internal temperature monitoring confirmed that the real temperatures attained in the reaction mixtures were significantly higher than the measured external IR surface temperatures (Fig. S2 in the ESI‡). It is therefore apparent that microwave reactors that rely exclusively on external IR temperature probes as lead sensors should not be used for transformations that involve ILs or other strongly absorbing materials as solvents or reagents. Although for experiments performed with the Biotage Initiator discussed above (Fig. 1) internal temperature monitoring in the standard configuration is not possible, it is more than likely that significant overshoots in temperature will also exist in these reactors. In addition, it should be noted that the factual overshoot in temperature is likely to be even higher than that displayed in Fig. 2 since the FO probes in these experiments need to be protected by immersion wells which also results in a delay time.5 Consequently, at least in terms of the reported reaction temperatures, previous results obtained using ILs as solvents/reagents in combination with microwave reactors based solely on external IR temperature control need to be treated with caution.13
Finally, the heating characteristics of bmimBr were evaluated utilizing the recently introduced Anton Paar Monowave 300 single-mode microwave reactor (850 W maximum magnetron output power).17 This instrument features simultaneous temperature monitoring by both external IR and internal fiber-optic probes, and allows either the IR or the FO sensor to be used as lead sensor, with the other temperature probe used as slave. The use of this methodology makes it possible to carefully scrutinize any problems associated with external versus internal temperature monitoring in microwave chemistry. With the Monowave 300 it is not possible to set different absorption levels (as with the Biotage Initiator) or manually control the maximum magnetron output power (as with the CEM Discover). Instead, controlled heating to a given set temperature is achieved either choosing the “as-fast-as-possible” mode, or by selecting an appropriate ramp time. As expected based on the results discussed above, for the heating of bmimBr to 100 °C using IR temperature control (ramp time 2 min), an overshoot of the internal FO sensor of ∼35 °C was detected (Fig. 3a). Gratifyingly, switching to FO temperature control under otherwise identical conditions, the selected target temperature of 100 °C was reached within ∼2.5 min without any detectable overshoot in temperature (Fig. 3b). Careful inspection of the power curves in Fig. 3 reveals that with the Monowave 300 the microwave irradiation is applied at a comparatively uniform and balanced level and is never reduced to 0 W as with the other two microwave systems.18
 | ||
| Fig. 3 FO/IR temperature (T) and power (P) profiles for 3 mL samples of bmimBr heated in an Anton Paar Monowave 300 single-mode reactor using either external IR (A) or internal FO temperature control (B). Set temperature 100 °C, ramp time 2 min, magnetic stirring on (600 rpm), 10 mL Pyrex vial. For heating profiles obtained in the “as-fast-as-possible” mode, see Fig. S3.‡ | ||
It should be emphasized that the overshoots using external IR sensors are generally only observed with extremely strongly microwave absorbing reaction media such as ILs. For standard organic solvents the agreement between external IR and internal FO temperature probes are excellent. Fig. S4 in the ESI displays some representing heating profiles obtained with the Monowave 300 utilizing the dual IR/FO temperature monitoring feature.‡
C. Synthesis of bmimBr under microwave conditions
Although promoted as environmentally benign reaction media, the synthesis of ILs often is not. Especially for the purification step, large volumes of organic solvents are typically required under conventional conditions. In general, halide-based ILs such as bmimBr can easily be prepared by reacting nitrogen-containing heterocyclic starting materials with an appropriate alkyl halide.7 To reduce the large excess of alkyl halides needed under traditional conditions, microwave-assisted methods have been developed.13 Much of the early work in this field was published by Varma and Namboodiri, applying open-vessel conditions.19 Later, Khadilkar and Rebeiro demonstrated that the preparation of ILs was also feasible applying closed-vessel microwave conditions, eliminating the dangers of working with toxic and volatile alkyl halides in the open atmosphere.20 One of the most comprehensive studies on the microwave-assisted preparation of ILs was published by Deetlefs and Seddon in 2003.14 A large number of ILs based on 1-alkyl-3-methylimidazolium (and related) cations were prepared by solvent-free alkylation of the corresponding basic heterocyclic cores using a dedicated multimode microwave reactor. Significantly reduced reaction times as compared to conventional methods and IL products in excellent yields and purities were obtained.14 Optimized conditions for the preparation of bmimBr utilized a 1.02 molar excess of butyl bromide and a reaction temperature of 80 °C (internal FO probe) for 6 min.14By and large following the procedure reported by Deetlefs and Seddon we first evaluated the exothermicity in the solvent-free generation of bmimBr from N-methylimidazole and butyl bromide (Scheme 1). For this purpose a 10 mL sealed Pyrex vial with an internal fiber-optic probe system immersed in a preheated oil bath at 100 °C was used (Fig. S5A‡).5 Performing the alkylation on a 15.9 mmol scale (∼3 mL reaction volume) with magnetic stirring an internal temperature overshoot of ∼60 °C was observed. At around 80 °C the reaction apparently becomes strongly exothermic leading to an internal temperature of nearly 160 °C within a few seconds (Fig. S5B‡). Visual inspection of the alkylation process revealed that the initially homogeneous mixture of N-methylimidazole and butyl bromide turned biphasic/turbid at 80 °C (after 1.5 min) and ultimately provided a viscous liquid (bmimBr) (Fig. S6‡). Clearly, under these circumstances intensive stirring of the reaction medium under microwave irradiation is required in order to obtain representative temperature data.4–6 If proper agitation can not be assured, selective heating of one of the two phases may result,4,5 in addition to erroneous temperature measurements resulting from the high viscosity of the medium.5 As already mentioned above, the change in the microwave absorptivity from moderate (starting materials) to high (ionic liquid) will create an additional problem in properly controlling the reaction.12,14
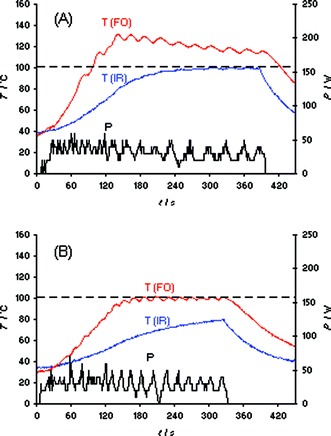
As with the heating of bmimBr described above, our initial evaluation of the IL synthesis in Scheme 1 involved the Initiator 2.5 instrument and a set temperature of 100 °C (IR temperature control). In contrast to the bmimBr heating experiment (Fig. 1B), the “very high” absorption level setting can not prevent the formation of a temperature overshoot of ∼60 °C in the IL synthesis, presumably due to the exothermicity of the reaction (Fig. S7‡). Using the Discover LabMate instrument, a similar effect was seen. Even applying only 10 W of maximum initial microwave power, a ∼80 °C internal temperature overshoot (FO) was experienced (Fig. 4A). It should be emphasized that this experiment was conducted in the so-called “flow valve off” mode.2 Using this setting, the active cooling feature on the Discover instrument—allowing compressed air to aid in the temperature control of the reaction—is not in operation. In the “flow valve on” mode, temperature control (IR) of the reaction is much improved as seen in Fig. 4B, with only a comparatively small difference between external IR and internal FO temperature. Apparently, intermittent bursts of compressed air aid in the control of the otherwise resulting strong exotherm by removing heat from the reaction mixture.21 With the Monowave 300, the overshoots resulting from the exotherm in the IL synthesis at 100 °C set temperature could not be controlled by either IR or FO temperature control (Fig. S8‡), since this microwave reactor—with the currently implemented temperature control algorithm—does not have the option of active cooling during the microwave irradiation process.
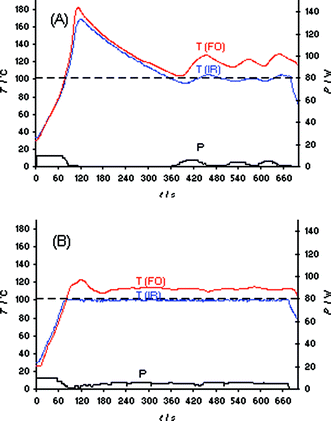 | ||
| Fig. 4 FO/IR temperature (T) and power (P) profiles for the solvent-free synthesis of ionic liquid bmimBr (Scheme 1). Experiments were performed using an IR controlled set temperature of 100 °C on a 15.9 mmol scale (1.02 equiv of BuBr). The internal reaction temperature was additionally monitored (slave) by an internal FO probe (OpSens fiber). CEM Discover LabMate, 10 W initial magnetron power, magnetic stirring (“high”), 10 mL Pyrex vial. (A) Flow valve off; (B) flow valve on (3.5 bar). | ||
At this stage we considered the use of a custom-made reaction vessel made out of sintered silicon carbide (SiC) to reduce the problem of exotherms in microwave-heated reactions. SiC is a strongly microwave absorbing chemically inert ceramic material that can be utilized at extremely high temperatures due to its high melting point (∼ 2700 °C) and very low thermal expansion coefficient.4,22 Microwave irradiation induces a flow of electrons in the semiconducting SiC that heats the reaction vessel very efficiently through resistance (ohmic) heating mechanisms.4,22 Due to the high microwave absorbtivity of SiC, any material (i.e. a reaction mixture) contained inside the vial will be effectively shielded from the electromagnetic field.23,24 In addition, because of its high thermal conductivity and effusivity (a measure for the ability to exchange thermal energy with its surroundings, Table 1) the heat flow through the 1.8 mm wall of the SiC reaction vessel is exceptionally fast in both directions.
| SiCa | Pyrexb | 18/8 Steelb | ||
|---|---|---|---|---|
| a SiC:Ekasic® F SSiC, ESK Ceramics.b Data from ref. 25.c The thermal effusivity e of a material is defined as the square root of the product of its thermal conductivity and volumetric heat capacity [e = (kρcp)0.5]. | ||||
| Thermal conductivity | λ/W m−1 K−1 | 125 | 1.2 | 30 |
| Thermal coeff. of expansion | α/m m−1 K−1 | 4.1 × 10−5 | 3.3 × 10−6 | 17.3 |
| Specific heat capacity | Cp [J kg−1 K−1 10−3] | 0.6 | 0.7 | 0.5 |
| Density | ρ [kg m−3 10−3] | 3.10 | 2.23 | 8.02 |
| Thermal effusivityc | e [J s−1/2 m−2 K−1] | 15000 | 1400 | 11000 |
Thus, in essence, using a SiC reaction vessel in combination with the Monowave 300 reactor a non-contact-heating autoclave experiment under very carefully controlled and monitored conditions can be performed.23 Importantly, such an experiment—although performed in a microwave reactor—does not involve dielectric heating effects on the ionic liquid formation since the SiC vial is effectively preventing microwave irradiation from penetrating to the reaction mixture.23 Therefore, the fact that the microwave absorptivity of the components is changing during the formation of the ionic liquid is irrelevant. Another important aspect is the ∼10 times higher thermal effusivity of SiC compared to Pyrex glass which aids in the prevention of exotherms. Comparing the synthesis of bmimBr performed in a standard Pyrex vial at 100 °C set temperature with the same experiment run in a SiC vial, the differences became immediately obvious (Fig. 5). While the experiment in the Pyrex vial leads to the expected overshoot (compare also Fig. S8‡), the use of the same reaction carried out in the SiC vessel of identical geometry prevents the occurrence of an overshoot completely since the heat generated during the ionic liquid formation is efficiently exchanged with the comparatively cool air in the microwave cavity via the SiC ceramic (Fig. 5).
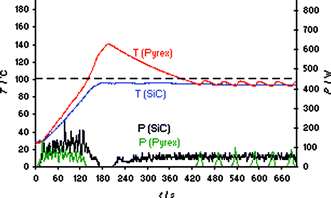 | ||
| Fig. 5 Internal fiber-optic temperature (T) and power (P) profiles for the solvent-free synthesis of ionic liquid bmimBr (Scheme 1) using Pyrex and SiC reaction vials. Anton Paar Monowave 300 single-mode reactor. Set temperature 100 °C, ramp time 2 min, magnetic stirring on (600 rpm). Experiments were performed using an IR controlled set temperature of 100 °C on a 10.6 mmol scale (1.02 equiv of BuBr). | ||
It would appear that an ideal set-up for controlling exotherms in the above reactions would be to use a SiC vial in combination with active cooling and internal temperature monitoring. Indeed, when active cooling is used in combination with FO temperature control and a SiC reaction vessel (CEM Discover) the exotherm and hold temperature can to some extent be controlled better than using a Pyrex vessel (Fig. S9‡). However, this method has the drawback of causing large oscillations while controlling the hold temperature, even when limiting the magnetron output power to 10 W. Another technique for controlling the exotherm in IL preparations is to carry out the process under continuous flow conditions, in particular using microreactors.26 Perhaps not surprisingly, microreactors made out of SiC ceramic have recently also been employed for other strongly exothermic reactions since the thermal effusivity of SiC is even higher than that of stainless steel (Table 1).27
Ultimately, optimization studies demonstrated that in our hands the best conditions to rapidly obtain the IL bmimBr under sealed vessel microwave conditions using only 2% excess of butyl bromide involved heating the reaction mixture at 145 °C for 20 min or at 160 °C for 6 min. These conditions provided bmimBr in high yield and purity with only small amounts of starting material being detected by 1H NMR (∼3–5%). Higher temperatures or increased reaction times led to thermolysis of the reaction product ultimately yielding mixtures of the two starting materials (Scheme 1).14,28 On the other hand, lower reaction temperatures or shorter times yielded incomplete conversions. For microwave heating experiments conducted at a set temperature of 145 °C or 160 °C no overshoots were observed (Monowave 300, FO temperature control, Pyrex vial) since the exotherm can efficiently be masked by the higher set temperatures in combination with choosing a suitable ramp time (Fig. S10‡). Reproduction of the internal temperature profile using conventional oil bath heating at 145 °C (Fig. S11‡) led to bmimBr of identical yield and purity as obtained by microwave heating, indicating that the rate enhancements observed for the preparation of ionic liquids under microwave conditions are of a purely thermal nature.13
D. Pulsed versus continuous microwave heating
Finally, having access to a dual external IR/–internal FO temperature measurement microwave system we wanted to study another unusual phenomenon in microwave chemistry. It has been reported that for some chemical transformations several shorter cycles (or pulses) of microwave heating were more efficient (e.g., result in a higher yield), than one continuous irradiation cycle at the same final target temperature.29,30 The most prominent example involves the Claisen rearrangement of an allyl aryl ether where three 15 min pulses at 220 °C provided a better conversion than one uninterrupted irradiation event at the same temperature.29 Conspicuously, this transformation was performed in a toluene–IL solvent system and was run with external IR temperature control.29 Based on the experiments described above, it appears likely that internal temperature overshoots were occurring in these transformations. Using several heating cycles, the thermal overshoot will not only be experienced once at the beginning of the microwave heating experiment, but will be continually repeated and thus ultimately will lead to a higher overall reaction temperature and thus a higher conversion. This hypothesis was supported by performing a pulsed heating experiment of bmimBr in the Monowave 300 reactor. Six heating cycles to 100 °C were programmed using the external IR probe as a lead sensor. As can be seen in Fig. 6, while the IR sensor displays the “correct” set temperature of 100 °C, the internal FO sensor shows repeated temperature overshoots to as high as 180–200 °C. Integration over the measured temperature curves for the full ∼20 min duration of the experiment reveals a significant difference of 45 °C between the average IR (80 °C) and internal FO temperature (125 °C). Importantly, conducting an experiment with one cycle of 20 min of microwave irradiation (Fig S12‡), the overall difference in between IR and FO temperatures was much smaller (19 °C, with an average IR temperature of 95 °C and an internal FO temperature of 114 °C). While the example shown herein involving ILs is clearly an extreme case, a difference between the externally measured IR and the factual internal reaction temperature will almost always exist, in particular in the initial heating-up phase.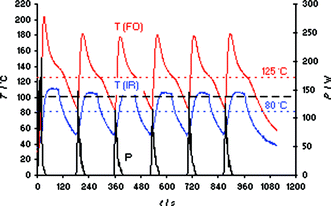 | ||
| Fig. 6 FO/IR temperature (T) and power (P) profiles for a 3 mL sample of bmimBr heated in an Anton Paar Monowave 300 single-mode reactor using external IR temperature control (FO sensor as slave). Six cycles applying a set temperature of 100 °C (1 min hold time) were programmed, magnetic stirring on (600 rpm), 10 mL Pyrex vial. | ||
An additional problem of IR sensors, not related to the heating of ILs, is that they tend to drift after prolonged use, in particular at high temperatures. This phenomenon is likely to be related to the warming of the cavity and therefore of the material surrounding the IR sensor itself. As shown in Fig. S13,‡ a 15 °C drift on an IR sensor was observed after heating NMP at 200 °C for 30 min.
Conclusions
In summary, we have shown that simultaneous infrared–fiber-optic temperature measurements can be important, if not critical, for the monitoring, control and understanding of microwave-assisted reactions. Due to the limitations of external IR sensors, essentially representing the outer surface temperature of a heavy-walled high thermal inertia Pyrex vessel, these sensors often do not represent the actual reaction temperatures inside the reaction vessel (Fig. S13‡). Internal FO temperature measurement devices are much better suited for this purpose and the simultaneous monitoring and evaluation from the output of both external IR and internal FO probes can lead to important information about the dielectric properties and exothermicity of a chemical reaction performed under microwave conditions. This is of particular importance for strongly microwave absorbing and/or viscous reaction media, and in those instances where the microwave absorption properties change during the transformation. In the case of ionic liquids, our experiments have shown that both for using ILs as reaction medium and particularly in their preparation, microwave instruments that rely on external IR sensors to control the heating process should not be used. Previous reports on the observation of “specific” or “non-thermal” microwave effects” applying this technology therefore have to be treated with caution.Experimental section
General
1H-NMR spectra were recorded on a Bruker 300 MHz instrument. Chemical shifts (δ) are expressed in ppm downfield from TMS as internal standard. Analytical HPLC (Shimadzu LC20) analysis was carried out on a C18 reversed-phase (RP) analytical column (150 × 4.6 mm, particle size 5 μm) at 25 °C using pure water (isocratic) as mobile phase at a flow rate of 0.5 mL min−1. The response factors of N-methylimidazole and butyl bromide at 215 nm were equal, as determined by 1H-NMR spectroscopy. The conversion of the crude reaction mixture was determined by HPLC at 215 nm. Conversions and purities of the purified product were determined by both analytical HPLC and 1H-NMR spectroscopy. N-Methylimidazole (>99%) and butyl bromide (99%) were obtained from Sigma-Aldrich and were used without any further purification. A sample of Ammoeng 100 was provided by Prof. A. Dondoni (Ferrara, Italy).Microwave irradiation experiments
Microwave irradiation experiments in the Biotage Initiator EXP 2.5 single-mode reactor (2.45 GHz, 400 W) used an external IR sensor and followed standard procedures.2 Similarly, reactions in the CEM Discover LabMate single-mode instrument (2.45 GHz, 300 W) were performed using IR temperature control.2 The procedure to use an added internal FO probe has been reported previously.5Experiments using simultaneous IR/FO temperature monitoring were performed using a Monowave 300 single-mode microwave reactor from Anton Paar GmbH (Graz, Austria).17 The instrument uses a maximum of 850 W magnetron output power and can be operated at 300 °C reaction temperature and 30 bar pressure. The reaction temperature is monitored by an external infrared sensor (IR) housed in the side-walls of the microwave cavity measuring the surface temperature of the reaction vessel, and/or by an internal fiber-optic (FO) temperature probe (ruby thermometer) protected by a borosilicate immersion well inserted directly in the reaction mixture. The measuring principle in the ruby thermometer is the decay time of the photoluminescence of a ruby (corundum, Al2O3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr) crystal. The crystal at the tip of the flexible fiber is excited by blinking light, causing the luminescence. The decay time is a temperature dependent physical constant of the material, therefore it can be used for temperature measurement. Once adjusted, this system is a calibration free tool for temperature measurement over the whole operation range and can also be used to adjust the IR sensor and the pressure sensor of the system. The magnetron output power can either be controlled by the FO probe (IR as slave) or by the IR sensor (FO as slave). Heating rates are controlled by either selecting the “as-fast-as-possible” or a ramp mode. Pressure sensing is achieved by a hydraulic sensor integrated in the swiveling cover of the instrument. The reusable Pyrex vials (10 mL and 30 mL) are sealed with PEEK snap caps and standard PTFE coated silicone septa. In case of FO temperature measurement punched seals to insert the immersing tube are employed. Seals and caps can be used for both Pyrex vial types and the 10 mL SiC vial as well. Precision of internal temperature measurement is provided by efficient stirring at a fixed rate of 600 rpm. Reaction cooling is performed by compressed air automatically after the heating period has elapsed. The required force of 6–8 bar is also used to pneumatically seal the vials tightly at the beginning to withstand 30 bar, and to ensure smooth release of potentially remaining pressure before opening the cover.
Cr) crystal. The crystal at the tip of the flexible fiber is excited by blinking light, causing the luminescence. The decay time is a temperature dependent physical constant of the material, therefore it can be used for temperature measurement. Once adjusted, this system is a calibration free tool for temperature measurement over the whole operation range and can also be used to adjust the IR sensor and the pressure sensor of the system. The magnetron output power can either be controlled by the FO probe (IR as slave) or by the IR sensor (FO as slave). Heating rates are controlled by either selecting the “as-fast-as-possible” or a ramp mode. Pressure sensing is achieved by a hydraulic sensor integrated in the swiveling cover of the instrument. The reusable Pyrex vials (10 mL and 30 mL) are sealed with PEEK snap caps and standard PTFE coated silicone septa. In case of FO temperature measurement punched seals to insert the immersing tube are employed. Seals and caps can be used for both Pyrex vial types and the 10 mL SiC vial as well. Precision of internal temperature measurement is provided by efficient stirring at a fixed rate of 600 rpm. Reaction cooling is performed by compressed air automatically after the heating period has elapsed. The required force of 6–8 bar is also used to pneumatically seal the vials tightly at the beginning to withstand 30 bar, and to ensure smooth release of potentially remaining pressure before opening the cover.
For the experiments described herein either a standard 10 mL Pyrex tube or a custom-made vessel made out of sintered silicon carbide (Ekasic® F SSiC, ESK Ceramics, Kempten, Germany) of the same geometry was used.22
Preparation of 1-butyl-3-methylimidazolium bromide (bmimBr) (Scheme 1)
The synthesis of bmimBr was performed following the procedure reported by Deetlefs and Seddon.14 A mixture of 1-methylimidazole (1.30 g, 15.9 mmol) and butyl bromide (2.22 g, 16.2 mmol) was placed in a 10 mL Pyrex reaction vessel equipped with a magnetic stir bar and fitted with an immersion well for the ruby thermometer. The Monowave 300 microwave reactor was programmed to ramp to 145 °C (ramp setting 2 min) using the ruby thermometer as lead sensor and applying a hold time of 20 min, after which the sample was externally cooled to 50 °C with pressurized air. A very small sample of the crude product was taken for HPLC monitoring, affording a conversion/purity of 95%. The resulting pale yellow viscous oil was diluted with 2 mL of MeCN and washed 3 times with EtOAc (5 mL). The lower phase was collected, the solvent removed under vacuo and dried for a further 6 h at 80 °C under reduced pressure (2 mbar). Upon cooling to room temperature, 1-butyl-3-methylimidazolium bromide (3.1 g, 90%; >99% HPLC homogeneity) was obtained as pale yellow crystals. 1H-NMR (300 MHz; CDCl3, 25 °C) δ (ppm) = 0.86 (3H, t, J = 7.5 Hz, N-(CH2)3CH2), 1.29 (2H, m, N-CH2-CH2–CH2-CH3), 1.82 (2H, m, N-CH2-CH2-CH2-CH3), 4.04 (3H, s, NCH3), 4.26 (2H, t, J = 7.5 Hz, N-CH2-(CH2-)2-CH3), 7.50 (1H, s, H-4), 7.62 (1H, s, H-5), 10.22 (1H, s, H-2).The same experimental procedure was used for the oil bath synthesis of bmimBr. Instead of the microwave reactor a preheated (145 °C) silicone oil bath with magnetic stirring (600 rpm) was used. A special customized sealing system with an immersion well for an OpSens fiber optic temperature sensor was used (see Fig S5A‡).5 After 20 min at 145 °C and decantation of the supernatant liquid, crude bmimBr was obtained as a viscous pale yellow oil (2.9 g, 84%; 93% HPLC homogeneity). The crude product was not further purified.
Acknowledgements
This work was supported by a grant from the Christian Doppler Society (CDG). We acknowledge Anton Paar GmbH for the provision of the Monowave microwave reactor and technical support. Bernhard Gutmann is thanked for early contributions to this work.Notes and references
- For a recent review with >900 references and a tabular survey of ca. 200 microwave chemistry review articles, books and book chapters, see: (a) C. O. Kappe and D. Dallinger, Mol. Diversity, 2009, 13, 71–193 Search PubMed; (b) S. Caddick and R. Fitzmaurice, Tetrahedron, 2009, 65, 3325–3355 CrossRef CAS and references cited therein.
- For a survey of commercially available microwave reactors, see: C. O. Kappe, D. Dallinger and S. S. Murphree, Practical Microwave Synthesis for Organic Chemists - Strategies, Instruments, and Protocols, Wiley-VCH, Weinheim, 2009, pp. 45–85 Search PubMed.
- (a) M. Nüchter, B. Ondruschka, W. Bonrath and A. Gum, Green Chem., 2004, 6, 128–141 RSC; (b) M. Nüchter, B. Ondruschka, D. Weiß, W. Bonrath and A. Gum, Chem. Eng. Technol., 2005, 28, 871–881 CrossRef CAS; (c) N. E. Leadbeater, S. J. Pillsbury, E. Shanahan and V. A. Williams, Tetrahedron, 2005, 61, 3565–3585 CrossRef CAS; (d) M. Hosseini, N. Stiasni, V. Barbieri and C. O. Kappe, J. Org. Chem., 2007, 72, 1417–1424 CrossRef CAS.
- J. M. Kremsner and C. O. Kappe, J. Org. Chem., 2006, 71, 4651–4658 CrossRef CAS.
- (a) M. A. Herrero, J. M. Kremsner and C. O. Kappe, J. Org. Chem., 2008, 73, 36–47 CrossRef CAS; (b) B. Bacsa, K. Horváti, S. Bősze, F. Andreae and C. O. Kappe, J. Org. Chem., 2008, 73, 7532–7542 CrossRef CAS.
- J. D. Moseley, P. Lenden, A. D. Thomson and J. P. Gilday, Tetrahedron Lett., 2007, 48, 6084–6087 CrossRef CAS.
- (a) Ionic Liquids in Synthesis, 2nd Edition, ed. P. Wasserscheid and T. Welton, Wiley-VCH, Weinheim, 2007 Search PubMed; (b) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772–3789 CrossRef; (c) M. A. P. Martins, Chem. Rev., 2008, 108, 2015–2050 CrossRef CAS; (d) W. Miao and T. H. Chan, Acc. Chem. Res., 2006, 39, 897–908 CrossRef CAS.
- For reviews on dielectric heating, see: (a) C. Gabriel, S. Gabriel, E. H. Grant, B. S. Halstead and D. M. P. Mingos, Chem. Soc. Rev., 1998, 27, 213–223 RSC; (b) D. M. P. Mingos and D. R. Baghurst, Chem. Soc. Rev., 1991, 20, 1–47 RSC.
- (a) J. Hoffmann, M. Nüchter, B. Ondruschka and P. Wasserscheid, Green Chem., 2003, 5, 296–299 RSC; (b) N. E. Leadbeater and H. M. Torenius, J. Org. Chem., 2002, 67, 3145–3148 CrossRef CAS; (c) E. Van Der Eycken, P. Appukkuttan, W. De Borggraeve, W. Dehaen, D. Dallinger and C. O. Kappe, J. Org. Chem., 2002, 67, 7904–7909 CrossRef CAS.
- M. Damm and C. O. Kappe, Mol. Diversity, 2009, 13, 529 Search PubMed.
- In general, the ability of a specific solvent to convert microwave energy into heat at a given frequency and temperature is determined by the so-called loss tangent (tanδ), expressed as the quotient, tanδ = ε′′/ε′. A reaction medium with a high tanδ at the standard operating frequency of a microwave synthesis reactor (2.45 GHz) is required for good absorption and, consequently, for efficient heating. Solvents used for microwave synthesis can be classified as high (tanδ > 0.5), medium (tanδ 0.1–0.5), and low microwave absorbing (tanδ < 0.1). See also: C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 Search PubMed.
- (a) S. Horikoshi, T. Hamamura, M. Kajitani, M. Yoshizawa-Fujita and N. Serpone, Org. Process Res. Dev., 2008, 12, 1089–1093 Search PubMed; (b) G. Dimitrakis, I. J. Villar-Garcia, E. Lester, P. Licence and S. Kingman, Phys. Chem. Chem. Phys., 2008, 10, 2947–2951 RSC.
- For reviews on the use of microwave irradiation in combination with ionic liquids, see: (a) R. Martinez-Palou, Mol. Diversity, 2009 DOI:10.1007/s11030-009-9159-3; (b) E. Boros, K. R. Seddon and C. R. Strauss, Chim. Oggi, 2008, 26(6), 28–30 Search PubMed; (c) N. E. Leadbeater and H. M. Torenius, in Microwaves in Organic Synthesis, 2nd edition, ed. A. Loupy, Wiley-VCH, Weinheim, 2006, pp. 327–361 Search PubMed; (d) J. Habermann, S. Ponzi and S. V. Ley, Mini-Rev. Org. Chem., 2005, 2, 125–137 Search PubMed; (e) N. E. Leadbeater, H. M. Torenius and H. Tye, Comb. Chem. High Throughput Screen., 2004, 7, 511–528 CAS.
- (a) M. Deetlefs and K. R. Seddon, Green Chem., 2003, 5, 181–186 RSC; (b) T. Erdmenger, R. M. Paulus, R. Hoogenboom and U. S. Schubert, Aust. J. Chem., 2008, 61, 197–203 CrossRef CAS.
- The details of this experimental set-up have been described in ref. 5. Although an internal FO temperature measurement system is also available from the instrument manufacturer for this reactor, it is not possible to display both external IR and internal FO temperatures simultaneously with the standard software.
- (a) C. Pretti, C. Chiappe, D. Pieraccini, M. Gregori, F. Abramo, G. Monni and L. Intorre, Green Chem., 2006, 8, 238–240 RSC; (b) A. Vecchi, B. Melai, A. Marra, C. Chiappe and A. Dondoni, J. Org. Chem., 2008, 73, 6437–6440 CrossRef CAS; (c) Ammoeng 100™ is a trademark of the Solvent Innovation GmbH, now part of Merck KGaA (solvent-innovation.com).
- For further details on the Monowave 300 single-mode microwave reactor (Anton Paar GmbH, Graz, Austria), see http://www.anton-paar.com/monowave300.
- It should be stressed that the nominal microwave magnetron output power values displayed by different microwave reactors can not be compared with each other.
- (a) R. S. Varma and V. V. Namboodiri, Chem. Commun., 2001, 643–644 RSC; (b) R. S. Varma and V. V. Namboodiri, Tetrahedron Lett., 2002, 43, 5381–5383 CrossRef; (c) V. V. Namboodiri and R. S. Varma, Chem. Commun., 2002, 342–343 RSC.
- B. M. Khadilkar and G. L. Rebeiro, Org. Process Res. Dev., 2002, 6, 826–828 Search PubMed.
- The “flow valve on” is the default method of operation for temperature control in the CEM Discover series of microwave reactors. This concept, although related, should not be confused with the “simultaneous cooling” method where a constant stream of compressed air is applied to the reaction vessel during the irradiation process. For details, see ref. 2 and 3.
- (a) Silicon Carbide: Recent Major Advances, ed. W. J. Choyke, H. Matsunami and G. Pensl, Springer, Berlin, 2004 Search PubMed; (b) Advances in Silicon Carbide Processing and Applications, ed. S. E. Saddow and A. Agarwal, Artech House Inc, Norwood, MA, 2004 Search PubMed; (c) Properties of Silicon Carbide, ed. G. L. Harris, Institute of Electrical Engineers, 1995 Search PubMed.
- For a preliminary report, see: D. Obermayer, B. Gutmann and C. O. Kappe, Angew. Chem., Int. Ed., 2009, 48, 8321 Search PubMed.
- For the application of SiC in microtiter plates, see: M. Damm and C. O. Kappe, J. Comb. Chem., 2009, 11, 460 Search PubMed , and ref. 10.
- (a) For further details, see: EKasic® technical data-sheet (http://www.esk.com) and the borosilicate-glass technical data sheet (http://www.duran-group.com); (b) R. C. Weast, CRC Handbook of Chemistry and Physics. 65th Edition, CRC Press, 1985 Search PubMed; (c) Y. A. Çengel, Heat Transfer. A Practical Approach, 2nd Edition, McGraw Hill Professional, 2003 Search PubMed.
- (a) D. A. Waterkamp, M. Heiland, M. Schlüter, J. C. Sauvageau, T. Beyersdorff and J. Thöming, Green Chem., 2007, 9, 1084–1090 RSC; (b) A. Renken, V. Hessel, P. Löb, R. Miszczuk, M. Uerdingen and L. Kiwi-Minsker, Chem. Eng. Process., 2007, 46(9), 840–845 CrossRef CAS; (c) D. Wilms, J. Klos, A. F. M. Kilbinger, H. Löwe and H. Frey, Org. Process Res. Dev., 2009, 13, 961 Search PubMed.
- (a) F. Meschke, G. Riebler, V. Hessel, J. Schürer and T. Baier, Chem. Eng. Technol., 2005, 28, 465–473 CrossRef CAS; (b) K. Ramadani, M. Ferrato, S. Elgue, C. Gourdon, V. Meille and C. Jolivet, Proceedings of the GPE-EPIC (2nd International Congress on Green Process Engineering—2nd European Process Intensification Conference), June 14–17, 2009, Venice, Italy Search PubMed.
- (a) C. G. Begg, M. R. Grimmet and P. D. Wethey, Aust. J. Chem., 1973, 26, 2435–2438 CAS; (b) A. G. Glenn and P. B. Jones, Tetrahedron Lett., 2004, 45, 6967–6969 CrossRef CAS; (c) B. K. M. Chan, N.-H. Chang and M. R. Grimmet, Aust. J. Chem., 1977, 30, 2005–2013 CAS.
- I. R. Baxendale, A.-L. Lee and S. V. Ley, J. Chem. Soc., Perkin Trans. 1, 2002, 1850–1857 RSC.
- (a) T. Durand-Reville, L. B. Gobbi, B. L. Gray, S. V. Ley and J. S. Scott, Org. Lett., 2002, 4, 3847–3850 CrossRef CAS; (b) I. R. Baxendale, S. V. Ley, M. Nessi and C. Piutti, Tetrahedron, 2002, 58, 6285–6304 CrossRef CAS; (c) W. Yin, Y. Ma, J. Xu and Y. Zhao, J. Org. Chem., 2006, 71, 4312–4315 CrossRef CAS.
Footnotes |
| † This paper is part of an Organic & Biomolecular Chemistry web theme issue on enabling technologies for organic synthesis. |
| ‡ Electronic supplementary information (ESI) available: Temperature/power profiles and experimental details. See DOI: 10.1039/b918407d |
| This journal is © The Royal Society of Chemistry 2010 |
