Adsorption and binding of capping molecules for highly luminescent CdSe nanocrystals – DFT simulation studies†
Hung-Lung
Chou
a,
Chih-Hsiang
Tseng
a,
K. Chandrasekara
Pillai
a,
Bing-Joe
Hwang
ab and
Liang-Yih
Chen
*a
aDepartment of Chemical Engineering, National Taiwan University of Science and Technology, 43, Section 4, Keelung Road, Taipei, 106, Taiwan. E-mail: sampras@mail.ntust.edu.tw
bNational Synchrotron Radiation Research Center (NSRRC), Hsinchu, 30076, Taiwan
First published on 19th October 2010
Abstract
During CdSe nanocrystal growth, loss of surface capping molecules occurs leading to a decrease of photoluminescence (PL) quantum yield. In general, aliphatic capping molecules are applied to passivate the surface of CdSe nanocrystals to modulate the optical properties of the CdSe. In this work, two kinds of alkylamine (n-butylamine (n-BA) and n-hexylamine (n-HA)) and oleic acid (OA) were used to modify the surfaces of the CdSe nanocrystals. From the PL spectra and quantum yield analyses, we observed that the PL emission peak positions of the modified CdSe nanocrystals have blue shifted for all three capping molecules. However, the PL quantum yield of the CdSe nanocrystals increased after introduction of the alkylamine molecules, but decreased with oleic acid. The detailed mechanism was not clear until now. In this study, a density function theory (DFT) simulation was employed to demonstrate binding energy and charge analyses of CdSe with n-BA, n-HA and OA. By comparing the binding energy of the bare CdSe nanocrystals to that of the CdSe with the capping molecules, it was shown that n-BA and n-HA as capping molecules help to increase the charge on Se and decrease it on cadmium of the CdSe.
Introduction
Semiconductor nanocrystals have attracted great interest over the past years because their properties are remarkably different from bulk semiconductors and can be tailored by controlling their composition, size and surface.1 These characteristics arise from several phenomena (viz. quantum confinement of charge carriers, surface effects, and geometrical confinement of phonons) and have turned semiconductor nanocrystals into promising materials for many applications, such as light-emitting diodes,2–4 lasers,5,6 photonic band gap crystals,7 ultrafast photonic switches, and biomedical tags for biological imaging, and nanosensors.8,9It has been established that the mechanism responsible for the photoluminescence (PL) of nano materials is electron-hole recombination, an event that takes place in the interior of the crystallites. Therefore, proper electronic passivation of the nanocrystal surface serves to reduce the number of surface defects, which are thought to be responsible for non-radiative recombination, to achieve a high quantum yield. While the surfaces capped by various organic or inorganic molecules appear to influence only moderately the absorption characteristics, it is well know that the emission efficiency and time evolution are very strongly affected by the surface treatment. Control of the surfaces is in particular the key to highly luminescent nanocrystals. Recently, several groups studied inorganic passivation of CdSe nanocrystals with higher band gap materials, such as CdSe/ZnS,10,11 CdSe/ZnSe,12,13 CdSe/CdS,14,15etc. Generally, choosing the relative band gap positions leads to enhanced charge transfer or improved luminescence. However, syntheses of core/shell nanocrystals go through additional coating procedures using highly active precursors, which have to be chosen very selectively for appropriate passivation and quantum confinement effects. Also, ideally epitaxial encasing would not be easily achieved and the bonding characteristics introduce additional difficulties to maintain size control or prevent alloying.16
In a previous report, capping molecule-protected CdSe nanocrystals consisting of semiconductor cores surrounded by organic monolayer have attracted considerable interest for applications in materials science and nanotechnology.17–19 Majeitch et al. showed increased PL of CdSe nanocrystals capped with butanethiolate.20 Talapin et al. reported blue shifts in the UV/visible absorption and PL spectra of the CdSe nanocrystals upon exchange of trioctylphosphine oxide (TOPO) with amines, such as n-hexadecylamine, n-dodecylamine or allylamine, producing roughly an order of magnitude increase in PL quantum yield.10 Although these studies enabled syntheses of stable capping molecule-protected semiconductor nanocrystals with various sizes, shapes, and compositions, very few studies have been conducted on the surface structures and properties of capping molecules. Not much is known regarding the nature and chemical properties of the binding between nanocrystals and their ligands. In selecting the capping molecules for the nanocrystals, a general practice is to extend the knowledge from traditional solution-coordination chemistry and surface chemistry on bulk materials. It must, however, be noted that compared to atoms on the flat surface of bulk substrates, the binding abilities of the atoms on the curved surfaces may be affected by their diverse structural environments and size-dependent electron configuration.
Despite the importance of surface exchange reactions with organic capping molecules for functionalization of semiconductor nanocrystals, information on the influence of the organic ligands on the optical properties of CdSe nanocrystals is fragmented. The knowledge of binding details is crucial for understanding of size and shape control during nanocrystal growth. In some cases, this lack of understanding may hinder the correct interpretation of experimental data. Computational approaches provide a useful tool for studying ligand adsorption.21–23 Classical simulation studies of bare CdSe nanocrystals have resolved the mechanism of surface relaxation23,24 and of pressure induced phase transitions.25,26 CdSe nanocrystals capped by TOPO were considered by Rabani,27 describing the surface packing and the total dipole moment of the capped nanocrystals. Ab initio calculations have the potential to deliver reliable results; but, they are limited to very small systems and do not account, typically, for thermal fluctuations. Thus, binding energies for capping molecules were computed for different surface sites of CdSe nanocrystals by classical simulation21,22 and density functional theory (DFT);28–30 and adsorption of ligands to bulk CdSe planes by DFT was investigated.31 In spite of a wealth of experimental data reported for CdSe nanocrystals, many fundamental questions regarding their structural and optoelectronic properties remain unanswered.
The present research systematically examines the consequences of exposure of CdSe nanocrystals to putative surface capping molecules on the optical properties of the CdSe nanocrystals. The synthesis of CdSe nanocrystals was conducted in non-coordinating solvent, 1-octadecene, in this work. In order to study the influence of the capping molecules on the luminescence properties of CdSe nanocrystals, the surface of CdSe nanocrystals was modified by n-butylamine (n-BA), n-hexylamine (n-HA) and oleic acid (OA). From DFT simulation, we demonstrate binding energy and charge analysis of CdSe with n-BA, n-HA and OA. By comparing the binding energy of the bare CdSe nanocrystals to that of the CdSe with the capping molecules, it was shown that n-BA and n-HA as capping molecules help to increase the charge on Se and decrease it on cadmium of the CdSe.
Experimental section
Synthesis of CdSe nanocrystals
Cadmium oxide (CdO, Alfa Aesar, 99.98%), selenium powder (Se, Alfa Aesar, 99.999%), 1-octadecene (1-ODE, Acros, 90%), tri-n-butylphosphine (TBP, Strem, 99%), oleic acid (OA, Showa, ACS), n-butylamine (n-BA, TCI, ACS reagent), n-hexylamine (n-HA, TCI, ACS reagent), Rhodamine 6G (R6G, Fluka), toluene, methanol and hexane (Tedia) were used without further purification.The synthesis of CdSe nanocrystals was conducted in a noncoordinating solvent, and 1-ODE was used in this work. A typical procedure for the synthesis of CdSe nanocrystals is as follows. CdO (0.16 mmol) was mixed with 0.7 mmol OA and 4.8 g 1-ODE in a 25 mL three-neck flask. The mixture was heated to 300 °C under Ar flow for 30 min, and Se stock solution (0.1 mmol of Se powder dissolved in 0.62 mmol of TBP and 1 g of ODE) was then injected. The solution mixture was cooled and the nanocrystals were allowed to grow at 260 °C to reach the desired size, as determined by UV-Visible absorption. To monitor CdSe nanocrystal growth, a small amount of sample (∼0.2 mL) was taken via a syringe and diluted to show an optical density between 0.1 and 0.2 by the addition of anhydrous toluene. The resulting CdSe nanocrystals were dissolved in toluene and the unreacted starting materials and side products were removed by extraction and precipitation procedures reported previously.32 No size sorting was performed in any of the samples reported here.
Ligand modification process for CdSe nanocrystals
An aliquot of CdSe nanocrystals solution was diluted with toluene to yield an optical density of approximately 0.1. A 3 mL portion of the nanocrystals solution was mixed with various capping molecules at a fixed concentration of 5 mM for surface modification. The solution mixture was stirred in the dark at room temperature for 1 h. Subsequently, CdSe nanocrystals were precipitated with methanol and re-dispersed in toluene for characterization of the change of PL quantum yield by UV/vis absorption and photoluminescence spectroscopy.Characterization
The room temperature UV-Visible absorption spectroscopy measurements were carried out from a Jasco V-560 on samples with low optical density (≤0.1 at the emission maximum) in order to minimize re-absorption and avoid absorption saturation. PL spectra were acquired from a Hitachi HF-7100 upon excitation at 350 nm, using a 300 W Xe lamp as the excitation source and a double grating monochromator. PL quantum yields were obtained according to reported procedures, using rhodamine 6G as standard with a quantum yield of 95% in ethanol. The size, size distribution, structure and orientation of the CdSe nanocrystals were analyzed by high resolution transmission electron microscopy (HR-TEM) in a Philip Tecnai-G2 operating at 200 kV. The TEM specimens were prepared by placing a drop of diluted CdSe nanocrystal solution onto a carbon/pioloform film supported on a copper mesh grid, and allowing it to dry under air at room temperature.Computational details
In DFT calculations, we used the VASP software, which uses plane waves as basis functions, and the ultrasoft (PW91) method generalized gradient approximation (GGA).33–36 In the calculation of the plane waves we used a cut-off energy of 400 eV, which was chosen by total energy convergence calculation for the CdSe cluster. The CdSe cluster was initially constructed on a wurtzite lattice with bulk Cd–Se bond lengths and then allowed to reach its lowest energy configuration by a relaxation procedure. By this optimization procedure, CdSe clusters attached with capping molecules n-BA, n-HA or OA were investigated, and the binding energy of the ligands and the charge transfer in CdSe nanocrystal-ligand composites were estimated.Results and discussion
Fig. 1(a) shows the TEM image of the as-grown CdSe nanocrystals synthesized by the non-coordinate method. It shows that the material has a very uniform size distribution and regular shape with 5 nm CdSe nanocrystals forming close-packed arrays. The high resolution TEM image in Fig. 1(b) also shows the crystallinity of CdSe nanocrystals. The corresponding fast Fourier transfer (FFT) diffraction pattern of Fig. 1(b), shown in Fig. 1(c), indicates the crystal structure of CdSe nanocrystals as zinc blend.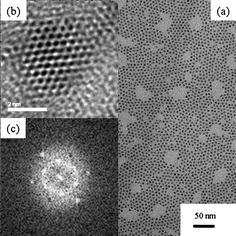 | ||
| Fig. 1 (a) TEM image of a sample of CdSe nanocrystals; (b) HRTEM image of a single CdSe nanocrystals and (c) the corresponding fast Fourier transfer diffraction pattern. | ||
The absorption and photoluminescence spectra of these CdSe nanocrystals varying with growth time are shown in Fig. 2(a), (b). The luminescence spectra from these CdSe nanocrystals are symmetric and narrow. However, the PL quantum yields decreased with growth time and were down to 5%, as shown in Fig. 2(c). It has been reported by Qu and Peng that the PL quantum yield of CdSe nanocrystals is not very sensitive to the imperfection of crystallinity of nanocrystals caused by the stacking faults, but it is still sensitive to the surface environment of nanocrystals, for instance, organic ligands or inorganic passivation on the surface of CdSe nanocrystals have improved the PL quantum yield dramatically.37 Talapin et al. also reported that a decrease of the PL efficiency of size-selected fractions in comparison with the initial crude solutions when the ligand molecule is more labile and can be washed out easily.38 In this study, only oleic acid and TBP were used to passivate the surfaces of CdSe nanocrystals and the surface coverage decreased with increasing growth time due to increasing size of CdSe nanocrystals. Our results also showed that the PL quantum yield is very sensitive to the surface environment of the CdSe nanocrystals. On the basis of the above observations, it is reasonable to rationalize that the control of the surface, probably a reconstructed surface of CdSe nanocrystals, may be very important for controlling and improving their PL properties.
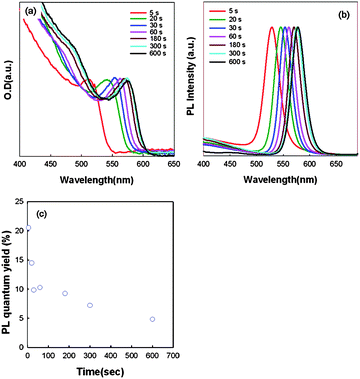 | ||
| Fig. 2 Temporal evolution of (a) UV-visible spectra; (b) PL spectra and (c) PL quantum yield of a growth reaction of CdSe nanocrystals. | ||
To understand the changes in the optical properties of the CdSe nanocrystals upon modification by the capping molecules, n-BA, n-HA or OA, were added to a solution of as-grown CdSe, and the PL and the UV/vis spectra were recorded before and after the addition. The PL spectra and the quantum yields of the CdSe nanocrystals modified by n-BA, n-HA or OA are shown in Fig. 3. We can observe that the positions of the luminescence emission peaks of the ligand-modified CdSe nanocrystals shifted to lower wave lengths for all the three capping molecules compared to the as-grown CdSe nanocrystals, Fig. 3(a). A similar blue-shift in emission following amine addition has also been observed in some other studies.10,39 Moreover, Fig. 3(b) shows that the PL quantum yield increased to 45% and 61% for the amines n-BA and n-HA, respectively, while OA showed a decrease to 5%. Generally, it is believed that the capping molecules effectively passivate the surface states and suppress the non-radiative recombination at surface vacancies, leading to enhanced PL quantum yield. But, contrary to this, of the three capping agents in our work, OA ligand exhibited an opposite behavior. Besides, some researchers reported that the quenching emission of the CdSe nanocrystals was observed at a relatively high concentration of capping molecules around 40 mM.40–42 But, we observed the variations in the PL quantum yield of the ligand-modified CdSe nanocrystals with relatively lower concentration of all the aliphatic organic compounds at ∼5 mM (Fig. 3(b)). These observations clearly indicate that the passivation effect of the capping molecules for CdSe nanocrystals are indeed more complex, suggesting a careful examination of the photoinduced charge transfer between CdSe nanocrystals and capping molecules. The present work was undertaken to unravel some of these aspects.
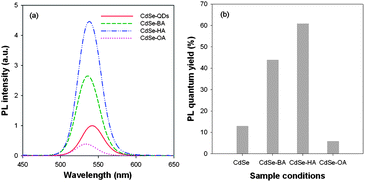 | ||
| Fig. 3 (a) Room temperature PL spectra of as-grown, n-BA, n-HA and OA capping molecule modified CdSe nanocrystals; (b) PL quantum yield of CdSe nanocrystals versus capping molecules. | ||
As the CdSe nanostructures are modified by the capping molecules, the capping effect of n-HA, n-BA and OA was derived in terms of the binding energy (Eb) of capping molecules on a CdSe cluster and the residual surface charge on the capping molecules and the CdSe, with the help of ab initio simulations. In the DFT calculation, amine and carboxylic acid were considered to be the key functional groups in obtaining the Eb on CdSe. To simply the simulations, we used a Cd4Se4 cluster to model the functional groups (see Fig. 4(a)). During simulations, the capping molecules were considered to adsorb and attach on the CdSe cluster and the binding energies of n-BA, n-HA, and OA ligands were computed using DFT simulations of an isolated cluster with one single ligand molecule. Representative snapshots for each of the capping molecules adsorbed on an isolated CdSe cluster are shown in Fig. 4(b)–(d). The binding energy, Eb, is defined as the sum of interactions between the capping molecule and cluster atoms, and it is given as Eb = Etotal − ECdSe − Ecapp, where Etotal, ECdSe and Ecapp are the total energy of the system, cluster energy, and capping molecule energy, respectively. The negative sign of Eb corresponds to the energy gain of the system due to ligand adsorption.
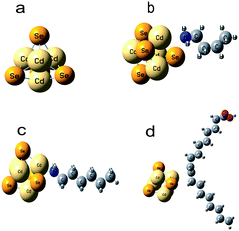 | ||
| Fig. 4 The optimized geometry of (a) CdSe cluster; (b) CdSe with n-BA; (c) CdSe with n-HA and (d) CdSe with OA capping molecule. | ||
Table 1 lists the Eb values calculated for the three capping ligands. We have verified that the main contribution to the Eb arose from the interactions of the CdSe with the capping molecules via the charges on the CdSe and the ligand functional groups. It was found that negatively charged atoms of amines adsorb on the CdSe cluster without changing the surface structure significantly. n-BA and n-HA were found to adsorb exclusively via its nitrogen atom with Eb values −0.99 and −0.93 eV for CdSe-BA and CdSe-HA, respectively. On the other hand, OA was found to yield an Eb of −0.21 eV for a CdSe cluster-OA combined with the conjugated bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of the alkyl chain adsorbed on CdSe. Our attempts for a CdSe cluster-OA combined with carboxyl function group adsorbed on CdSe resulted in a high energy, unstable configuration with the distance between the reactive centers varying continuously. This is reasonable, since as per the well-known hard and soft acids and bases theory,43 the Cd2+ and Se2− soft ions could not interact with hard –COOH of OA, as suggested by Chen et al.44 Indeed, the charge analysis shows that the charge transfer between OA and CdSe is quite small, as will be explained below.
C) of the alkyl chain adsorbed on CdSe. Our attempts for a CdSe cluster-OA combined with carboxyl function group adsorbed on CdSe resulted in a high energy, unstable configuration with the distance between the reactive centers varying continuously. This is reasonable, since as per the well-known hard and soft acids and bases theory,43 the Cd2+ and Se2− soft ions could not interact with hard –COOH of OA, as suggested by Chen et al.44 Indeed, the charge analysis shows that the charge transfer between OA and CdSe is quite small, as will be explained below.
| Capping molecule | E b /eV |
|---|---|
| (HA)C6NH2 | −0.99 |
| (BA)C4NH2 | −0.93 |
| OA | −0.21 |
Puzder et al. obtained ca. 0.91 eV from DFT calculations for trimethylamine on a (CdSe)15 NC.21 Similar values (0.89–1.02 eV) have been reported in the DFT study of amines at a CdSe surface.45 Our results for organic amines are in good agreement with these values. It must be noted that the more negative the Eb value the stronger is the adsorption. Indeed, in the simulation work, the more negative Eb value was used as optimum to represent the adsorption strength of the functional group on a given cluster, when several other possible configurations for the adsorption on the CdSe cluster existed. Now, concerning the three capping molecules, the higher negative Eb values for the amine derivatives clearly indicate a stronger adsorption of these compounds on CdSe than the OA acid ligand with a lesser negative Eb.
The Bader charge analyses were carried out for CdSe, n-BA, n-HA, OA, CdSe-BA, CdSe-HA and CdSe-OA to examine the variations in Eb in terms of charge transfer between CdSe and capping molecules, and the charge results are listed in Table 2. In CdSe-BA and CdSe-HA systems, the charge of selenium was increased from 6.614 e (for the bare CdSe) to 6.696 e and 6.700 e, respectively. This is quite reasonable since the donation of charge of nitrogen atom of capping molecules to Se would easily occur. On the other hand, in the CdSe-OA system, the charge of selenium atom was increased to 6.637 e less than that of the selenium atom in the CdSe-BA and CdSe-HA systems.
| Species | Charge (e) | Charge difference (e) |
|---|---|---|
| CdSe | Se: 6.614 | |
| Cd: 11.404 | ||
| BA | N: 7.787 | |
| HA | N: 7.801 | |
| OA | O: 7.921 | |
| CdSe-BA | Se: 6.696 | Se: 6.614–6.696 = −0.082 |
| Cd: 11.388 | Cd: 11.404–11.388 = +0.016 | |
| N: 7.561 | N: 7.787–7.561 = +0.226 | |
| CdSe-HA | Se: 6.700 | Se: 6.614–6.700 = −0.086 |
| Cd: 11.375 | Cd: 11.404–11.375 = +0.029 | |
| N: 7.561 | N: 7.801–7.561 = +0.24 | |
| CdSe-OA | Se: 6.637 | Se: 6.614–6.637 = −0.023 |
| Cd: 11.267 | Cd: 11.404–11.267 = +0.137 | |
| O: 7.906 | O: 7.921–7.906 = +0.015 |
Interestingly, the charges for Cd in CdSe-BA, CdSe-HA and CdSe-OA show a decrease from 11.404 e for a bare CdSe, indicating a net electron transfer from Cd atom to Se for all the three capping molecules. Since Cd has less electronegativity (1.7) than that for nitrogen (3.0), greater electron donation to Se from N atom of the capping molecules than that from Cd can be inferred. While the amine molecules adsorb strongly with facile electron donation from their “-NH2” functional group to Se of CdSe nanocrystals (with higher Eb values), the conjugated bond (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) of OA forms weak bonding with CdSe and lessens the molecule's capping effect (with lower Eb values). Additionally, the OA molecule with linear carbon-carbon structure can easily form a dense and stable cover layer on the surface of CdSe, preventing other molecules from approaching the CdSe QDs.44 Moreover, the structure of the OA molecule has large stereo-hindrance. All these factors severely reduce effective bonding between the OA molecule and CdSe. Thus, the present study clearly demonstrates that the improved charge transfer of amine-capped CdSe mainly arises due to higher value of Bader charge, implying a larger charge donation than OA, and the modification of CdSe QD by molecular capping plays an important role for improvement of CdSe charge donation.
C) of OA forms weak bonding with CdSe and lessens the molecule's capping effect (with lower Eb values). Additionally, the OA molecule with linear carbon-carbon structure can easily form a dense and stable cover layer on the surface of CdSe, preventing other molecules from approaching the CdSe QDs.44 Moreover, the structure of the OA molecule has large stereo-hindrance. All these factors severely reduce effective bonding between the OA molecule and CdSe. Thus, the present study clearly demonstrates that the improved charge transfer of amine-capped CdSe mainly arises due to higher value of Bader charge, implying a larger charge donation than OA, and the modification of CdSe QD by molecular capping plays an important role for improvement of CdSe charge donation.
Fig. 5 illustrates the PL intensity dependence on charge donation between CdSe and ligands. At present, we are interested in a preliminary estimation of how atomic charges may change with the HA ligand.
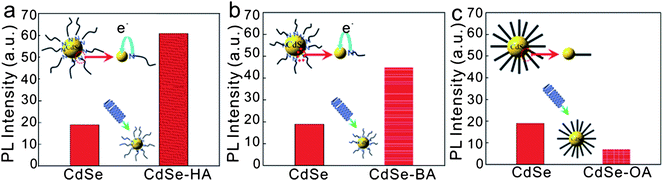 | ||
| Fig. 5 Schematic illustration of PL intensity dependence on charge donation between CdSe and ligands (a) CdSe-HA and (b) CdSe-BA and (c) CdSe-OA. | ||
Conclusions
In conclusion, we have demonstrated that amines can be used as the capping molecules for CdSe nanocrystals to largely enhance PL quantum yield based on the DFT computation. We propose that the interactions between capping molecules and CdSe nanocrystals may be attributed to the NH2 group and CdSe cluster. A better understanding of this interaction will facilitate the design of better conjugates for CdSe nanocrystals for various applications.Acknowledgements
We thank the NCHC and NTUST for providing massive computing time. Financially support from the National Science Council under Contract No. NSC 99-2811-M-011-005 is gratefully acknowledged.References
- J. Van Embden, J. E. Sader, M. Davidson and P. Mulvaney, J. Phys. Chem. C, 2009, 113, 16342–16355 CrossRef CAS.
- V. C. Colvin, M. C. Schlamp and A. P. Alivisatos, Nature, 1994, 370, 354 CrossRef CAS.
- M. Cao, C. Lesser, S. Kirstein, H. Mohwald, A. L. Rogach and H. Weller, J. Appl. Phys., 2000, 87, 2297 CrossRef CAS.
- V. C. Sundar, K. Lee, J. R. Heine, M. G. Bawendi and J. F. Jensen, Adv. Mater., 2000, 12, 1102 CrossRef CAS.
- V. I. Klimov, A. A. Mikhailovsky, S. Xu, A. Malko, J. A. Hollingsworth, C. A. Leatherdale, H. J. Eisler and M. G. Bawendi, Science, 2000, 290, 314–317 CrossRef CAS.
- M. Kazes, D. Y. Lewis, Y. Ebenstein, T. Mokari and U. Banin, Adv. Mater., 2002, 14, 317–321 CrossRef CAS.
- Y. A. Vlasov, N. Yao and D. J. Norris, Adv. Mater., 1999, 11, 165–169 CrossRef CAS.
- M. Bruchez Jr, M. Moronne, P. Gin, S. Weiss and A. P. Alivisatos, Science, 1998, 281, 2013–2016 CrossRef CAS.
- D. Gerion, F. Pinaud, S. C. Williams, W. J. Parak, D. Zanchet, S. Weiss and A. P. Alivisatos, J. Phys. Chem. B, 2001, 105, 8861–8871 CrossRef CAS.
- D. V. Talapin, A. L. Rogach, A. Kornowski, M. Haase and H. Weller, Nano Lett., 2001, 1, 207–211 CrossRef CAS.
- C. D. Heyes, A. Y. Kobitski, V. V. Breus and G. U. Nienhaus, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 75, 125431 CrossRef.
- S. A. Ivanov, A. Piryatinski, J. Nanda, S. Tretiak, K. R. Zavadil, W. O. Wallace, D. Werder and V. I. Klimov, J. Am. Chem. Soc., 2007, 129, 11708–11719 CrossRef CAS.
- U. T. D. Thuy, N. Q. Liem, D. X. Thanh, M. Protiere and P. Reiss, Appl. Phys. Lett., 2007, 91.
- D. E. Gomez, J. Van Embden, P. Mulvaney, M. J. Fernee and H. Rubinsztein-Dunlop, ACS Nano, 2009, 3, 2281–2287 CrossRef CAS.
- D. E. Gomez, J. Van Embden, J. Jasieniak, T. A. Smith and P. Mulvaney, Small, 2006, 2, 204–208 CrossRef CAS.
- M. A. Hines and P. Guyot-Sionnest, J. Phys. Chem., 1996, 100, 468–471 CrossRef CAS.
- A. Hoshino, K. Fujioka, T. Oku, M. Suga, Y. F. Sasaki, T. Ohta, M. Yasuhara, K. Suzuki and K. Yamamoto, Nano Lett., 2004, 4, 2163–2169 CrossRef CAS.
- T. Tsuruoka, K. Akamatsu and H. Nawafune, Langmuir, 2004, 20, 11169–11174 CrossRef CAS.
- F. Dubois, B. Mahler, B. Dubertret, E. Doris and C. Mioskowski, J. Am. Chem. Soc., 2007, 129, 482–483 CrossRef CAS.
- S. A. Majetich and A. C. Carter, J. Phys. Chem., 1993, 97, 8727–8731 CrossRef CAS.
- A. Puzder, A. J. Williamson, N. Zaitseva, G. Galli, L. Manna and A. P. Alivisatos, Nano Lett., 2004, 4, 2361–2365 CrossRef CAS.
- S. Kilina, S. Ivanov and S. Tretiak, J. Am. Chem. Soc., 2009, 131, 7717–7726 CrossRef CAS.
- A. Kasuya, R. Sivamohan, Y. A. Barnakov, I. M. Dmitruk, T. Nirasawa, V. R. Romanyuk, V. Kumar, S. V. Mamykin, K. Tohji, B. Jeyadevan, K. Shinoda, T. Kudo, O. Terasaki, Z. Liu, R. V. Belosludov, V. Sundararajan and Y. Kawazoe, Nat. Mater., 2004, 3, 99–102 CrossRef CAS.
- B. J. Morgan and P. A. Madden, Phys. Chem. Chem. Phys., 2007, 9, 2355–2361 RSC.
- M. Grünwald, E. Rabani and C. Dellago, Phys. Rev. Lett., 2006, 96, 255701 CrossRef.
- B. J. Morgan and P. A. Madden, J. Phys. Chem. C, 2007, 111, 6724–6731 CrossRef CAS.
- E. Rabani, J. Chem. Phys., 2001, 115, 1493–1497 CrossRef CAS.
- C. De Mello Donega, S. G. Hickey, S. F. Wuister, D. Vanmaekelbergh and A. Meijerink, J. Phys. Chem. B, 2003, 107, 489–496 CrossRef.
- P. Schapotschnikow, R. Pool and T. J. H. Vlugt, Comput. Phys. Commun., 2007, 177, 154–157 CrossRef.
- H. Liu, J. Guo, Y. Yin, A. Augustsson, C. Dong, J. Nordgren, C. Chang, P. Alivisatos, G. Thornton, D. F. Ogletree, F. G. Requejo, F. De Groot and M. Salmeron, Nano Lett., 2007, 7, 1919–1922 CrossRef CAS.
- I. Csik, S. P. Russo and P. Mulvaney, J. Phys. Chem. C, 2008, 112, 20413–20417 CrossRef CAS.
- W. W. Yu and X. Peng, Angew. Chem., Int. Ed., 2002, 41, 2368–2371 CrossRef CAS.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter, 1993, 47, 558 CrossRef CAS.
- J. P. Perdew, J. A. Chevary, S. H. Vosko, K. A. Jackson, M. R. Pederson, D. J. Singh and C. Fiolhais, Phys. Rev. B: Condens. Matter, 1992, 46, 6671 CrossRef CAS.
- D. Vanderbilt, Phys. Rev. B: Condens. Matter, 1990, 41, 7892 CrossRef.
- G. Kresse and J. Furthmuller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- L. Qu and X. Peng, J. Am. Chem. Soc., 2002, 124, 2049–2055 CrossRef CAS.
- D. V. Talapin, A. L. Rogach, E. V. Shevchenko, A. Kornowski, M. Haase and H. Weller, J. Am. Chem. Soc., 2002, 124, 5782–5790 CrossRef CAS.
- C. Bullen and P. Mulvaney, Langmuir, 2006, 22, 3007–3013 CrossRef CAS.
- C. Landes, C. Burda, M. Braun and M. A. El-Sayed, J. Phys. Chem. B, 2001, 105, 2981–2986 CrossRef CAS.
- C. F. Landes, M. Braun and M. A. El-Sayed, J. Phys. Chem. B, 2001, 105, 10554–10558 CrossRef CAS.
- C. Landes, M. Braun, C. Burda and M. A. El-Sayed, Nano Lett., 2001, 1, 667–670 CrossRef CAS.
- R. G. Pearson, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- J. Chen, J. L. Song, X. W. Sun, W. Q. Deng, C. Y. Jiang, W. Lei, J. H. Huang and R. S. Liu, Appl. Phys. Lett., 2009, 94, 153115-153111–153115-153113.
- P. Schapotschnikow, B. Hommersom and T. J. H. Vlugt, J. Phys. Chem. C, 2009, 113, 12690–12698 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Figure S1. See DOI: 10.1039/c0nr00569j |
| This journal is © The Royal Society of Chemistry 2010 |
