Analytical electron tomography mapping of the SiC pore oxidation at the nanoscale†
Ileana
Florea
a,
Ovidiu
Ersen
*a,
Charles
Hirlimann
a,
Lucian
Roiban
a,
Adrien
Deneuve
b,
Matthieu
Houllé
b,
Izabela
Janowska
b,
Patrick
Nguyen
c,
Charlotte
Pham
c and
Cuong
Pham-Huu
b
aInstitut de Physique et Chimie des Matériaux de Strasbourg, CNRS - Université de Strasbourg (UDS) UMR 7504, 23, rue du Loess, BP 43, 67037, Strasbourg cedex 02, France. E-mail: ovidiu.ersen@ipcms.u-strasbg.fr; Fax: +33(0) 03 88 10 72 48; Tel: +33(0) 03 88 10 70 28
bLaboratoire des Matériaux, Surfaces et Procédés pour la Catalyse, CNRS - Université de Strasbourg (UDS), UMR 7515, 25, rue Becquerel, 67087, Strasbourg cedex 08, France
cTechnical Center, Sicat, 1, rue du Broetch, 67700, Otterswiller, France
First published on 11th October 2010
Abstract
Silicon carbide is a ceramic material that has been widely studied because of its potential applications, ranging from electronics to heterogeneous catalysis. Recently, a new type of SiC materials with a medium specific surface area and thermal conductivity, called β-SiC, has attracted overgrowing interest as a new class of catalyst support in several catalytic reactions. A primary electron tomography study, performed in usual mode, has revealed a dual surface structure defined by two types of porosities made of networks of connected channels with sizes larger than 50 nm and ink-bottled pores with sizes spanning from 4 to 50 nm. Depending on the solvent nature, metal nanoparticles could be selectively deposited inside one of the two porosities, a fact that illustrates a selective wetting titration of the two types of surfaces by different liquids. The explaining hypothesis that has been put forward was that this selectivity against solvents is related to the pore surface oxidation degree of the two types of pores. A new technique of analytical electron tomography, where the series of projections used to reconstruct the volume of an object is recorded in energy filtered mode (EFTEM), has been implemented to map the pore oxidation state and to correlate it with the morphology and the accessibility of the porous network. Applied, for the first time, at a nanoscale resolution, this technique allowed us to obtain 3D elemental maps of different elements present in the analysed porous grains, in particular oxygen; we found thus that the interconnected channel pores are more rapidly oxidized than the ink-bottled ones. Alternatively, our study highlights the great interest of this method that opens the way for obtaining precise information on the chemical composition of a 3D surface at a nanometer scale.
Introduction
Silicon carbide is a ceramic material that has been widely studied because of its potential applications, ranging from electronics to heterogeneous catalysis.1 Alpha silicon carbide (α-SiC) is the most common crystalline form of the material exhibiting a wurtzite like hexagonal crystalline structure. The beta polymorph of silicon carbide (β-SiC), that exhibits a zinc blend crystal structure, has attracted a growing interest during the last decades as a new class of catalyst support owing to its relatively large specific surface area (>20 m2 g−1) as compared to α-SiC synthesized via the Acheson process that leads to low specific surface areas (0.1–1 m2 g−1).2 The SiC material is also thermally conductive compared to traditional catalyst supports such as alumina or silica and that could allow a rapid heat transfer throughout the catalyst body and thus, prevent hot spots formation in exothermic reactions which is detrimental for the reaction selectivity.1 The medium specific surface area allows for the deposition of metallic active phases with reasonably high dispersion while the good thermal conductivity of the substrate strongly influences the selectivity of the catalyst for exothermic reactions like the Fischer–Tropsch synthesis (FTS),3n-butane partial oxidation,4 H2S selective oxidation5 or methanol dehydration to dimethylether.6 These properties are attributed to the synthesis method based on a gas-solid reaction involving SiC vapour and solid carbon at moderate temperature (about 1400 °C) that avoids the sintering of the material, as it is the case of high-temperature synthesis.7 Previous analysis based on gas adsorption and mercury intrusion methods have shown that this specific preparation generates a dual porosity inside the material, made of mesopores (4–50 nm) and macropores (>50 nm), giving rise to two types of SiC surfaces. One should expect that the reactivity of these spatially separated surfaces, especially towards oxygen, depends strongly on the morphology, the accessibility and the hydrophilic-hydrophobic character of the corresponding pores. A recent electron tomography study has shown that beta SiC exhibits a dual surface structure corresponding to a porosity made of a network of connected channels (C-pore) with typical sizes larger than 50 nm and ink-bottled pores (R-pore) with sizes spanning from 4 to 50 nm.8 The channel pores have been observed to be easily wetted by water, i.e. they have a hydrophilic surface behaviour, whereas the ink-bottled pores are wetted by alcoholic or organic solvents, i.e. their surface has a hydrophobic behaviour. From this observation the hypothesis has been put forward that this selective wetting against solvents could be related to the oxidation degree of the two classes of pores: hydrophilic surface containing a higher concentration of the oxygenated functional groups whereas hydrophobic surface having less oxygenated functional groups. Such surface behaviour is very similar to the one occurring on carbon nanotubes surfaces where the hydrophilic and hydrophobic character can be controlled at will as a function of the chemical or thermal treatment.9 In the catalysis field, the oxidative resistance of the support is of tremendous importance, as at an extensive oxidation degree the influence of pore migration and plugging and active phase encapsulation become predominant contributing to an activity loss. The oxygen concentration on the support surface is also of great importance as it allows the anchorage and dispersion of the metal or oxide active phase on the support surface. However, the oxygen concentration, i.e. the thickness of the oxide layer, on each type of pore is not easy to map using traditional techniques at hand. Indeed, high oxygen concentration or thick layers of oxide could plug the pore or encapsulate the active phases that are deposited on the surface deteriorating the overall catalyst activity. Up to now, no technique was able to give a direct access to such pore surface behavior. It is thus of great interest to find a new and appropriate characterization technique allowing to selectively map the pore oxidation degree in the two classes of pores of beta SiC, as well as to correlate this parameter to the morphology and to the accessibility of the porous network at a nanoscale spatial resolution. Comparing the various existing techniques able to provide specific information at a nanometer scale, it appears that one of the best ways to acquire 3D compositional mapping is the Energy Filtered TEM (EFTEM) tomography. In that regard, the first goal of this paper is to demonstrate the feasibility of the electron tomography in the analytical mode with a resolution down to the nanometer. Applied in a quantitative way, this technique was subsequently used to determine the pore oxidation degree, or in other words, the thickness of the oxide layer, at the surfaces defined by the two types of pores present in β-SiC. The characterization of such pore surfaces could be helpful for the understanding of the support behavior towards oxidation reactivity which, in turn, would represent a powerful tool for controlling the active phase localization and its accessibility as a function of the pore encapsulation by the oxide layer. The developed technique could also be further applied to the study of other one-dimensional catalyst supports, i.e. inner versus outer surface reactivity of carbon nanotubes, as well as to other porous catalytic systems, either pure or doped with foreign elements, in order to build-up the relationship between the catalytic performance and surface composition.Emergence and state-of-the-art of the EFTEM tomography
Generally, to obtain chemically selective information at the nanoscale, the electron energy loss spectroscopy (EELS) technique is commonly used and allows the mapping of the elemental composition (in both image and spectroscopic mode), the chemical state or the phase distribution.10–12 Related to the Si–C–O-based materials, a typical example of EELS analysis concerns the investigation of the chemical bonding in bulk ceramics prepared by pyrolysis technique as a function of O content.13 However, by recording individual images or spectra, this technique remains a 2D analysis tool because the projection effect gives only access to thickness-integrated information along the direction of observation. Interpretation needs the setting of hypothesis about the studied nano-object nature and can be sometimes difficult or ambiguous, especially for objects with complex 3D morphologies or random spatial distribution of their components. From the morphological point of view, the analysis of objects at a nanometer scale implies the use of electron microscopy in the tomographic mode, which produces 3D reconstructions of the said objects and thus, eliminates the hypothetical interpretations. Electron tomography in a bright-field mode has been extensively employed in the field of biology research and it has only recently been translated in the field of materials at a nanoscale, since the pioneering work published by de Jong and co-workers.14 More recently, a new experimental mode has been implemented, using for the volume reconstruction, images recorded with a high angle annular dark-field detector in the scanning mode (STEM-HAADF). This mode is indeed extremely valuable to characterize crystalline systems, because it avoids diffraction contrast and allows the recording of images mainly sensitive to the atomic number. However, the difference in atomic number, especially for light elements, of the chemical elements present in the sample frequently leads to insufficient contrast preventing the use of this density-sensitive method. To overcome this drawback, a third chemically selective mode was recently implemented based on energy-filtered TEM (EFTEM) imaging to acquire the tilt projection series used subsequently to compute the reconstruction. The individual images are formed across a spectrometer that selects inelastic scattered electrons with a given energy loss allowing the collection of signals emitted from a specific chemical element amongst the others. The EFTEM tomography method has been first used to study nanocrystals15 or composite materials.16 Recent results17 were obtained from tilt series of EFTEM images on the basis of an appropriate choice of materials (constituting elements with very distinct energy losses: Fe, Ni, O) and with a compromise between data acquisition speed and resolution. In another work, filtered images recorded in the plasmon region of an EELS spectrum have been used to reconstruct a 3D signal sensitive to local changes in the specimen density.18 Some specific applications have also recently been published in the field of biology, either in zero-loss mode selecting only elastically scattered electrons in order to enhance the contrast,19 or in a more quantitative way to provide 3D maps of phosphorous in nucleic acid, by recording L2,3 pre-edge and post-edge images.20 However, exploiting a unique 3D chemical map is not accurate enough, since the method is very sensitive to various parameters, including extraction of the background (for a proper extraction, it is certainly better to use two pre-edge images than only one), change in specimen thickness when tilting, and data processing. A more valuable analysis can be performed when all the chemical elements present in the specimen are mapped altogether, yielding relative 3D chemical maps with unknown parameters. Thomas et al.21 have shown that it is possible to perform chemical tomography using the spectroscopic mode instead of the image mode of EELS spectroscopy. This technique, referred to as 4D STEM-EELS tomography, requires a 360° rotation holder and pillar-shaped samples to minimize artifacts, thus limiting its applications in a routine way. However, in the more convenient EFTEM mode the current spatial resolution reported up to now is limited to only a few tens of nm. In this framework, reaching the nanometer range represents a great breakthrough for extending this technique to full characterization of complex nano-objects. In this way, correlations between the distributions of the chemical elements can be obtained, as for example the oxygen distribution in the vicinity of a surface having a complex 3D morphology.Even being beneficial as a characterization technique in the materials field, the EFTEM technique implementation and application at the nanoscale remain slow to show up. Similarly as in the case of classical EFTEM imaging, the first concern about the technique is the high doses of electrons needed to obtain series of chemical projections with a significant signal to noise ratio (SNR). Furthermore, in the tomographic mode, in order to obtain relevant 3D chemical maps, the number of projections (N) must be higher than 50 and each projection is obtained from three filtered images. Even if the sample under study is not known to be sensitive to electron irradiations, long exposition times can produce small damages accumulation leading to non-reliable 3D reconstructions. The second obstacle in this technique is that the image intensities in the so-called “chemically selective projections” must satisfy the projection requirement for tomography that stipulates that the signal must vary monotonically through the structure to be suitable for reconstruction.21 This consideration generally requires the acquisition of three energy-filtered images for each chemical element at each tilt angle, two before the chosen edge to estimate the background and one on the edge from which the background will be extracted. Once the tilt series of chemical projections and the corresponding elemental reconstructions are computed, the combined analysis of the obtained 3D maps for the major elements present in the specimen becomes possible. The main practical difficulties and drawbacks of the technique are further discussed in the Experimental Methods section.
In this context, the challenge was here to adapt the acquisition and the data treatment to insure accurate 3D chemical maps constructions with a nanometer resolution which was not achieved so far. Indeed, as predicted by some authors,16,22 improving the spatial resolution seems to be possible if the sampling of the angular range is performed with an increased pixel and angular resolution. Considerable work has therefore been devoted in this study to carefully optimise the acquisition parameters, computing and analysis processes in order to achieve chemical mapping with a nanometer-scale resolution. In particular, for the system studied in this work, the analytical electron tomography is the unique method which allows performing a quantitative analysis of the oxygen content on the two types of surfaces. More generally, it is thought that such result could be extremely helpful for studying the real chemical composition of the active phases in the heterogeneous catalysis field, especially their composition with spatial resolution on the topmost surface where the catalytic steps do occur, and to determine the relationship between the chemical composition of the active phases and the performance of the catalyst.
Results
Classical analysis by 3D-TEM and XPS: selective localization of nanoparticles versus porous structure and surface chemistry
In this work the β-SiC material was synthesized by allowing solid carbon and SiO to react in an argon flow at a moderate temperature (∼1400 °C).23 The low synthesis temperature avoided specific surface area loss through surface pore diffusion. The as-synthesized material was further calcined in air at 800 °C for 2 h in order to remove residual carbon inside the matrix. Using the Brunauer, Emett, and Teller technique (BET), we measured a specific surface area of 25 m2 g−1, a reasonably large value due to the presence of both meso- and macropores.A meticulous analysis using 3D-TEM imaging revealed that the resulting ceramic material possesses two types of porosities with different surface wetting behavior.8 The main results obtained by analyzing the 3D reconstruction computed from a tomographic record following the procedure described in the Experimental Method section are summarized in Fig. 1A, B and C, which highlight the presence of both channel pores with typical size larger than 50 nm on average and ink-bottled pores with sizes spanning from 4 to 50 nm. In particular, Fig. 1B shows a typical slice through the reconstructed volume on which both the interconnected channel pores (C-pores) and the ink-bottled pores (R-pores) are observable. In Fig. 1C, a modelling of the 3D reconstruction of the SiC grain is shown that gives a better intuitive perception of the two different types of pores defining the two spatially separated surfaces.
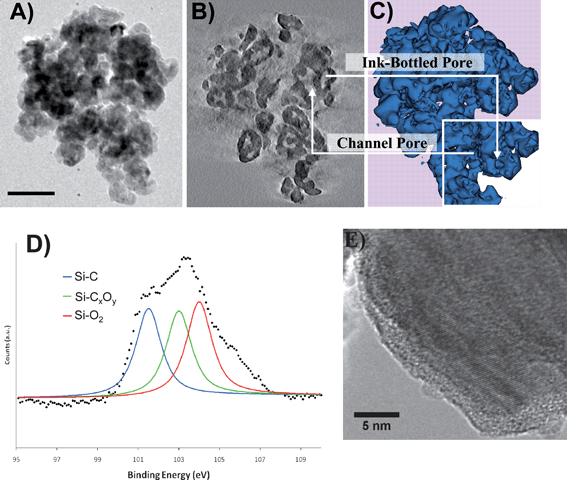 | ||
| Fig. 1 (A) Example of a 2D TEM image from the tilt series recorded in Bright Field mode on a typical grain of SiC. The scale bar is 100 nm. (B) Typical slice through the volume reconstruction of the sample showing the presence of the two types of pore: interconnected channel pores and ink-bottled pores. (C) Modeling of the reconstruction showing the global morphology of the pores and their spatial distribution. Inset: Closer view of the porous structure. (D) XPS Si2p spectrum of the SiC sample after synthesis and after air calcination at 800 °C to remove the unreacted carbon. (E) High resolution TEM image showing the presence of an oxide layer on the C-pore surface. | ||
The averaged surface oxygen content on the two surfaces which is the core of our questioning has been first characterized using XPS analysis. The XPS Si2p spectrum recorded on the SiC sample after synthesis and air calcination at 800 °C is presented in Fig. 1D. The decomposition of the raw spectrum indicates that at least three Si-based species are present on the SiC surface. The Si2p peak located at low binding energy, i.e. 101 eV, could be attributed to the Si atoms engaged in the SiC phase, whereas the two other Si2p peaks located at higher binding energies were attributed to the Si atoms engaged in the SiCxOy and SiO2 phases.24 According to the relative intensity of the different Si2p peaks, one can conclude that oxygen-containing Si compounds were present in a large amount on the topmost surface of the SiC material after the calcination step. Silicon carbide is well-known to be highly reactive with oxygen and, thus, soon after its exposure to air the SiC surface was steadily covered with a thin layer of oxygen-containing species, i.e. SiCxOy and SiO2.25–27 Mozdzierz et al.28 have proposed the following chemical formula for the silicon oxycarbide phase: SiO1.52C0.6–1.05. In the present work, the concentration of these various species on the SiC surface after air calcination at 800 °C, determined from the XPS decomposition, is 34 at. % for SiC, 33 at. % for SiCxOy and 33 at. % for SiO2. Such results are in good agreement with the HRTEM analysis that reveals an amorphous layer on the SiC surface (Fig. 1E), as well with the previously reported results on the oxidation state of the SiC surface upon air calcination.24 The next question addresses the localization of the various species either on the whole surface of the material or onto specific localizations depending on the nature of the pores.
The total amount of the SiCxOy and SiO2 species present on the topmost surface of SiC is determined by submitting the sample to a soda (20 wt. %) treatment at 80 °C that removes the oxygen-containing species. According to the results the oxygen concentration on the SiC material was found to be 3.5 wt. %. The complete removal of the oxygen-containing species on the SiC after the soda treatment was already shown in a previous reported study.29 Similar results have also been observed in our previous work using a Zeta potential technique.8
Another accurate determination of the oxygenated groups present on a SiC surface and, in turn, of the hydrophilic/hydrophobic character of the material surface, has recently been reported by Keller et al.30,31 using H/D exchange between gaseous D2O and a SiC material. H/D titration to selectively titrate the oxygen sites present on the fresh SiC surface is given to be 4.0 ± 0.3 μmol/m2 (Table 1). On SiC treated with soda solution at 80 °C, no H/D exchange was observed confirming the oxygen-free nature of the material. The same SiC sample after oxidizing in air at higher temperature, i.e. 1000 °C has its whole surface covered with a layer of oxygen-containing species similar to that of pure silica, i.e. 11.7 ± 1.0 μmol/m2 for SiC-oxidized and 11.6 ± 0.7 μmol/m2 for silica. This is confirmed by XPS results reported in the literature indicating a homogeneous surface coverage of SiC with a SiO2 layer.32,33 Oxidizing SiC material in air at 1000 °C leads to complete surface oxidation and in that case, only hydrophilic surfaces are detected. However, depending to the hydrophilic or hydrophobic nature of the original surface, the thickness of the oxygen-containing layer can be different. Characterizing the nature of the surface is the objective of the present EFTEM tomographic analysis.
| Fresh SiC | Soda treated SiC | Oxidized SiC (1000 °C - 2h) | SiO2 | |
|---|---|---|---|---|
| Number of –OH surface groups (μmol/g) | 115 ± 10 | 0 | 282 ± 25 | 1390 ± 85 |
| Number of –OH surface group (μmol/m2) | 4.0 ± 0.3 | 0 | 11.7 ± 1.0 | 11.6 ± 0.7 |
| Surface coverage by oxygenated species (%) | 34 ± 4 | 0 | 100 ± 8 | 100 |
| Hydrophilic-hydrophobic surface estimation from H/D titration (%) | 34–66 | 0–100 | 100–0 | 100–0 |
| Hydrophilic-hydrophobic surface estimation from tomography (%) | 48–52 |
Taking into account the fact that on the oxidized SiC sample the totality of the surface is covered with a homogeneous thin layer of oxygen, one can estimate the oxygen surface (hydrophilic surface) coverage on the fresh SiC sample to be 34%. The oxygen-free pure SiC surface (hydrophobic surface) represents then 66% of the total surface of the SiC material. According to these results one can state that SiC material, after synthesis and air calcinations at 800 °C, exhibits a hybrid surface containing both oxygen and oxygen-free fractions; contrary to bulk SiC covered with a SiO2 shell that only exhibits hydrophilic surface character as does bulk SiO2. These results are somewhat different from those obtained using tomography analysis, i.e. 48 and 52%, respectively. In this particular case, they were deduced by assigning, in a first approximation, the hydrophilic and hydrophobic surfaces to the R-pore and C-pore surfaces.8
Another typical finding which has been inferred from our previous observations is that, when this system was used as a catalytic support and Pd nanoparticles were deposited inside the porous network by means of an incipient wetness impregnation method, the localization of Pd seems to be closely depended on the nature of the solvent, ethanol or water, which selectively wets one or the other porous system.8 To confirm this observation, additional 3D analysis were performed on a similar system composed by Fe3O4 nanoparticles inserted in the SiC matrix using ethanol as solvent (see ESI, SI-1).† Once again, the particles were mostly localized in the R-porosity when ethanol was used as a solvent. As a consequence, we concluded that the selective localization of the active phase deposited by impregnation is only due to a selective wetting titration of the two surfaces by different liquids, the channel C pores being wetted by water whereas the ink-bottled R pores were preferentially wetted by alcoholic or organic solvents.
In order to explain such a difference, we propose the following hypothesis about the pore surface reactivity: the channelled pores are mostly decorated with oxygenate functional groups exhibiting a hydrophilic behavior, while the ink-bottled pores that seem to have less affinity with oxygen exhibit a hydrophobic character. According to this assumption one should expect the oxygen content on the surface of the C pores to be larger than the oxygen content in the R channel pores after a thermal treatment in air, which typically leads to an enhanced layer of oxygenated compounds on the surface. That unveils a difference in oxygen affinity between the two pores surfaces. However, no study dealing with the 3D surface mapping of the pore oxidation degree at the nanoscale has been reported up to now and it is the purpose of this work to bridge this gap.
The existence of a difference in chemical composition between the R-pore and C-pore surfaces is primarily suggested by accurately analyzing the SiC volume reconstruction obtained by classical tomography in the vicinity of the surface (for more details, see ESI, SI-2).† Indeed, a relatively thick fringe-like contrast can be observed at the SiC surface and especially at that defined by the C-pores. The difference in contrast with bulk SiC could be related to a slightly different chemical composition, and could be associated to a relatively thick oxide layer present on the SiC surface. The presence of such fringes, especially at the C-pore surface, suggests a difference in oxidation rate between the two pore surfaces, in agreement with the previous hypothesis.
Analysis by STEM-EELS: 2D chemical composition
In order to obtain more chemical selective information on the oxidation state at the two surfaces, we first performed 2D analytical TEM measurements using the STEM-EELS mode. In that regard, several EELS spectra were recorded for various positions of the electron beam focused probe (0.25 nm in diameter). Fig. 2A shows an image recorded in the dark-field mode of the analyzed grain. The electron beam of the microscope was then scanned along a line (green line on Fig. 2B) crossing a SiC aggregate presenting a roughly cylindrical R-pore having its surface perpendicular to the beam. The scan direction was chosen in order to chemically investigate small areas going from the outside of the SiC aggregate to the inside of the considered R-pore, allowing the simultaneous probing of the two types of surfaces. A typical EELS spectrum recorded with a 0.3 eV energy dispersion showing the K-edge of oxygen is shown in the ESI (SI-3).† The as-deduced relative concentration of oxygen for the probed areas is drawn on Fig. 2C and clearly shows that it is present on the two surfaces (R-pore and C-pore). However, this information remains insufficient to precisely compare the concentration of oxygen present on the two surfaces, due partly to the poor quality of the signal to noise ratio (SNR) which does not allow quantitative analysis. But the main reason is that chemical fluctuations can occur through the direction of the electron beam, because the analyzed surfaces are certainly not perfectly perpendicular to this direction and have also a 3D morphology.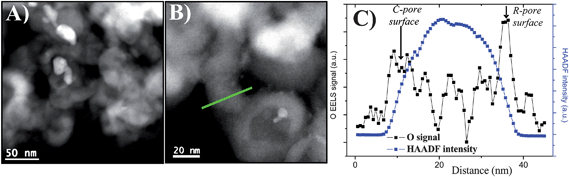 | ||
| Fig. 2 EELS analysis on a typical SiC aggregate with an R-pore roughly perpendicular to the electron beam, suggesting the presence of the oxygen mainly on the two surfaces. (A) General STEM image recorded in the annular dark-field (ADF) mode. (B) STEM-ADF image taken with a higher magnification showing the scan direction of the electron beam (in green), for the recording of the successive EELS spectra. (C) Mass-sensitive intensity (in blue) and oxygen relative concentration deduced from the EELS spectra (in black), along this direction going from the outside of SiC to the inside of the R-pore. | ||
3D oxygen mapping in SiC through EFTEM tomography
Fig. 3A and B present a typical 2D projection of the analyzed SiC fragment recorded in the Zero Loss mode and the corresponding EELS spectrum recorded with a 0.1 eV energy dispersion on the whole object, showing the K-edges of carbon and oxygen. The positions and widths of the three energy windows used to record the energy filtered images are also represented for the oxygen, as well as the corresponding chemical signal (see inset), after the background extraction. Fig. 3C and D show three energy filtered images recorded at the O K-edge (left) and the resulting 2D oxygen elemental map. In the same manner a 2D mapping of carbon is obtained for each tilt angle (the corresponding images are given in the ESI, SI3).† At the end of a recording series and elemental mapping calculation, three correlated tilt series are obtained: one with the mean density and the two others giving the elemental projections of the oxygen and carbon. From these tilt series two 3D reconstruction volumes are calculated that contain two mappings of the elements of interest. Alternatively, a third reconstruction corresponding to the mean density volume is obtained from the Zero Loss tilt series, from which a typical image is presented in Fig. 4A.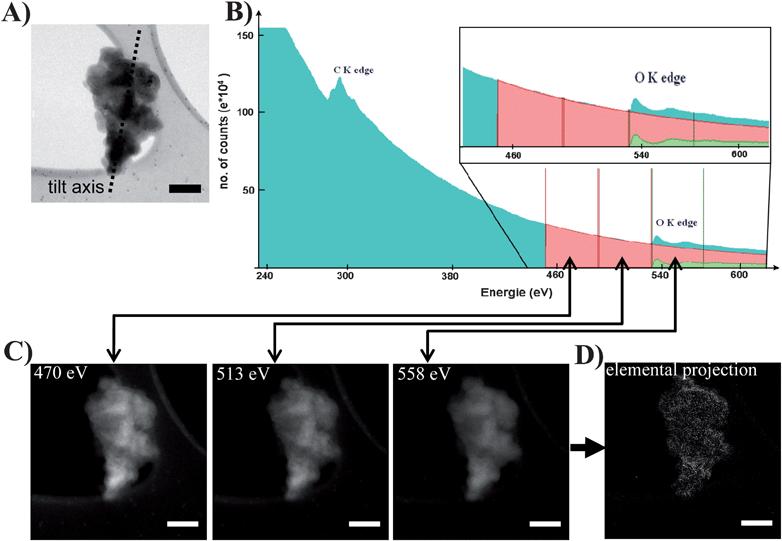 | ||
| Fig. 3 (A) Image of a β-SiC nanoparticle recorded in the Zero Loss mode on the SiC grain selected for the analytical tomographic analysis. (B) EELS spectrum recorded on the whole object, illustrating the C K-edge (at 284 eV) and O K-edge (at 532 eV), as well as the characteristics of the energy windows used to acquire the tilt series of filtered images for the oxygen. The insert shows the resulting oxygen signal after the background extraction. (C) Corresponding filtered images recorded at the K-edge of the oxygen at a given tilt angle. (D) Elemental projection of the oxygen obtained from the three filtered images, after the background extraction using the three-window method. The scale bar is 100 nm for all images. | ||
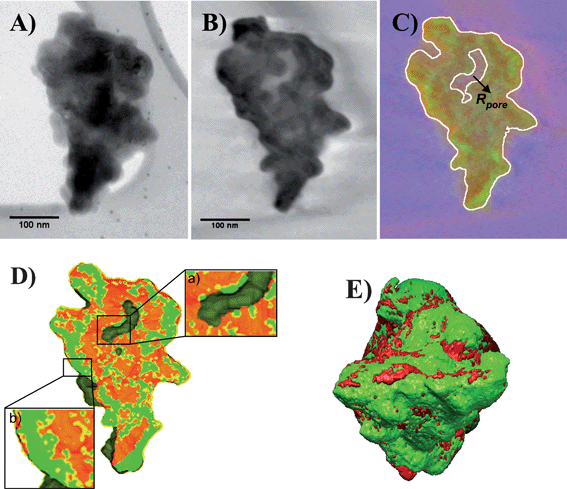 | ||
| Fig. 4 (A) 2D-TEM image at 0° tilt from the tilt series used to reconstruct the mean density volume. (B) Section through the reconstructed volumes corresponding to the mean density. (C) Cross-section, taken at the same depth and angle with respect to the initial orientation as that of Fig. 4A, through the 3D relative map of SiC (red) with respect to SiOx (green), in white the guide for the external surface and the pore. (D) Threshold sorting modeling of the 3D SiC/SiOx map showing the spatial spread of the SiOx on the two surfaces; inset a: zoom in the vicinity of the R-pore showing the presence of less oxygen on the R-pore surface; inset b: zoom near the external C-pore surface illustrating its high oxidation degree. (E) Global modeling of the analyzed SiC grain. | ||
A representative slice extracted from the mean density reconstruction is shown in Fig. 4B. In such image the thickness of the slice, given by the pixel size in the initial projection images (1.1 nm), is thin enough to insure that one can assume the absence of overlapping effects along the sample thickness.
Analyzing the reconstruction corresponding to the mean density we can easily identify the two types of surfaces proposed by the analyzed SiC fragment. We observe thus a R-pore inside the grain that contributes to the R-porosity, whereas the external surface of the grain defines its C-porosity. Comparing the amount of oxygen present on the two surfaces by analyzing only the slices from the oxygen volume is not quite convincing, as the signal to noise ratio (SNR) remains relatively low in a unique chemical reconstruction and thus, leads to a relatively large error on the oxygen localization. However, since the three volumes are spatially correlated, they can be analyzed simultaneously, by superposing the 3D maps of carbon and oxygen in order to obtain a 3D relative map of SiOx compared to SiC. A cross-section extracted from the obtained 3D relative map taken through the R-pore is presented in Fig. 4C, with SiC colored in red pixels and SiOx colored in green pixels. As can be observed, the determination of the relative spatial spread of the two different chemical phases is much more accurate compared to the results obtained by analyzing the individual elemental reconstructions. In order to get a more intuitive view of the oxygen distribution and to perform a quantitative analysis, a modeling of the 3D relative map was realized using a criterion based on the comparison of the intensities of individual elemental maps, pixel by pixel. A typical view of the modeling is showed in Fig. 4D, giving a clearer view on the difference in terms of oxidative resistance between the R-pore and C pore surfaces (see insets a and b). Note that the spatial resolution in this analytical tomographic mode estimated by taking into consideration the experimental conditions and specimen characteristics, is of the order of 7 nm. In addition, the quantitative analysis of the 3D relative map allows us to determine the mean concentration in oxygen in the analyzed grain to be of the order of 10 wt. %, in agreement with the global value obtained by the thermo-gravimetric analysis.
Discussion
High-temperature oxidative treatment slowly leads to the complete oxidation of the SiC surface leading to a hydrophilic behavior. However, the thickness of the oxygen-containing layer largely varies in a great manner depending on the hydrophilic or hydrophobic nature of the original SiC surface. According to the presented slice and to the 3D modeling, one can conclude that the oxidation mostly starts on the surface of the C-pores whereas it is less important on the surface exposed in the R-pores. That clearly evidences, in the limit of our spatial resolution (7 nm), a different oxidative behavior induced by the pore morphology. Increasing the oxidation treatment duration leads to a more complete transformation of SiC into SiO2 especially on the C-pore surface. Classical TEM micrograph evidences the progressive channel pore (C-pore) plugging by the extensive growth of SiO2 away from the SiC surface which bridges the pore gap (see ESI, SI-3).† Such pore plugging is also at the origin of the overall specific surface area decrease after oxidation. It is worth noting that during the oxidation process, the R-pore surface also undergoes oxidizing as well but probably with a lower rate due to the hydrophobic character of the original surface.The results obtained in this work clearly evidence the fact that β-SiC having a dual porosity exhibits an atypical behavior against oxidation. The interconnected channel pores (C-pore) are more affected by the calcinations step due to their high oxygen affinity provided by the presence of a higher defect density. The C-pores result from the aggregation of several SiC grains stacking along a channel where grain boundaries do generate topological defects, which make the pores more reactive against oxygen. On the other hand, the ink-bottled pores are much less affected by the oxidative treatment due to the following points: (i) an ink-bottled aperture is less prone to oxygen diffusion and is therefore less oxidized than the more accessible channel pores, (ii) the less defective surface characteristics of the ink-bottled pores prevents further oxygen adsorption and consecutive oxidation. It appears that an active catalytic phase specifically localized inside an ink-bottled pore is less affected during oxidative treatments or reaction and this improves the catalyst life-time.
The low oxygen content on the R-pore surface also significantly modifies their wetting behavior against liquid media. It is expected that organic liquid media will have a strong wetting behavior with such a surface and thus, will significantly influence the catalytic activity as well. Previous results have shown that despite the larger size of palladium particles localized within the R-pore, they display a higher hydrogenation activity for cinnamaldehyde than the smaller ones localized on the C-pore surface.
Conclusion
In conclusion, it can be stated that the analytical electron tomography technique is a powerful characterization tool for imaging pores oxidation states in SiC, as the thickness of oxygen-containing layers, and the connectivity of a porous material. In this work it has been applied to porous β-SiC that was submitted to a thermal treatment. It showed that interconnected channel pores are more affected by an oxidative treatment due to the presence of high defects density with a high affinity for oxygen adsorption and to their open access, than ink-bottled pores that exhibit a higher oxidative resistance owing to the presence of a lower defects density and a constrained access. Such results are of great interest for the subsequent use of the material in catalysis. According to our results, the most accessible pore allowing the reactants access to the active phase is the first to be plugged after prolonged oxidative treatment due to the extensive development of a thick layer of SiO2 over the pore channel, which blocks the access to the reactants.From a general point of view, the development of this new and powerful technique with a nanometer resolution will have an important impact in the field of nanomaterials, as it allows one to get access to two most critical parameters: (i) the 3D structure of the sample in the tomographic mode and (ii) the chemical composition of the analyzed material through the electron-energy-loss signature. The technique used in the present work can be employed for analyzing other catalytic systems, where the access to the topmost surface chemical composition represents an important parameter for understanding their catalytic performances, as for instance intimate Si-Al catalysts or zeolite materials, or the chemical composition of various surfaces in carbon nanotubes, inner versus outer, as a function of either a chemical or a thermal treatment.
Experimental methods
Set-up of EFTEM tomography at the nanoscale
EFTEM tomography uses a tilt series of elemental projections to compute an elemental selective reconstruction volume. For our studied system, i.e. β-SiC, the elements of interest are oxygen, present especially on the surface as SiOx compounds, and carbon, present in the bulk as SiC. As the global concentration of oxygen is rather low in an individual SiC aggregate since it is only present as a native oxide layer on the surface, we performed our analysis on oxidized SiC samples, containing 10% of oxygen in weight in order to reach a reasonable signal to noise ratio (SNR) and therefore minimizing the recording duration.For the electron tomography analysis, the β-SiC samples were crushed in a mortar into a very fine powder and dispersed in ethanol in an ultrasonic bath for a few minutes. The solution was then deposited on a copper grid recovered by a holey carbon membrane where finally a drop of a solution containing gold calibrated nanoparticles of 5 nm was deposited. These nanoparticles will be used as fiducial markers in the data treatment process, in particular for the fine alignment of the tilt series before the reconstruction.
The energy filtered images of the tilt series were obtained using a JEOL 2100F (FEG) TEM/STEM electron microscope operating at 200 kV, equipped with a TRIDIEM post-column imaging filter of the Gatan Company. The images acquired on a 2048*2048 pixel cooled CCD detector were hardware-binned to 512*512 pixels with a pixel size of about 1.1 nm. The EFTEM tomography software, implemented as a plug-in in Digital Micrograph, provides automated acquisition of data by controlling the spectrum energy offset and the width of the energy selecting slit. In addition, the software allows to automatically change the specimen tilt step by step, to correct the defocusing and the specimen drift. In order to limit the irradiation damage, a total of 57 angle positions were selected in the angular range between −70 and 70°, with projections taken every 2.5°.
In order to ensure a monotonic dependence of the chemical signal as a function of thickness and concentration, we used the three-window method for the two elements of interest C and O.34,35 This allows a proper extraction of the background in the considered energy range and furnishes elemental projections with a quite good approximation. In that regard, eight images were recorded at each tilt angle: a first Zero-Loss image collecting the elastic scattered electrons, three filtered images at the K-edge of carbon (theoretical position 284 eV) used as a signature of SiC, three energy filtered images at the K-edge of oxygen (theoretical position 532 eV) used as a signature of SiOx, and finally a second Zero-Loss image, used to check the immobility of the sample during the acquisition procedure of the eight filtered images. The energy positions of the filtered images for the carbon atoms were 244, 270 and 300 eV for the two pre-edge windows and the post-edge window respectively, with an energy slit of 24 eV and an exposure time of 5 s for each image; for the oxygen, the energy positions were 470, 513 and 558 eV, with an energy slit of 40 eV and an exposure time of each image of 12 s. A 120 μm diameter objective aperture was used to record all these images.
After the acquisition process of the tilt series is finished, the pre-processing steps and the reconstruction procedure were performed using the IMOD and TOMOJ/EFTETJ softwares.36,37 In a first step, two series of chemical 2D maps (one for the oxygen and the other for the carbon, so-called “elemental projections”), were computed from the tilt series of filtered images, tilt angle by tilt angle. For one element, starting from the three tilt series corresponding to the three chosen energy windows (previously aligned to correct for the specimen drift between their successive acquisitions), a series of chemical projections were computed by extracting from the post-edge image the background estimated from the two pre-edge images using an exponential law. The tilt series are further aligned, in order to set all the projections, elemental or mass-sensitive, in the same frame of reference. Since the signal to noise ratio (SNR) in the chemical projections of C and O is relatively poor, the usual alignment procedure based on cross-correlation or fiducial markers gives poor results. However, in the Zero-Loss tilt series the signal to noise ratio (SNR) is high enough as to allow the implementation of cross-correlation algorithms. In addition, the gold fiducial markers deposited on the TEM grid membrane supporting the object are clearly observable in that case and can be used as reference points to make a more accurate alignment of the tilt series. After checking the stability of the sample during the acquisition process of the successive images recorded at the same tilt angle (by comparing the two Zero-Loss images recorded at the beginning and at the end of the energy record set), the spatial parameters used to align the Zero-Loss tilt series can be applied to align the two elemental projection series. We obtained thus three projection series aligned in a unique frame of reference: the first one corresponds to the Zero-Loss images, which are mass-sensitive, and the two others to the elemental projection series for O and C, which are chemically sensitive. These three projection series are spatially correlated one relative to the others and, as a consequence, the corresponding reconstruction volumes are also spatially correlated, allowing performing a combined analysis. To reduce the typical artifacts appearing in the reconstruction, due to the limited maximum tilt angle and number of projections,15 the three reconstructed volumes were computed using iterative algorithms.38 With respect to the simple back-projection method, the use of these algorithms is also strongly recommended for the tilt series with poor signal to noise ratio (SNR), as it gives a refined solution.39 To extract from the whole reconstruction useful information, a segmentation procedure is needed consisting on a threshold sorting of the voxels of the reconstructed volumes as a function of their grey-level intensity. In our case, for the chemical selective volumes, this procedure is difficult to apply due to a poor resulting signal to noise ratio (SNR). However, as all the volumes are spatially correlated, one can use the segmentation obtained from the mean density volume (with high signal to noise ratio (SNR)) and apply the mask of the analyzed grain, which delimits the borders of the object, to the elemental volumes. In that regard, one can consider for the analysis of elemental maps only the voxels corresponding to the full material. A relative 3D map can finally be deduced, by superposing with different colors the intensities of the considered elemental 3D maps.
To estimate the spatial resolution in the analytical tomographic mode, we have used a method simply based on the superposition of the 2D analytical resolution and of that of the tomographic approach. The first, which depends mainly on the microscope aberrations and experimental conditions used in the acquisition of the filtered images, was estimated by using the relations given by Egerton,40 resulting in a resolution limit of about 5 nm. Furthermore, as usual in the field of electron tomography, the resolution of the tomographic approach was determined by using the analytical formula incorporating the parameters of the acquisition process.15 Using this mathematical approach, the value obtained for the tomographic-specific resolution was about 5.5 nm. By combining this resolution with that of the initial 2D elemental maps deduced previously, we found a lower limit for the spatial resolution of the order of 7 nm, which allows to comparatively analyze the presence of a silicon oxide layer on the R-pore and C-pore surfaces.
Drawbacks and practical difficulties of the EFTEM tomography technique
In addition to the usual artifacts of the electron tomography due to an incomplete sampling of the angular range, another limitation of the EFTEM tomography comes out from the poor signal to noise ratio (SNR) in the chemical projections, which degrades the accuracy of the reconstructions in terms of difference between the 3D solution and the chemical distribution in the original object. To obtain significant results, it is recommended to analyze simultaneously several complementary chemical volumes of the same specimen, in a combined manner. Apart from the problem related with the signal to noise ratio (SNR), other main practical difficulty of this analytical mode is related to the calculation of the elemental projections, in particular to the extraction of the background from the pre-edge filtered images. For instance, one must totally prevent the existence of a negative signal in all the pixels of the 2D elemental maps corresponding to a negative number of atoms. For this reason, with respect to the other methods used to compute the 2D elemental maps as for instance the jump-ratio method,34 the use of the three-windows method is the most suitable. It requires recording three filtered images for each chemical element, but allows a better estimation of the background, pixel by pixel, and furnishes intensities in the elemental projections that monotonically depend on the number of atoms. The effect of the thickness variation when tilting the sample must also be taken into account, as the plural inelastic scattering affects the shape of the spectrum and consequently the background calculation. Such an effect can be neglected in a first approximation, provided that the three windows method is used and especially if the thickness of the object does not change drastically when tilting, as in the case of the grain analyzed in this study. In addition, in order to fulfil the projection requirement for the tomography technique, the effect of the plural scattering on the 2D elemental maps must be neglected in a first approximation. This is generally the case when the sample thickness is lower than the effective inelastic mean free path in any point of the specimen and that for all angular positions of the tilt series.21 In that regard, one has to systematically compare the maximum specimen thickness to the inelastic mean free path for the analyzed specimen. However, if the maximum thickness is reached only on a limited area of the specimen and it is not to different from the estimated mean free path, the contribution of the single scatterings to the total spectrum is still predominant40 and thus the projection requirement for the tomography is fulfilled in a first approximation.Concerning the pre-processing steps of the elemental tilt series before the reconstruction, owing to their poor signal to noise ratio (SNR), the alignment must be performed in an indirect manner, using the parameters deduced from the alignment of the Zero-Loss tilt series. These projections have high SNR and furthermore the presence of the fiducial markers allows to precisely refining the rough cross-correlation solution. Finally, a last experimental limitation is the presence of the irradiation damages in the specimen, especially if long acquisition duration is employed with the general aim to increase the SNR. The optimization of all these parameters, from the acquisition conditions to the parameters and methods for the data treatment and analysis, is needed for obtaining realistic and significant 3D chemical maps with a nanometer spatial resolution.
Acquisition parameters for the STEM-EELS mode
The STEM-EELS mode was locally employed to perform 2D analytical measurement for accessing the pore oxidation degree. The recording of the EELS spectra near the O-K edge was performed using Gatan DigiScan in combination with Spectrum Imaging (SI) plug-in in Digital Micrograph software. The diameter of the probe, used to raster the sample in the chosen direction was about 0.2 nm. The final spectrum was obtained from 70 EELS SI spectra with 2 s exposure time per spectra using a 2 mm spectrometer aperture and a dispersion of 0.3 eV/channel.Acknowledgements
This work was financially supported by SiCat Co. The SEM and TEM experiments were carried out at the facilities of the IPCMS (UMR 7504 CNRS). XPS experiments were carried out at the facilities of the LMSPC (UMR 7515 CNRS) and P. Bernhardt is gratefully acknowledged for performing the experiments.References
- M. J. Ledoux and C. Pham-Huu, Silicon Carbide a novel catalyst support for heterogeneous catalysis, CATTECH, 2001, 5, 226–246 CrossRef CAS.
- G. C. T. Wei, Beta Sic Powders Produced by Carbothermic Reduction of Silica in a High-Temperature Rotary Furnace, J. Am. Ceram. Soc., 1983, 66, c111–113 CrossRef CAS.
- S. Sarin-Poncet; M. J. Ledoux; C. Pham-Huu; J. Bonsquet; B. Madani, Pat. Appl., WO 2005/013345 A1, 2005.
- M. J. Ledoux, C. Crouzet, C. Pham-Huu, V. Turines, K. D. Kourtakis, P. L. Mills and J. J. Lerou, High-Yield Butane to Maleic Anhydride Direct Oxidation on Vanadyl Pyrophosphate Supported on Heat-Conductive Materials: SiC, Si3N4, and BN, J. Catal., 2001, 203, 495–508 CrossRef CAS.
- P. Nguyen, D. Edouard, J. M. Nhut, M. J. Ledoux and C. Pham-Huu, High thermal conductive β-SiC for selective oxidation of H2S: A new support for exothermal reactions, Appl. Catal., B, 2007, 76, 300–310 CrossRef CAS.
- S. Ivanova, E. Vanhaecke, B. Louis, S. Libs, M. J. Ledoux, S. Rigolet, C. Marichal, C. Pham, F. Luck and C. Pham-Huu, Efficient Synthesis of Dimethyl Ether over HZSM-5 Supported on Medium-Surface-Area Beta-SiC Foam, ChemSusChem, 2008, 1, 851–857 CrossRef CAS.
- M. J. Ledoux, S. Hantzer, C. Pham-Huu, J. Guille and M. P. Desaneaux, New synthesis and uses of high-specific-surface SiC as a catalytic support that is chemically inert and has high thermal resistance, J. Catal., 1988, 114, 176 CAS.
- I. Florea, M. Houlle, O. Ersen, L. Roiban, A. Deneuve, I. Janowska, P. Nguyen, C. Pham and C. Pham-Huu, Selective Deposition of Palladium Nanoparticles inside the Bimodal Porosity of SiC Investigated by Electron Tomography, J. Phys. Chem. C, 2009, 113, 17711–17719 CrossRef CAS.
- J. P. Tessonnier, O. Ersen, G. Weinberg, C. Pham-Huu, D. Sheng Su and R. Schlögl, Selective Deposition of Metal Nanoparticles Inside or Outside Multiwalled Carbon Nanotubes, ACS Nano, 2009, 3, 2081–2089 CrossRef CAS.
- C. Colliex, T. Manoubi and O. L. Krivanek, EELS in the Electron Microscopy- A review of present trends, Journal of Electron Microscopy, 1986, 35, 307–313 Search PubMed.
- P. Bayle-Guillemaud, R. Radtke and M. Sennour, Electron spectroscopy imaging to study ELNES at a nanoscale, J. Microsc., 2003, 210, 66–73 CrossRef CAS.
- P. Bayle-Guillemaud, A. Barbier and C. Mocuta, Development of quantitative energy filtering TEM method to study a reactive NiO/80Ni20Fe interface, Ultramicroscopy, 2001, 88, 99–110 CrossRef CAS.
- K. Kaneko and K.-I. Kakimoto, HRTEM and ELNES analysis of polycarbosilane-derived Si–C–O bulk ceramics, J. Non-Cryst. Solids, 2000, 270, 181–190 CrossRef CAS.
- A. J. Koster, U. Ziese, A. J. Verkleij, A. H. Janssen and K. P. De Jong, Three-Dimensional Transmission Electron Microscopy: A Novel Imaging and Characterization Technique with Nanometer Scale Resolution for Material Science, J. Phys. Chem. B, 2000, 104, 9368–9370 CrossRef CAS.
- P. A. Midgley and M. Weyland, 3D electron microscopy in the physical sciences: The development of Z-contrast and EFTEM tomography, Ultramicroscopy, 2003, 96, 413–431 CrossRef CAS.
- G. Mobus and B. J. Inkson, Three-dimensional reconstruction of buried nanoparticles by element-sensitive tomography based on inelastically scattered electrons, Appl. Phys. Lett., 2001, 79, 1369–1371 CrossRef CAS.
- M. Weyland, T. J. V. Yates, R. E. Dunin-Borkowski, T. Laffont and P. A. Midgley, Nanoscale analysis of three-dimensional structures by electron tomography, Scripta Materialia, 2006, 55, 29–33 CrossRef CAS.
- M. H. Gass, K. K. K. Koziol, A. H. Windle and P. A. Midgley, Four-Dimensional Spectral Tomography of carbonaceous Nanocomposites, Nano Lett., 2006, 6, 376–379 CrossRef CAS.
- R. D. Leapmann, E. Kocsis, G. Zhang, T. L. Talbot and P. Laquerriere, Three-dimensional distribution of elements in biological samples by energy-filtered electron tomography, Ultramicroscopy, 2004, 100, 115–125 CrossRef CAS.
- M. A. Aronova, Y. C. Kim, R. Harmon, A. A. Sousa, A. Zhang and R. D. Leapman, Three-dimensional elemental mapping of phosphorus by quantitative electron spectroscopic tomography (QuEST), J. Struct. Biol., 2007, 160, 35–48 CrossRef CAS.
- K. Jarausch, P. Thomas, D. N. Leonard, R. Twesten and C. R. Booth, Four-dimensional STEM-EELS: Enabling nano-scale chemical tomography, Ultramicroscopy, 2009, 109, 326–337 CrossRef CAS.
- G. Mobus, R. C. Doole and B. J. Inkson, Spectroscopic electron tomography, Ultramicroscopy, 2003, 96, 433–451 CrossRef CAS.
- C. Pham-Huu; O. Ersen; M. J. Ledoux, in Nanoparticles and Catalysis, D. Astruc (Ed.), Wiley-VCH, 2008 Search PubMed.
- N. Keller, C. Pham-Huu, S. Roy, M. J. Ledoux, C. Estournés and Guille, Influence of the preparation conditions on the synthesis of high surface area SiC for use as a heterogeneous catalyst support, J. Mater. Sci., 1999, 34, 3189 CrossRef CAS.
- R. Pampuch, W. Ptak, S. Jonas and J. Stoch, Mater. Sci. Monographs, 1980, 6, 435 Search PubMed.
- L. Porte and A. Sartre, J. Mater. Sci., 1989, 24, 271 CAS.
- R. Moene, M. Makkee and J. A. Moulijin, High Surface Area Silicon Carbide as catalyst support characterization and stability, Appl. Catal., A, 1998, 167, 321 CrossRef CAS.
- N. Mozdzierz, M. Backhaus-Ricoult and D. Imhoff, Proc. 13th Int. Congr. On Electron Microscopy, Les Editions de Physique, 1994, 325 Search PubMed.
- N. Keller, C. Pham-Huu, M. J. Ledoux, C. Estournès and G. Ehret, Preparation and characterization of SiC microtubes, Appl. Catal., A, 1999, 187, 255–268 CrossRef CAS.
- N. Keller, G. Koehl, F. Garin and V. Keller, Chem. Commun., 2005, 201 RSC.
- N. Keller, F. Di Grégorio, C. Pham-Huu and V. Keller, Diamond Relat. Mater., 2008, 17, 1867–1870 CrossRef CAS.
- N. Keller, C. Pham-Huu and M. J. Ledoux, Low temperature use of SiC-supported NiS2-based catalysts for selective H2S oxidation Role of SiC surface heterogeneity and nature of the active phase, Appl. Catal., A, 2002, 234, 191 CrossRef CAS.
- S. Ivanova, B. Louis, B. Madani, J. P. Tessonnier, M. J. Ledoux and C. Pham-Huu, ZSM-5 Coatings on a SiC Monoliths: Possible New Structured Catalyst for the Methanol-to-Olefins Process, J. Phys. Chem. C, 2007, 111, 4368 CrossRef CAS.
- P. J. Thomas and P. A. Midgley, An introduction to energy-filtered transmission electron microscopy, Top. Catal., 2002, 21, 109–138 CrossRef CAS.
- J. Veerbeck, D. Van Dyck and G. Van Tendeloo, Energy-filtered transmission electron microscopy: an overview, Spectrochim. Acta, Part B, 2004, 59, 1529–1534 CrossRef.
- D. N. Mastronarde, Dual-axis tomography: An approach with alignment methods that preserve resolution, J. Struct. Biol., 1997, 120, 343–352 CrossRef CAS.
- C. Messaoudii, T. Boudier, C. O. Sanchez Sorzano and S. Marco, TomoJ: tomography software for three-dimensional reconstruction in transmission electron microscopy, BMC Bioinformatics, 2007, 6, 288 CrossRef.
- R. Gordon, R. Bender and Gabor T. Herman, Algebraical Reconstruction Technique (ART) for Three-Dimensional Electron Microscopy and X-Rays Photography, J. Theor. Biol., 1970, 24, 471–481 CrossRef.
- H. Friedrich, M. R. McCartney and P. R. Buseck, Comparison of intensity distribution in tomograms from BF TEM, ADF STEM, HAADF STEM, and calculated tilt series, Ultramicroscopy, 2005, 106, 18–27 CrossRef CAS.
- R. F. Egerton; Electron Energy-Loss Spectroscopy in the Electron Microscope Second Edition, Plenum PressNew York and London Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: SI-1–SI-4. See DOI: 10.1039/c0nr00449a |
| This journal is © The Royal Society of Chemistry 2010 |
