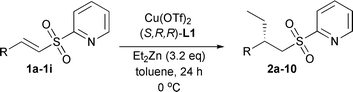
Catalytic asymmetric conjugate addition of dialkylzinc reagents to α,β-unsaturated sulfones†
Pieter H. Bos, Beatriz Maciá, M. Ángeles Fernández-Ibáñez, Adriaan J. Minnaard and Ben L. Feringa*
Stratingh Institute for Chemistry, University of Groningen, Nijenborgh 4, 9747 AG Groningen, The Netherlands. E-mail: b.l.feringa@rug.nl; Fax: +31 50 363 4278; Tel: +31 50 363 4296
First published on 1st October 2009
Abstract
An efficient method is reported for the highly enantioselective copper-catalyzed conjugate addition of dialkylzinc reagents to α,β-unsaturated sulfones using a monodentate phosphoramidite ligand.
The conjugate addition of organometallic reagents to α,β-unsaturated compounds is one of the most versatile and widely used synthetic methods for carbon–carbon bond formation.1 Enantioselective metal-catalyzed versions of this transformation are used as key-steps in the synthesis of numerous natural products and biologically active compounds and have been studied extensively over the past decade.2
An important goal in extending the current methodology is the development of an enantioselective catalytic method for the addition of organometallic reagents to α,β-unsaturated sulfones. Optically active sulfones with a stereocenter at the β-position are valuable intermediates in organic chemistry due to their ease of derivatization yielding a variety of building blocks, including aldehydes or ketones, alkenes, alkynes, alkanes and haloalkanes.3 In 2007, Carretero et al.4 and Charette and Desrosiers5 both reported methodology for the catalytic asymmetric conjugate reduction of β,β-disubstituted α,β-unsaturated sulfones leading to alkyl aryl sulfones. Using a hydrosilylation approach excellent yields and enantiomeric excesses were obtained.
A complementary approach to sulfones bearing β stereocenters is the asymmetric conjugate addition of organometallic reagents to α,β-unsaturated sulfones. In 2004, Mauleón and Carretero reported a rhodium-catalyzed asymmetric conjugate addition of organoboronic acids to α,β-unsaturated sulfones giving high to excellent yields and enantioselectivities.6
Recently, Charette et al. reported a method for the catalytic asymmetric conjugate addition of diorganozinc reagents to β-substituted vinyl sulfones using a bidentate phosphorus based ligand (Binap) and elevated temperatures (60 °C) with good results.7 Performing the reaction in benzene gave high yields and lower enantioselectivities while using THF gave an increase in ee, but lower yields. Our group recently developed a copper-catalyzed asymmetric conjugate addition of Grignard reagents to α,β-unsaturated sulfones giving excellent results using a bidentate phosphorus based ligand (Tol-Binap) and aliphatic substrates.8
In this paper we describe the first asymmetric conjugate addition of diorganozinc reagents to α,β-unsaturated sulfones using a monodentate phosphoramidite ligand (L1). Among all the ligands used so far in the field of copper-catalyzed conjugate addition of organometallic reagents, phosphoramidites stand out as readily accessible, inexpensive and easily modified.9 The excellent enantioselectivities that phosphoramidites give in the conjugate addition of dialkylzinc reagents to a variety of substrates10 encouraged us to use them as ligands in the conjugate addition of diorganozinc reagents to α,β-unsaturated sulfones.
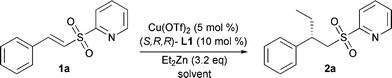
Initially, we studied the addition of diethylzinc to α,β-unsaturated sulfone 1a using phosphoramidite L1 (Fig. 1) at various temperatures and in different solvents (Table 1).
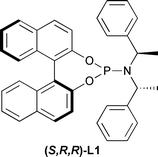 | ||
| Fig. 1 Structure of ligand (S,R,R)-L1. | ||
| ||||
|---|---|---|---|---|
| Solvent | Temperature (°C) | Conversionb (%) | eec,d (%) | |
| a Conditions: 1 (1 equiv., 0.1 mmol), Et2Zn (3.2 equiv.), Cu(OTf)2 (5 mol%), (S,R,R)-L1 (10 mol%).b Conversion determined by GC-MS.c Enantiomeric excess determined by chiral HPLC (See the ESI1).d Determined by comparison with literature data based on the sign of optical rotation.e Slow addition of the substrate 1a over 5 h. | ||||
| 1e | Toluene | −40 | 100 (72 h) | 94 (S) |
| 2e | Toluene | −25 | 100 (48 h) | 95 (S) |
| 3e | Toluene | −10 | 100 (24 h) | 95 (S) |
| 4e | Toluene | 0 | 100 (24 h) | 92 (S) |
| 5 | Toluene | 0 | 100 (24 h) | 88 (S) |
| 6 | THF | 0 | 0 (24 h) | nd |
| 7 | Et2O | 0 | 25 (24 h) | nd |
| 8 | t-BuOMe | 0 | 30 (24 h) | nd |
| 9 | DCM | 0 | 12 (24 h) | nd |
At −40 °C in toluene, full conversion was obtained after 3 days giving the product with excellent ee (entry 1). Upon increasing the reaction temperature full conversion was obtained in 24 h while the enantioselectivity remained excellent (entries 2–4). Upon direct addition of the substrate to the reaction mixture the enantiomeric excess dropped only slightly (entry 5). Use of either ethereal solvents or DCM resulted in a large decrease in conversion (entries 6–9).
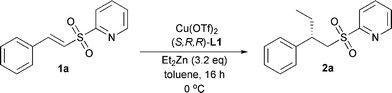
After optimization of reaction temperature and solvent the influence of the copper source was examined (Table 2).
| |||
|---|---|---|---|
| [Cu] source | Conversionb (%) | eec,d (%) | |
| a Conditions: 1 (1 equiv., 0.1 mmol), Et2Zn (3.2 equiv.), Cu salt (5 mol%), (S,R,R)-L1 (10 mol%) in toluene at 0 °C.b Conversion determined by GC-MS after 24 h.c Enantiomeric excess determined by chiral HPLC (See the ESI1).d Determined by comparison with literature data based on the sign of optical rotation. | |||
| 1 | Cu(OTf)2 | 100 | 88 (S) |
| 2 | CuI | 0 | nd |
| 3 | CuTC | 0 | nd |
| 4 | CuBr2 | 5 | nd |
| 5 | CuOTf·benzene | 40 | 83 (S) |
The catalyst based on copper(II) triflate gave full conversion after 24 h and high enantiomeric excess. Changing the counterion of the copper source had a dramatic effect on the conversion of substrate 1a. This effect was observed for both copper(I) and copper(II) salts and almost no conversion was obtained after 24 h (entries 2–4). When copper(I) triflate was used the conversion increased again, but both the conversion and the enantiomeric excess were lower than the result obtained with copper(II) triflate (entry 5). The nature of the counterion is of extreme importance and it seems that the triflate counterion is necessary in order to give good results.11
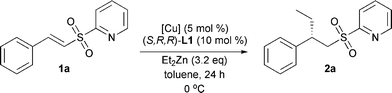
Changing the metal to ligand ratio did not have any effect on the enantioselectivity, but upon using a ratio of 1:1.5 of copper to ligand the conversion after 24 h increased slightly (Table 3).
| |||||
|---|---|---|---|---|---|
| Cu(OTf)2 (mol%) | L1 (mol%) | [Cu]/L1 | Conversionb (%) | eec,d (%) | |
| a Conditions: 1 (1 equiv., 0.1 mmol), Et2Zn (3.2 equiv.), Cu(OTf)2, (S,R,R)-L1 in toluene at 0 °C.b Conversion determined by GC-MS after 16 h.c Enantiomeric excess determined by chiral HPLC (See the ESI1).d Determined by comparison with literature data based on the sign of optical rotation. | |||||
| 1 | 5 | 11 | 1:2.2 | 82 | 88 (S) |
| 2 | 11 | 11 | 1:1 | 82 | 88 (S) |
| 3 | 7.5 | 11 | 1:1.5 | 85 | 88 (S) |
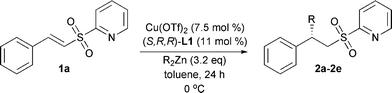
After establishing the optimal conditions for the addition of diethylzinc, several other diorganozinc reagents were examined (Table 4). With the less reactive dimethylzinc no conversion was obtained after 24 h (entry 2). Di n-butylzinc gave less than 10% conversion after 24 h. Even after 5 days the conversion was not complete, nevertheless the product could be isolated in good yield and with moderate enantiomeric excess (entry 3). The more reactive diisopropylzinc gave full conversion after 24 h and both the isolated yield and enantiomeric excess dropped slightly compared with diethylzinc (entry 4). Unfortunately, the use of diphenylzinc did not give any reaction (entry 5).
| ||||
|---|---|---|---|---|
| R | Product | Conversionb (%) | eec,d (%) | |
| a Conditions: 1 (1 equiv., 0.25 mmol), R2Zn (3.2 equiv.), Cu(OTf)2 (7.5 mol%), (S,R,R)-L1 (11 mol%) in toluene at 0 °C.b Conversion determined by GC-MS after 24 h. Isolated yields in parentheses.c Enantiomeric excess determined by chiral HPLC (See the ESI1).d Determined by comparison with literature data based on the sign of optical rotation.e Slow addition of the substrate over 5 h.f Conversion determined by GC-MS after 5 days.g In this case 2-naphthyl substituted sulfone 1i was used. | ||||
| 1 | Et | 2a | 100 (70) | 92(S)e |
| 2 | Me | 2b | 0 | nd |
| 3 | n-Bu | 2c | 84 (73)f | 66(+) |
| 4 | i-Pr | 2d | 100 (61) | 85(S) |
| 5 | Phg | 2e | 0 | nd |
Using optimized conditions we then studied the influence of the substitution pattern of the substrate on the conjugate addition of diethylzinc (Table 5). Because excellent results have been obtained using Grignard reagents on aliphatic substrates,8 we focused our efforts on a range of aromatic substrates for which excellent ee's were found. Introduction of a chloride at the ortho position gave a drop in ee (entry 2). Moving the chloride further away from the reaction centre increased the enantiomeric excess (entries 2–4). We attribute this effect to steric hindrance.
| ||||
|---|---|---|---|---|
| R | Product | Yieldb,c (%) | eed,e (%) | |
| a Conditions: 1 (1 equiv., 0.25 mmol), Et2Zn (3.2 equiv.), Cu(OTf)2 (7.5 mol%), (S,R,R)-L1 (11 mol%) in toluene at 0 °C.b Full conversion after 24 h determined by GC-MS.c Isolated yield.d Enantiomeric excess determined by chiral HPLC (See the ESI1).e Determined by comparison with literature data based on the sign of optical rotation.f Slow addition of the substrate over 5 h.g Incomplete conversion. | ||||
| 1 | Ph | 2a | 70 | 92 (S)f |
| 2 | o-Cl Phenyl | 3 | 85 | 70 (+) |
| 3 | m-Cl Phenyl | 4 | 66 | 84 (+) |
| 4 | p-Cl Phenyl | 5 | 68 | 89 (+) |
| 5 | p-Me Phenyl | 6 | 46g | 92 (+) |
| 6 | p-CF3 Phenyl | 7 | 84 | 96 (S) |
| 7 | p-i-Pr Phenyl | 8 | 52g | 90 (+) |
| 8 | p-Br Phenyl | 9 | 86 | 96 (+) |
| 9 | 2-Naphthyl | 10 | 83 | 93 (S) |
In the instance of electron donating substituents such as the para methyl and para isopropyl substituted substrates full conversion was not achieved after 24 h giving lower isolated yields. Fortunately, the lower reaction rate did not negatively influence the enantiomeric excess and excellent ee's were obtained (entries 5 and 7). Furthermore, para trifluoromethyl-, p-bromo- and 2-naphthyl- substituted substrates gave good yields and excellent enantiomeric excesses (entries 6, 8 and 9).
In summary, we have developed a highly enantioselective copper-catalyzed conjugate addition of dialkylzinc reagents to a range of aromatic α,β-unsaturated sulfones using a monodentate phosphoramidite ligand. This procedure provided β-substituted 2-pyridyl sulfones in moderate to good yields (46–86%) and good to excellent enantiomeric excess (70–96%) and is complementary to our recent publication on the addition of Grignard reagents to aliphatic substrates.8 These enantioenriched sulfones are potentially useful intermediates in the preparation of a wide variety of functionalized chiral building blocks.
Acknowledgements
Financial support from The Netherlands Organization for Scientific Research (NWO-CW) is acknowledged. We thank T. D. Tiemersma-Wegman (GC and HPLC) and A. Kiewiet (MS) for technical assistance (Stratingh Institute for Chemistry, University of Groningen).Notes and references
- (a) P. Perlmutter, Conjugate Addition Reactions in Organic Synthesis; Tetrahedron Organic Chemistry Series 9, Pergamon Press, Oxford, U.K., 1992 Search PubMed; (b) B. E. Rossiter and N. M. Swingle, Chem. Rev., 1992, 92, 771–806 CrossRef CAS.
- (a) For reviews, see: K. Tomioka and Y. Nagaoka, in Comprehensive Asymmetric Catalysis, ed. E. N. Jacobsen, A. Pfaltz, H. Yamamoto, Springer, New York, 1999, Vol. 3, pp. 1105–1120 Search PubMed; (b) B. L. Feringa, Acc. Chem. Res., 2000, 33, 346–353 CrossRef CAS; (c) N. Krause and A. Hoffmann-Röder, Synthesis, 2001, 171–196 CrossRef CAS; (d) B. L. Feringa, R. Naasz, R. Imbos and L. A. Arnold, in Modern Organocopper Chemistry, ed. N. Krause, VCH, Weinheim, Germany, 2002, pp. 224–258 Search PubMed; (e) A. Alexakis and C. Benhaim, Eur. J. Org. Chem., 2002, 3221–3236 CrossRef CAS; (f) T. Hayashi and K. Yamasaki, Chem. Rev., 2003, 103, 2829–2844 CrossRef CAS; (g) S. Woodward, Angew. Chem., Int. Ed., 2005, 44, 5560–5562 CrossRef CAS; (h) F. Lopéz, A. J. Minnaard and B. L. Feringa, in The Chemistry of Organomagnesium Compounds, ed. Z. Rappoport, I. Marek, Wiley, Chichester, U. K., 2008, Part 2, Chapter 17 Search PubMed; (i) S. R. Harutyunyan, T. den Hartog, K. Geurts, A. J. Minnaard and B. L. Feringa, Chem. Rev., 2008, 108, 2824–2852 CrossRef CAS.
- (a) E. N. Prilezhaeva, Russ. Chem. Rev., 2000, 69, 367 RSC; (b) S. E. Kelly, in Comprehensive Organic Synthesis, ed. B. M. Trost, I. Flemming, Pergamon Press, Oxford, 1991, Vol. 1, pp. 792 Search PubMed; (c) B. M. Trost, Bull. Chem. Soc. Jpn., 1988, 61, 107 CAS; (d) N. S. Simpkins, Sulphones in Organic Synthesis; Tetrahedron Organic Chemistry Series 10, Pergamon Press, Oxford, U.K., 1993 Search PubMed.
- T. Llamas, R. G. Arrayás and J. C. Carretero, Angew. Chem., Int. Ed., 2007, 46, 3329–3332 CrossRef CAS.
- J.-N. Desrosiers and A. B. Charette, Angew. Chem., Int. Ed., 2007, 46, 5955–5957 CrossRef CAS.
- (a) P. Mauleón and J. C. Carretero, Org. Lett., 2004, 6, 3195–3198 CrossRef CAS; (b) P. Mauleón and J. C. Carretero, Chem. Commun., 2005, 4961–4963 RSC; (c) P. Mauleón, I. Alonso, M. R. Rivero and J. C. Carretero, J. Org. Chem., 2007, 72, 9924–9935 CrossRef CAS.
- J.-N. Desrosiers, W. S. Bechara and A. B. Charette, Org. Lett., 2008, 10, 2315–2318 CrossRef CAS.
- P. H. Bos, A. J. Minnaard and B. L. Feringa, Org. Lett., 2008, 10, 4219–4222 CrossRef CAS.
- (a) B. L. Feringa, Acc. Chem. Res., 2000, 33, 346–353 CrossRef CAS; (b) A. J. Minnaard, B. L. Feringa, L. Lefort and J. G. de Vries, Acc. Chem. Res., 2007, 40, 1267 CrossRef CAS; (c) J. G. de Vries and L. Lefort, in Handbook of Homogenous Hydrogenation, ed. J. G. De Vries, C. J. Elsevier, Wiley-VCH Verlag, Weinheim, Germany, 2007, pp. 1245–1278 Search PubMed; (d) L. Lefort and J. G. de Vries, in Phosphorus Ligands in Asymmetric Catalysis, ed. A. Börmer, Wiley-VCH Verlag, Weinheim, Germany, 2008, pp. 1348–1376 Search PubMed; (e) R. B. C. Jagt, P. Y. Toullec, E. P. Schudde, J. G. de Vries, B. L. Feringa and A. J. Minnaard, J. Comb. Chem., 2007, 9, 407 CrossRef CAS.
- (a) A. H. M. de Vries, A. Meetsma and B. L. Feringa, Angew. Chem., Int. Ed. Engl., 1996, 35, 2374 CrossRef CAS; (b) B. L. Feringa, M. Pineschi, L. A. Arnold, R. Imbos and A. H. M. de Vries, Angew. Chem., Int. Ed. Engl., 1997, 36, 2620 CrossRef CAS; (c) A. Alexakis, D. Polet, S. Rosset and S. March, J. Org. Chem., 2004, 69, 5660 CrossRef CAS; (d) T. Jerphagnon, M. G. Pizzuti, A. J. Minnaard and B. L. Feringa, Chem. Soc. Rev., 2009, 38, 1039–1075 RSC.
- This dependence is in contrast to that reported for the asymmetric conjugate addition of Grignard reagents to α,β-unsaturated sulfones where the nature of the counterion hardly had an effect on conversion and enantioselectivity, see ref. 8.
Footnote |
| † Electronic supplementary information (ESI) available: General procedures for the synthesis of substrates and products; 1H and 13C NMR spectral data of all products. See DOI: 10.1039/b917269f |
| This journal is © The Royal Society of Chemistry 2010 |