Carbamate complexation by urea-based receptors: studies in solution and the solid state†
Peter R. Edwards, Jennifer R. Hiscock, Philip A. Gale* and Mark E. Light
School of Chemistry, University of Southampton, Southampton, UK. E-mail: philip.gale@soton.ac.uk; Fax: +44 2380 80596805
First published on 22nd October 2009
Abstract
The interactions of a series of urea based neutral hydrogen bond donor anion receptors have been investigated with i) alkylcarbamate anions formed by the reaction of carbon dioxide with primary aliphatic amines and ii) the zwitterionic species formed by the reaction of carbon dioxide with 1,4,5,6-tetrahydropyrimidine. Significant downfield chemical shift changes were observed for the urea NH protons in many cases, consistent with host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) anion hydrogen bonding interactions, and thus stabilisation of the carbon dioxide bound species. In the case of the alkylammonium-alkylcarbamate salts, this represents successful competition with electrostatic interactions between the alkylcarbamate and alkylammonium components of the salt. A synchrotron structure of a ternary complex formed by an amide appended diindolylurea, the ammonium carbamate salt formed by 1,3-diaminopropane and CO2 and 18-crown-6, was elucidated and shows the carbamate group bound by six hydrogen bonds (accepting five and donating one) to the functionalised diindolylurea.
anion hydrogen bonding interactions, and thus stabilisation of the carbon dioxide bound species. In the case of the alkylammonium-alkylcarbamate salts, this represents successful competition with electrostatic interactions between the alkylcarbamate and alkylammonium components of the salt. A synchrotron structure of a ternary complex formed by an amide appended diindolylurea, the ammonium carbamate salt formed by 1,3-diaminopropane and CO2 and 18-crown-6, was elucidated and shows the carbamate group bound by six hydrogen bonds (accepting five and donating one) to the functionalised diindolylurea.
Introduction
The emission of the green-house gas carbon dioxide has increased greatly over the past 200 years as a result of the increased use of fossil fuels.1 This has significantly raised the atmospheric concentration of carbon dioxide, which is anticipated to have widespread detrimental effects on the global climate.2 Much recent work has focused on the fixation,3 activation or stabilisation4 of carbon dioxide, with the aim of either removing excess carbon dioxide from the gas phase/atmosphere,5 or using it as a green chemical feedstock for the synthesis of specific chemical intermediates.6The use of primary amines as carbon dioxide “scrubbers” is extensive in industry due to their wide availability, low cost and the high stability of the alkylammonium-alkylcarbamate (AAAC) salt.7 It is also possible to release the carbon dioxide by moderate temperature elevation.8 Weiss and co-workers have previously shown that the two components of the salt exchange carbon dioxide and a proton very rapidly on the NMR timescale, (Scheme 1).9
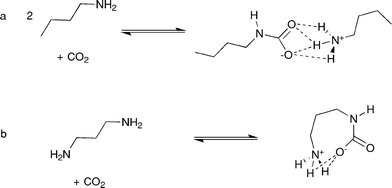 | ||
| Scheme 1 Formation of AAAC salts by reaction of a) n-butylamine and b) 1,3-diaminopropane, with carbon dioxide. | ||
Cyclic amidines such as 1,4,5,6-tetrahydropyrimidine (THP) have also attracted much attention as carbon dioxide fixation agents, due to their charge neutral products.10 The adduct formed by reaction of THP with carbon dioxide (THP-CO2), is zwitterionic, and can be thought of as analogous to the alkylcarbamate component of the AAAC salt, (Scheme 2).
 | ||
| Scheme 2 Formation of THP-CO2 by reaction of 1,4,5,6-tetrahydropyrimidine with carbon dioxide. | ||
We have recently reported the anion binding affinities of receptors 5–8 in H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO-d6 mixtures.11 Receptor 6 for example, binds acetate with a stability constant > 104 M−1 in 0.5% H2O
DMSO-d6 mixtures.11 Receptor 6 for example, binds acetate with a stability constant > 104 M−1 in 0.5% H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
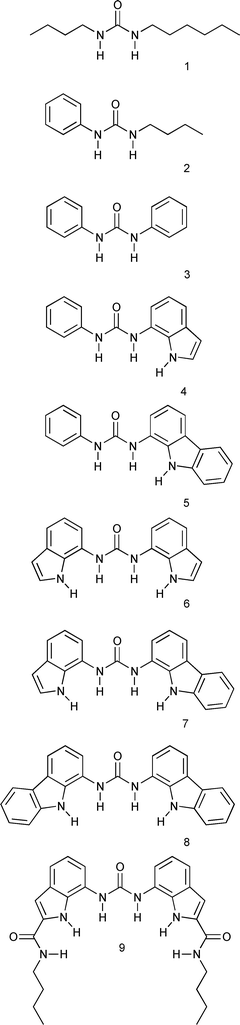
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO-d6. We hypothesised that these receptors and receptors 4.12 and 9,11d would be capable of binding and hence stabilising the alkylcarbamate anion component of AAAC salts and THP-CO2, via hydrogen bond formation in organic solution. Proton NMR experiments in DMSO-d6 were used to assess the interactions. Three other simple ureas, 1,13214 and 315 were prepared according to literature procedures and have been studied for comparison. Aspects of our work on AAAC salt complexation have been communicated previously.12
DMSO-d6. We hypothesised that these receptors and receptors 4.12 and 9,11d would be capable of binding and hence stabilising the alkylcarbamate anion component of AAAC salts and THP-CO2, via hydrogen bond formation in organic solution. Proton NMR experiments in DMSO-d6 were used to assess the interactions. Three other simple ureas, 1,13214 and 315 were prepared according to literature procedures and have been studied for comparison. Aspects of our work on AAAC salt complexation have been communicated previously.12
When AAAC salts are formed in solution with a suitable receptor, it is anticipated that the anionic component of the salt will form hydrogen bonding interactions with the receptor. We hypothesised that addition of 18-crown-6 would result in complex formation with the alkylammonium cation of the AAAC salt16 and hence reduce the degree of ion-pairing in solution between the ammonium and carbamate groups (Scheme 3).
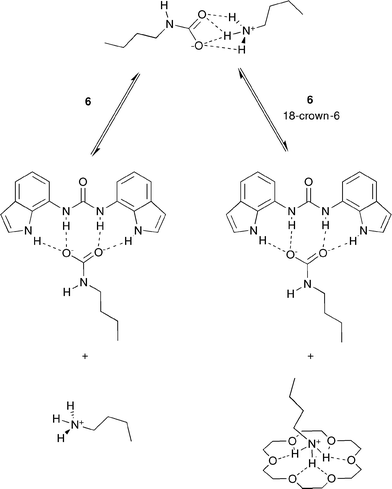 | ||
| Scheme 3 Expected binding modes between 6 and AAAC salts in the presence and absence of 18-crown-6. | ||
In the case of THP-CO2 there is expected to be a lower degree of ion-pairing in solution as the adduct is a neutral zwitterion. This will lead to an enhanced interaction between the zwitterion and urea-based receptor and hence stronger binding to the receptors than was observed with the AAAC salts, (Scheme 4).
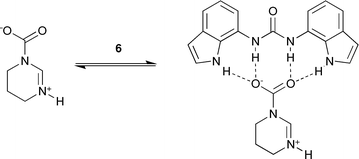 | ||
| Scheme 4 Expected binding mode between 6 and THP-CO2. | ||
Results and discussion
Initially, we attempted to measure stability constants between receptors 1–9 and the activated CO2 species using 1H NMR titration techniques. However, we found that the data obtained could not be reproduced reliably. This is presumably due to the loss of carbon dioxide from solution over the timescale of the titration, leaving unknown concentrations of the AAAC or THP-CO2 adduct in solution.We have observed that when one equivalent of AAAC salt or THP-CO2 adduct is formed in solution in the presence of a receptor, carbon dioxide is not readily lost from solution as evidenced by the high reproducibility of the chemical shift of the urea NH protons of 1–9 in the presence of AAAC salts or THP-CO2. We have observed that the solutions produce identical spectra 24 h after preparation.
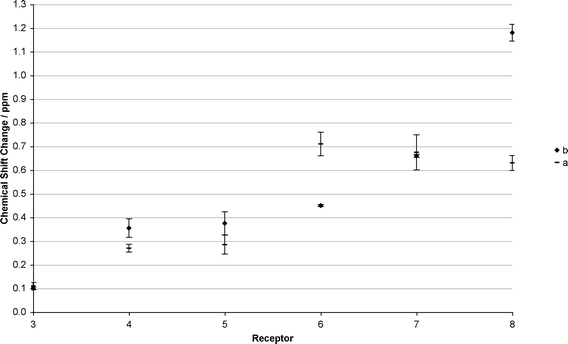
We looked at the correlation between chemical shift upon addition of one equivalent of guest and stability constant for titrations of the receptors with tetrabutylammonium benzoate which had been conducted previously11 and also conducted new titrations with receptors 1, 2 and 4 and tetrabutylammonium benzoate. The results (shown in the ESI) show an average shift of 1.2 ppm for the urea protons of compounds 1, 2 and 3 which have stability constants between 17 M−1 and 674 M−1, 1.75 ppm for compounds 5, 7 and 8 which have stability constants between 3400 and 5880 M−1, and 2.4 ppm for compounds 4, 6 and 9 with stability constants > 104 M−1. It may be that the different families of compounds here (e.g. simple ureas, ureas with one extra hydrogen bond donor, ureas with two extra hydrogen bond donors and compound 9) have different binding modes with the carbamate guests. However, the correspondence between chemical shift of the urea NH groups and Ka observed with the compounds in the presence of one equivalent of benzoate is evidence that leads us to expect at least some degree of correlation between the chemical shift of the urea NH protons in the absence and presence of one equivalent of carbamate and the stability constant with carbamate.
A series of solutions were prepared in DMSO-d6 containing 1 mM of receptor 1–9 and either, a) 2 eq. n-butylamine, b) 1 eq. 1,3-diaminopropane, c) 2 eq. n-butylamine + 1 eq. 18-crown-6, d) 1 eq. 1,3-diaminopropane + 1 eq. 18-crown-6, or e) 1 eq. THP. The compounds were dissolved and subjected to bubbling of carbon dioxide for 3 min. Mean chemical shift changes of three repeats are presented, (Tables 1–5).
| Urea NH (downfield resonance) | Urea NH (upfield resonance) | |
|---|---|---|
| Chemical shift changes presented are the mean of three repeats. | ||
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 0.11 (13) | |
| 4 | 0.26 (6) | 0.28 (6) |
| 5 | 0.27 (13) | 0.30 (15) |
| 6 | 0.71 (7) | |
| 7 | 0.67 (10) | 0.68 (12) |
| 8 | 0.63 (5) | |
| Urea NH (downfield resonance) | Urea NH (upfield resonance) | |
|---|---|---|
| Chemical shift changes presented are the mean of three repeats. | ||
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 0.08 (23) | |
| 4 | 0.28 (14) | 0.30 (15) |
| 5 | 0.37 (9) | 0.42 (10) |
| 6 | 0.70 (10) | |
| 7 | 0.50 (7) | 0.51 (6) |
| 8 | 0.56 (6) | |
| Urea NH (downfield resonance) | Urea NH (upfield resonance) | |
|---|---|---|
| Chemical shift changes presented are the mean of three repeats. | ||
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 0.10 (6) | |
| 4 | 0.34 (15) | 0.37 (7) |
| 5 | 0.36 (13) | 0.39 (13) |
| 6 | 0.45 (1) | |
| 7 | 0.66 (1) | 0.66 (1) |
| 8 | 1.18 (3) | |
| Urea NH (downfield resonance) | Urea NH (upfield resonance) | |
|---|---|---|
| Chemical shift changes presented are the means of three repeats. | ||
| 1 | 0 | 0 |
| 2 | 0 | 0 |
| 3 | 0.15 (6) | |
| 4 | 0.43 (4) | 0.46 (5) |
| 5 | 0.40 (2) | 0.45 (2) |
| 6 | 0.59 (6) | |
| 7 | 0.78 (5) | 0.78 (5) |
| 8 | 0.77 (10) | |
| Urea NH (downfield resonance) | Urea NH (upfield resonance) | |
|---|---|---|
| Chemical shift changes presented are the means of three repeats. | ||
| 1 | 0.03 (37) | 0.03 (37) |
| 2 | 0.22 (13) | 0.23 (13) |
| 3 | 0.75 (4) | |
| 4 | 1.09 (12) | 1.32 (10) |
| 5 | 1.06 (4) | 1.25 (4) |
| 6 | 1.17 (5) | |
| 7 | 1.31 (4) | 1.34 (4) |
| 8 | 1.42 (3) | |
| 9 | 0.87 (6) | |
Downfield chemical shift changes were observed for the urea NH proton resonances for many receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest combinations. The smallest magnitude chemical shift changes were observed with the AAAC salts in the absence of 18-crown-6. Slightly larger chemical shift changes were observed with the AAAC salts in the presence of 18-crown-6, whilst larger chemical shift changes were observed in the presence of the THP-CO2 adduct. Receptors 1–3 contain two hydrogen bond donors and showed the smallest chemical shift changes with each AAAC salt and the THP-CO2 adduct. Receptors 4–5, which contain three hydrogen bond donors, have slightly larger chemical shift changes, whilst receptors 6–8, which contain four hydrogen bond donors, have the largest chemical shift changes of this series of receptors. Chemical shift changes could not be obtained for receptor 9 with either AAAC salt, in either the presence or absence of 18-crown-6, due to resonance broadening in the 1H NMR spectrum. With THP-CO2, a moderate chemical shift change was observed.
guest combinations. The smallest magnitude chemical shift changes were observed with the AAAC salts in the absence of 18-crown-6. Slightly larger chemical shift changes were observed with the AAAC salts in the presence of 18-crown-6, whilst larger chemical shift changes were observed in the presence of the THP-CO2 adduct. Receptors 1–3 contain two hydrogen bond donors and showed the smallest chemical shift changes with each AAAC salt and the THP-CO2 adduct. Receptors 4–5, which contain three hydrogen bond donors, have slightly larger chemical shift changes, whilst receptors 6–8, which contain four hydrogen bond donors, have the largest chemical shift changes of this series of receptors. Chemical shift changes could not be obtained for receptor 9 with either AAAC salt, in either the presence or absence of 18-crown-6, due to resonance broadening in the 1H NMR spectrum. With THP-CO2, a moderate chemical shift change was observed.
Alkylammonium![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h3_char_2009.gif) alkylcarbamate salts
alkylcarbamate salts
The chemical shift changes of the urea NH protons with AAAC salts are relatively small compared to those observed with tetrabutylammonium oxo-anion salts or THP-CO2. We propose that this can be attributed to the non-innocent nature of the ammonium cation, resulting in a large proportion of the alkylcarbamate anion bound via hydrogen bonds and electrostatic interactions to the alkylammonium cation. The neutral receptors compete with the ion-pairing interaction to different extents, dependent on their relative affinities for carbamate anions.In the absence or presence of 18-crown-6 no chemical shift changes are observed for receptors 1 or 2 (Fig. 1 and 2). This is attributed to their poor oxo-anion binding affinities in the competitive solvent, DMSO-d6. With receptor 3 (which binds benzoate with Ka = 674 M−1 in 0.5% H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO-d6)11c there are chemical shift changes of around 0.08–0.15 ppm for the urea NH protons (Fig. 1 and 2). With receptors 4 and 5 (5 binds benzoate with Ka = 3420 M−1 in 0.5% H2O
DMSO-d6)11c there are chemical shift changes of around 0.08–0.15 ppm for the urea NH protons (Fig. 1 and 2). With receptors 4 and 5 (5 binds benzoate with Ka = 3420 M−1 in 0.5% H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO-d6)11b there are larger chemical shift changes of around 0.26–0.45 ppm (Fig. 1 and 2). With receptors 6, 7, and 8 (which bind benzoate 5880 M−1 < Ka in 0.5% H2O
DMSO-d6)11b there are larger chemical shift changes of around 0.26–0.45 ppm (Fig. 1 and 2). With receptors 6, 7, and 8 (which bind benzoate 5880 M−1 < Ka in 0.5% H2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [D6]DMSO)11 the chemical shift changes are all larger, around 0.45–1.18 ppm (Fig. 1 and 2).
[D6]DMSO)11 the chemical shift changes are all larger, around 0.45–1.18 ppm (Fig. 1 and 2).
 | ||
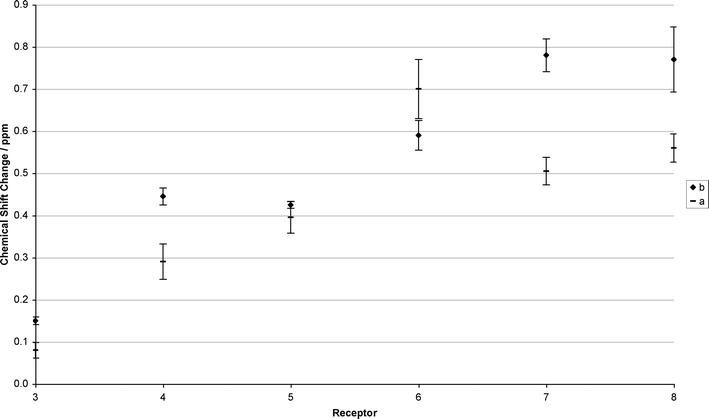
| Fig. 1 Chemical shift changes of one or two urea NH proton(s) (averaged with errors) observed for receptors 3–8 upon addition of a) 2 eq. n-butylamine bubbled with carbon dioxide for three minutes, and b) 2 eq. n-butylamine + 1 eq. 18-crown-6, bubbled with carbon dioxide for three minutes. | ||
 | ||
| Fig. 2 Chemical shift changes of one or two urea NH proton(s) (averaged with errors) observed for receptors 3–8 upon addition of a) 1 eq. 1,3-diaminopropane bubbled with carbon dioxide for three minutes, and b) 1 eq. 1,3-diaminopropane + 1 eq. 18-crown-6, bubbled with carbon dioxide for three minutes. | ||
It can be seen from this data, that the larger the stability constant for tetrabutylammonium benzoate complexation, the larger the chemical shift changes with alkylcarbamate anions. Whilst care should be taken when attempting to draw comparisons between chemical shift changes and binding affinities, analogy with our previous work suggests that receptors 6–8 will bind AAAC salts most strongly. It is reasonable to conclude that when looking across this series of structurally related receptors when following a similar urea NH group, that a high affinity receptor should exhibit larger chemical shift changes than a low affinity receptor in general.
The addition of 18-crown-6 has the effect of increasing the chemical shift changes for the majority of the receptors compared to the absence of 18-crown-6 and is particularly evident with receptor 8 (Fig. 1). This observation appears to confirm the hypothesis that addition of 18-crown-6 will reduce the degree of ion-pairing in solution between the ammonium cations and carbamate anions.
However, interestingly, the chemical shift change of receptor 6decreases upon addition of 18-crown-6. This leads to a trend where the chemical shift changes are related to the number of hydrogen bond donors and acidity of the indole or carbazole pendant groups (Fig. 1 and 2). Both of these increase from receptor 1 to receptor 8 (pKa indole NH = 21.0 in DMSO, pKa carbazole NH = 19.9 in DMSO)17 causing increasingly larger chemical shift changes. It is not yet clear why compound 6 undergoes a larger chemical shift in the absence of 18-crown-6 anions than more acidic compounds 7 and 8. The decrease in chemical shift of the urea protons of compound 6 upon addition of 18-crown-6 brings the chemical shift into line with what would be expected considering the acidity of the receptor relative to the other receptors studied.
THP-CO2 adduct
The THP-CO2 adduct can be thought of as a zwitterionic AAAC analogue. The species is neutral and therefore there is less scope for ion pairing in solution. It is therefore expected that THP-CO2 will cause greater chemical shift changes of the urea NH protons of receptors 1–8, than AAAC salts due to the lower degree of ion-pairing. It was found that THP-CO2 causes much larger chemical shift changes than the AAAC alkylcarbamate anions. THP-CO2 causes a small chemical shift change with receptor 1, and causes chemical shift changes of 0.22 and 0.23 ppm for the two urea NH protons of receptor 2 (Fig. 3). The chemical shift changes of the urea NH protons then increase from 0.75 ppm (3), to between 1.06-1.42 ppm (4–8) (Fig. 3). As with AAAC salts in the presence of 18-crown-6, receptors 7 and 8 have the largest chemical shift changes, (Fig. 3). The urea NH protons of receptor 9 undergo smaller chemical shift changes than those in compounds 6–8 with THP-CO2 under identical conditions.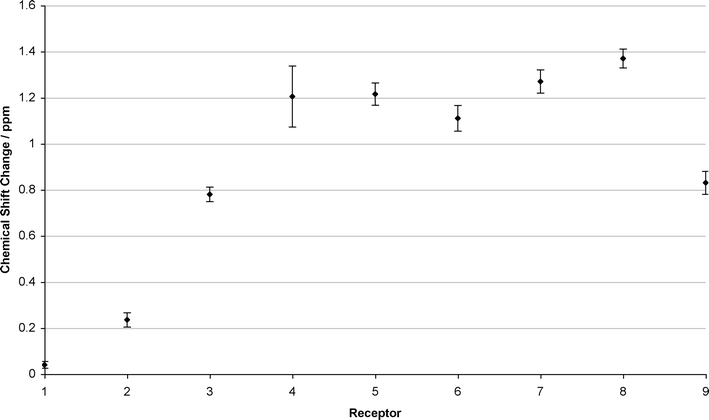 | ||
| Fig. 3 Chemical shift changes of one or two urea NH proton(s) (averaged with errors) observed for receptors 1–9 in the presence of 1 eq. 1,4,5,6-tetrahydropyrimidine, bubbled with carbon dioxide for 3 min. | ||
Solid state studies
Crystals of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ternary complex of 9, 18-crown-6 and the AAAC salt formed from carbon dioxide and 1,3-diaminopropane were obtained by slow evaporation of a DMSO solution of the complex. The structure (Fig. 4) shows the zwitterion bound to both receptor 9 (complexing the carbamate group by donating five hydrogen bonds and accepting one) and 18-crown-6 (binding the ammonium group). There are two such complexes in the asymmetric unit. The amide groups in receptor 9 are oriented such that one NH is oriented into the binding site whilst the other amide is oriented such that the C
1 ternary complex of 9, 18-crown-6 and the AAAC salt formed from carbon dioxide and 1,3-diaminopropane were obtained by slow evaporation of a DMSO solution of the complex. The structure (Fig. 4) shows the zwitterion bound to both receptor 9 (complexing the carbamate group by donating five hydrogen bonds and accepting one) and 18-crown-6 (binding the ammonium group). There are two such complexes in the asymmetric unit. The amide groups in receptor 9 are oriented such that one NH is oriented into the binding site whilst the other amide is oriented such that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O groups are oriented into the binding site. This allows one amide to donate a hydrogen bond to a carbamate oxygen whilst the other amide C
O groups are oriented into the binding site. This allows one amide to donate a hydrogen bond to a carbamate oxygen whilst the other amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O can accept a hydrogen bond from the carbamate NH group. The structure of the complex is also shown schematically (Fig. 5). Please see the supplementary information for additional views and a table of hydrogen bonding interactions present in this complex.‡
O can accept a hydrogen bond from the carbamate NH group. The structure of the complex is also shown schematically (Fig. 5). Please see the supplementary information for additional views and a table of hydrogen bonding interactions present in this complex.‡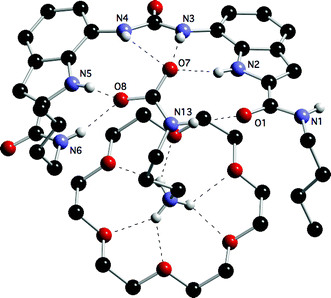 | ||
| Fig. 4 A view of one of the two complexes in the asymmetric unit of the ternary complex of the AAAC salt formed by 1,3-diaminopropane and carbon dioxide bound to receptor 9 and 18-crown-6. Solvent and non-acidic hydrogen atoms have been omitted for clarity. | ||
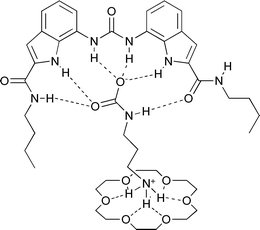 | ||
| Fig. 5 Diagram of one of the two complexes in the asymmetric unit of the ternary complex of the AAAC salt formed by 1,3-diaminopropane and carbon dioxide bound to receptor 9 and 18-crown-6. | ||
Conclusions
We have shown that it is possible to bind the alkylcarbamate portion of alkylammonium alkylcarbamate salts in solution using a series of urea-based receptors under competitive conditions. An indication of the relative strengths of these interactions can be gained by considering the magnitude of the chemical shift changes of the urea NH protons that are involved in the binding. We have also demonstrated that it is possible to stabilise the alkylammonium cation with a crown ether, which in most cases leads to enhanced hydrogen bonding between the neutral carbamate receptor and the alkylcarbamate anion. Decreasing the degree of ion-pairing in solution by using the zwitterionic species THP-CO2 instead of alkylammonium alkylcarbamate salts causes significantly larger chemical shift changes, consistent with a greater effective concentration of anionic species in solution. The crystal structure of the AAAC salt bound to receptor 9 and 18-crown-6 illustrates how a combination of anion and cation receptors can be employed to complex species formed from the reaction of diamines and carbon dioxide.Acknowledgements
PAG thanks the EPSRC (C-Cycle) for funding. The authors would like to thank the EPSRC for funding and Professor W. Clegg and members of the National Crystallography Service for data collection at Diamond.18Notes and references
- M. Aresta and A. Dibendetto, Dalton Trans., 2007, 2975 RSC.
- The Third Assessment Report of the Intergovernmental Panel on Climate Change, B. Mertz, O. Davidson, R. Sware and J. Pan, Cambridge University Press, Cambridge, 2001 Search PubMed.
- (a) R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, Science, 2008, 319, 939 CrossRef CAS; (b) Z. Chen, H.-S. Song, M. Portillo, C. Jim Lim, J. R. Grace and E. J. Anthony, Energy Fuels, 2009, 23, 1437 CrossRef CAS; (c) X.-X. Zhao, X.-L. Zu, S.-B. Sun, L.-L. Zhang and X.-Q. Liu, Energy Fuels, 2009, 23, 1534 CrossRef CAS; (d) C. Zhao, X. Chen and Y. Liu, Energy Fuels, 2009, 23, 1766 CrossRef CAS; (e) J. L. Atwood, L. J. Barbour and J. Jerga, Angew. Chem., Int. Ed., 2004, 43, 2948 CrossRef CAS; (f) S. J. Dalgarno, J. Tian, J. E. Warren, T. E. Clark, M. Makha, C. L. Raston and J. L. Atwood, Chem. Commun., 2007, 4848 RSC; (g) H. Zhou, W.-Z. Ziang, Y.-M. Wang, J.-P. Qu and X.-B. Lu, Macromolecules, 2009, 42, 5419 CrossRef CAS.
- (a) B. Verdejo, S. Blasco, J. Gonzalez, E. García-España, P. Gavina, S. Tatay, A. Domenech, M. T. Domenech-Carbo, H. R. Jimenez and C. Soriano, Eur. J. Inorg. Chem., 2008, 84 CrossRef CAS; (b) E. García-España, P. Gavina, J. Latorre, C. Soriano and B. Verdejo, J. Am. Chem. Soc., 2004, 126, 5082 CrossRef CAS; (c) S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2006, 4344 RSC.
- (a) Z. Liang, M. Marshall and A. L. Chaffee, Energy Fuels, 2009, 23, 2785 CrossRef CAS; (b) Z. Q. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368 CrossRef CAS; (c) O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705 CrossRef CAS; (d) B. Arstad, H. Fjellvag, K. O. Kongshuang, O. Swang and R. Blom, Adsorption, 2008, 14, 755 CrossRef CAS; (e) S. Couck, J. F. M. Denayer, G. V. Baron, T. Rémy, J. Gascon and F. Kapteijn, J. Am. Chem. Soc., 2009, 131, 6326 CrossRef CAS; (f) B. Mu, F. Li and K. S. Walton, Chem. Commun., 2009, 2493 RSC; (g) L. Natrajan, J. Pécaut and M. Mazzanti, Dalton Trans., 2006, 1002 RSC; (h) S. J. Brooks, P. A. Gale and M. E. Light, Chem. Commun., 2006, 4344 RSC; (i) J. A. Tossell, Inorg. Chem., 2009, 48, 7105 CrossRef CAS; (j) S. J. Brooks, S. E. García-Garrido, M. E. Light, P. A. Cole and P. A. Gale, Chem.–Eur. J., 2007, 13, 3320–3329 CrossRef.
- (a) X. Zheng, Z. Luo, L. Zhang and J.-P. Cheng, Green Chem., 2009, 11, 455 RSC; (b) Y. O. Sharma and M. S. Degani, Green Chem., 2009, 11, 526 RSC; (c) J. Song, Z. Zhang, S. Hu, T. Wu, T. Jiang and B. Han, Green Chem., 2009, 11, 1031 RSC.
- (a) A. Diaf, L. J. Garcia and E. J. Beckmann, J. Appl. Polym. Sci., 1994, 53, 857 CrossRef CAS; (b) A. Diaf, R. M. Enick and E. J. Beckamnn, J. Appl. Polym. Sci., 1993, 50, 835 CrossRef CAS; (c) G. Sartori, W. S. Ho and D. W. Savage, Sep. Purif. Rev., 1987, 16, 171 CrossRef CAS.
- N. Belman, J. N. Israelachvili, Y. Li, C. R. Safinya, J. Bernstein and Y. Golan, J. Am. Chem. Soc., 2009, 131, 9107 CrossRef CAS.
- M. George and R. G. Weiss, Langmuir, 2003, 19, 8168 CrossRef CAS.
- (a) T. Endo, D. Nagai, H. Yamaguchi and B. Ochiai, Macromolecules, 2004, 37, 2007 CrossRef CAS; (b) T. Seçkin, B. Alici, E. Çetinkaya and I. özdemir, J. Polym. Sci., Part A: Polym. Chem., 1997, 35, 2411 CrossRef CAS.
- (a) C. Caltagirone, J. R. Hiscock, M. B. Hursthouse, M. E. Light and P. A. Gale, Chem.–Eur. J., 2008, 14, 10236 CrossRef CAS; (b) J. R. Hiscock, C. Caltagirone, M. E. Light, M. B. Hursthouse and P. A. Gale, Org. Biomol. Chem., 2009, 7, 1781 RSC; (c) C. Caltagirone, P. A. Gale, J. R. Hiscock, S. J. Brooks, M. B. Hursthouse and M. E. Light, Chem. Commun., 2008, 3007 RSC; (d) P. A. Gale, J. R. Hiscock, S. J. Moore, C. Caltagirone, M. B. Hursthouse and M. E. Light, Chem. Asian J. DOI:10.1002/asia.200900230. For our other work on indole containing anion receptors see: (e) G. W. Bates Triyanti, M. E. Light, M. Albrecht and P. A. Gale, J. Org. Chem., 2007, 72, 8921 CrossRef CAS; (f) C. Caltagirone, P. A. Gale, J. R. Hiscock, M. B. Hursthouse, M. E. Light and G. J. Tizzard, Supramol. Chem., 2009, 21, 125 CrossRef CAS; (g) D. Makuc, M. Lenarčič, G. W. Bates, P. A. Gale and J. Plavec, Org. Biomol. Chem., 2009, 7, 3505 RSC.
- P. R. Edwards, J. R. Hiscock and P. A. Gale, Tetrahedron Lett., 2009, 50, 4922 CrossRef CAS.
- N. R. McElroy and P. C. Jurs, J. Med. Chem., 2003, 46, 1066 CrossRef CAS.
- T. Mizuno, T. Kino, T. Ito and T. Miyata, Synth. Commun., 2000, 30, 1675 CrossRef CAS.
- M. E. Sitzmann and W. H. Gilligan, J. Org. Chem., 1985, 50, 5879 CrossRef CAS.
- R. M. Izatt, J. D. Lamb, N. E. Izatt, B. E. Rossiter Jr., J. J. Christensen and B. L. Haymore, J. Am. Chem. Soc., 1979, 101, 6273 CrossRef.
- F. E. Bordwell, G. E. Drucker and H. E. Fried, J. Org. Chem., 1981, 46, 632 CrossRef CAS.
- W. Clegg, J. Chem. Soc., Dalton Trans., 2000, 3223 RSC.
Footnotes |
† Electronic supplementary information (ESI) available: Representative 1H NMR spectra for each combination of receptor![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest. Additional views of crystal structure, and table of hydrogen bonding interactions. CCDC reference number 745012. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b917140a guest. Additional views of crystal structure, and table of hydrogen bonding interactions. CCDC reference number 745012. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/b917140a |
| ‡ Crystallographic data were collected at Diamond beamline I19 using a Rigaku Saturn 724+ detector on a Crystal Logics diffractometer. Crystal data for 9[H3N+(CH2)3NHCO2−][18-crown-6]: 2(C27H32N6O3) 2(C12H24O6) 2(C4H10N2O2) 0.5(C2H6OS), M = 1781.14, triclinic, a = 12.626(7), b = 14.165(9), c = 27.564(17) Å, α = 84.204(10)°, β = 77.246(8)°, γ = 72.121(9)°, U = 4573(5) Å3, T = 120(2) K, space group P |
| This journal is © The Royal Society of Chemistry 2010 |

![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ,
,